Abstract
Background
Cyclins regulate the cell cycle in association with cyclin dependent kinases (CDKs). CDKs are under inhibitory control of cyclin dependent kinase inhibitors (CDKIs).
Method
In this study we tested the expression of CDKIs p15, p16, p21 and p27 by immunohistochemistry to determine the role of CDKIs in the initiation of primordial follicle growth. Ovaries were collected from 60-day-old cycling B6D2F1/J mice (n = 16).
Results
Expression of p15, p16, p21 and p27 did not vary in granulosa and theca cells by the follicle stage. However, p16 staining was stronger (++) in the oocytes of all primordial, and 57.4 ± 3.1% of primary follicles compared to the remaining primary and more advanced follicles (+). Interestingly, primary follicles with weaker (+) oocyte staining for p16 had significantly larger mean follicle diameter compared to the primary and primordial follicles with stronger (++) oocyte staining (55.6 ± 2.1 vs. 32.0 ± 1.0 and 26.5 ± 0.7 μm, respectively, p < 0.0001). This difference in follicle diameter was mainly due to a larger mean oocyte diameter (primary follicles, stronger vs. weaker, 19.6 ± 0.6 vs. 31.5 ± 1.4 μm, p < 0.0001). Oocytes of atretic follicles showed stronger staining with all four CDKIs.
Conclusions
These preliminary findings suggest that the initiation of oocyte growth, which seems to lead follicle growth, is associated with diminished p16 expression in the mouse ovary. Further studies are needed to investigate the factors that regulate the expression of p16 in the oocyte, which might also govern the initiation of primordial follicle growth.
Similar content being viewed by others
Introduction
The onset of growth of an individual primordial follicle is unpredictable; some beginning shortly after formation while others may remain "quiescent" for many years. By which mechanism the primordial follicles are selected to grow is unknown. We previously showed that the Proliferating Cell Nuclear Antigen (PCNA) is expressed in the rodent ovarian follicles at the earliest sign of growth [1]. PCNA is a co-factor of cyclin-D and it makes a complex with cyclin-D, a cyclin dependent kinase (CDK), and a cyclin dependent kinase inhibitor (CDKI). The progression of cells through the cell cycle is regulated by a family of protein kinases known as the cyclin-dependent kinases (CDKs). The sequential activation of the members of this family and their phosphorylation of certain substrates promotes the progression through the cell cycle. Cyclins function as the positive regulators of CDKs. D-type and E-type cyclins assemble with CDKs during the G1 phase and these holoenzymes act as rate-limiting controllers to regulate passage through the restriction point and the subsequent onset of DNA replication [2, 3]. D-type cyclins are usually synthesized by mid-G1 phase and accumulate to a maximum as cells advance through the G1/S boundary. D-type cyclins act as regulatory subunits for two cyclin-dependent kinases CDK4 and CDK6 [4, 5]. The synthesis of another G1 phase cyclin, cyclin E, increases in late G1 and decreases once DNA replication is initiated. Cyclin E forms complexes during this interval with CDK2. Cyclins and CDKs assemble into complexes with one another as cells progress through G1 phase, cyclins being required to activate the serine-threonine kinase activity of their catalytic partners. Furthermore, CDK-activating kinase (CAK) phosphorylates cyclin-bound CDKs on a single threonine residue, a modification that is essential for their activity [6–9].
Cyclin-dependent kinase inhibitors (CDKIs) are proteins that bind to and inhibit the activity of CDKs. Two major classes of CDK inhibitors have been identified. The p16 family (p15, p16, p18 and p19) binds to and inhibits the activities of CDK4 and CDK6. The p21 family (p21, p27, p28 and p57) can bind to broad range of CDK-cyclin complexes and inhibit their activities. CDKIs are capable of suppressing growth, and several lines of evidence strongly suggest that at least some CDKIs may be tumor suppressor proteins [10, 11].
In this study, we studied the expression of four CDKIs; p15, p16, p21 and p27 in mouse ovaries by immunohistochemistry to assess whether the initiation of primordial follicle growth was associated with the expression of CDKIs.
Materials and Methods
The study was approved by the institutional animal care committee at the State University of New York Health Science Center at Brooklyn.
Tissue Preparation and Immunohistochemistry
Ovaries were collected from 60-day-old cycling B6D2F1/J mice (n = 16) during estrus. Ovaries were fixed in Bouin's solution for 6 hours and transferred into 70% alcohol and incubated for 24 hours at room temperature. After 24 hours, ovaries were embedded in paraffin and serially sectioned at 5 μm. Sections were mounted on coated slides (Vectabond; Vector Laboratories, Burlingame, CA).
Forty sections were randomly selected from one ovary in each animal and stained for CDKIs p15, p16, p21 and p27 (Vector Laboratories, Burlingame, CA). Sections were deparaffinized in xylene for 10 min, gradually rehydrated in ethanol for 9 min. After washes with water and TBS either normal goat serum for p16 and p27 (rabbit polyclonal antibodies) or normal rabbit serum for p15 and p21 (goat polyclonal antibodies) were added for 20 min at 37°C to prevent non-specific binding. Then first antibodies were added (1/50) for p15, p21, p27 and (1/100) for p16 in TBS and sections were incubated for 90 min at 37°C. After a wash in TBS, sections were quenched in 3% hydrogen peroxidase to block endogenous peroxidase activity. Then sections were immersed in water and TBS and second antibodies were added at a dilution of 1/100 for 30 min at 37°C. Anti-rabbit antibody for p16 and p27 (rabbit polyclonal antibodies) and anti-goat antibody for p15 and p21 (goat polyclonal antibodies) were used for second antibodies. After a wash in TBS, avidin-biotin complex was added for 20 min at 37°C followed by diaminobenzadine (DAB) solution for 4 min. Sections were then counterstained with hematoxylin. The immunostaining intensity was quasi-quantified using a "-" to "+++" scale. This was a scale comparing relative staining intensity within each antibody amongst cell types and follicle stages.
Morphometry
All sections were examined under 100× to 1000× magnification under light microscopy to determine immunostaining characteristics and the follicular stages. Earlier stages of follicles were classified as described previously [1]. Primordial follicle, an ungrown oocyte encapsulated by flattened or squamous cell; and primary follicle, with a single layer of cuboidal granulosa cells.
One section from each p16-stained slide was randomly selected for oocyte and follicle measurements. There were 2 sections per animal for a total of 32 sections. All primordial (n = 249) and primary follicles (n = 160) with the oocytes that had a nucleus were examined in each section. Oocyte and follicle diameters were measured by a micrometer. Mean oocyte and follicle diameters in each follicle category were compared in relation to the intensity of the staining for p16.
Statistical Analysis
Statistical analysis was performed using a two-way analysis of variance. Statistical significance was set at p = 0.05.
Results
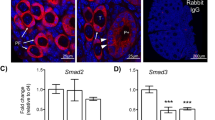
Quasiquantification of immunostaining intensities for each CDKI is shown in table 1. Expression of p15, p16, p21 and p27 did not vary in granulosa and theca cells by the follicle stage. However, p16 staining was stronger (++) in the oocytes of all primordial (Figure 1A and 1E) and 57.4 ± 3.1% of primary follicles (Figure 1B,1C) compared to the remaining primary (Figure 1D,1E) and more advanced follicles (+) (Figure 2A,2B). Interestingly, primary follicles with weaker (+) oocyte staining for p16 (Figure 1D,1E) had significantly larger mean follicle diameter compared to the primary and primordial follicles with stronger (++) stained oocytes (55.6 ± 2.1 vs. 32.0 ± 1.0 and 26.5 ± 0.7 μm, respectively, p < 0.0001). This difference in follicle diameter was mainly due to a larger mean oocyte diameter (primary follicles, stronger vs. weaker, 19.6 ± 0.6 vs. 31.5 ± 1.4 μm, p < 0.0001). The oocytes of atretic follicles showed stronger staining with p16 (Figure 2C) as well as other CDKIs (not shown).
Changing expression of p16 in oocytes of follicles initiating growth. Original magnification 1000X. (A)Increased expression of p16 in primordial follicle oocytes (arrows); (B&C) Increased expression of p16 in primary follicles with unenlarged oocytes (arrows); (D) Diminished expression of p16 after enlargement of the oocyte in primary follicles. (E) Note the primordial follicle (arrow) with significantly higher expression of p16 in its oocyte compared to the adjacent primary follicle with an enlarged oocyte. Bar = 50 μm.
Discussion
Here we showed that p16 is strongly expressed in the oocytes of primordial and early primary follicles (primary follicles with "ungrown" oocytes) in comparison to primary follicles. The change in the expression of p16 appears to coincide with the first measurable sign of oocyte growth. These findings suggest that the initiation of oocyte growth, which seems to lead follicle growth, is associated with diminished p16 expression in the mouse ovary.
Oocyte in adult mouse ovary has entered the first meiotic prophase and arrested in early M-phase. Oocyte in adult mice has already finished DNA replication (S-phase). It is, therefore curious that p16, a factor controlling G1/S, plays a role in healthy oocyte. However recent studies have shown that TGF-B family member ligands such as GDF-9, which influence cell proliferation and differentiation, are involved in follicle growth initiation and oocyte growth [12, 13]. Interestingly, other studies have shown that TGF-B's antiproliferative effects on some cell types is mediated by the p16 family CDKIs [14]. Since GDF-9 is only synthesized in the oocyte, and since it is not expressed in the oocyte until primary follicle stage, a different member of the TGF-B family such as Activin-A [15] may be influencing oocyte p16 expression via paracrine mechanisms.
Role of p16 in primordial follicle quiescence might be through an entirely different mechanism. CDKIs also play role in contact inhibition [16]; and thus high expression of p16 in ungrown oocytes may also reflect inhibition bestowed on them by pregranulosa cells. It is therefore plausible that p16's role in oocyte growth may be different than its general role in cell cycle control.
We also found that atretic follicles strongly expressed all four CDKIs tested. This finding is consistent with the previous work in other cell types which indicated the involvement of CDKIs in apoptosis [17]. P16 expression was also high in theca cells regardless of follicle stage. This is consistent with the low mitotic activity of these cells.
In addition, granulosa cells of healthy growing follicles showed weaker staining. This finding complements the finding from our previous study that PCNA is strongly expressed in granulosa cells starting from the primary follicle stage [1]. PCNA is a cofactor of Cyclin D, and p16 family of kinase inhibitors inhibit the activity of CDKs associated with this cyclin. Downregulation of p16 would result in activation of this CDK and increased PCNA and Cyclin D expression, which result in the progression of cell cycle through the G1/S phase. There is also an inverse relationship between PCNA and p16 expression in oocyte growth. PCNA is expressed after the oocyte enlargement in primary follicles, exactly the same stage when p16 expression disappears.
Factors regulating the expression of p16 are unknown. Recent work has shown that p16 expression may be regulated by Jun family of transcription factors. Interestingly, jun family of transcription factors play an important role in cell proliferation [18]. These transcription factors are activated by the enzyme Jun amino terminal kinase (JNK), the expression of which can be regulated by UV, genotoxic stress, and cytokines. Our current research focuses on these and other potential upstream regulators of p16.
In conclusion, this preliminary study suggests that quiescence of primordial follicles might be maintained by high expression of p16. Cyclin dependent kinase inhibitors have been shown to be growth suppressors, and the absence or decreased expression of p16 strongly coincides with the first measurable follicle growth. Further studies will be helpful in determining the factors that regulate the expression of p16, which might also govern the initiation of follicular growth.
References
Oktay K, Schenken RS, Nelson JF: Proliferating cell nuclear antigen marks the initiation of follicular growth in the rat. Biol Reprod. 1995, 53: 295-301.
Hunter T: Braking the cycle. Cell. 1993, 75: 839-841.
Sherr CJ: Mammalian G1 cyclins. Cell. 1993, 73: 1059-1065.
Matsushime H, Ewen ME, Strom DK, Kato J, Hanks SK, Roussel MF, Sherr CJ: Identification and properties of an atypical catalytic subunit (p34 PSKJ3 /CDK4) for mammalian D-type G1 cyclins. Cell. 1992, 71: 323-334.
Meyerson M, Harlow E: Identification of a G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994, 14: 2077-2086.
Sherr CJ: Mammalian G1 cyclins and cell cycle progression. Proc Assoc Am Physicians. 1995, 107: 181-186.
Koff A, Cross F, Fischer A, Schumacher J, Leguellec K, Philippe M, Roberts JM: Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991, 66: 1217-1228.
Koff A, Giordano A, Desai D, Yamashita K, Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR, Roberts JM: Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992, 257: 1689-1694.
Dulic V, Lees E, Reed SI: Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992, 257: 1958-1961.
Xiong Y, Hannon G, Zhang H, Casso D, Kobayashi R, Beach D: p21 is a universal inhibitor of cyclin kinases. Nature. 1993, 366: 701-704. 10.1038/366701a0.
Grana X, Reddy EP: Cell cycle control in mammalian cells: role of cyclins, cyclin-dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene. 1995, 11: 211-219.
Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM: Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol. 1999, 13: 1018-34.
Hayashi M, McGee EA, Min G, Klein C, Rose UM, van Duin M, Hsueh AJ: Recombinant growth differentiation factor-9 (GDF-9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology. 1999, 140: 1236-1244.
Hummer BT, Barlett C, Henry E, Weissman BE: Expression of Smad4 in the FaDu cell line partially restores TGF-beta growth inhibition but is not sufficient to regulate fibronectin expression or suppress tumorigenicity. J Cell Physiol. 2003, 194: 289-302. 10.1002/jcp.10202.
Oktay K, Karlikaya G, Akman O, Ojakian G, Oktay M: Interaction of Extracellular Matrix and Activin-A in the initiation of follicle growth in the mouse. Biol Reprod. 2000, 63: 457-461.
Joyce NC, Harris DL, Mello DM: Mechanism of mitotic inhibition in corneal endothelium: contact inhibition and TGF-beta2. Invest Ophtalmol Vis Sci. 2002, 43: 2152-2159.
Kim M, Katayose Y, Rojanala L, Shas S, Sgagias M, Jang L, Jung YJ, Lee SH, Hwang SG, Cowan KH: Induction of apoptosis in p161 ink4A mutant cell lines by adevovirus-mediated overexpression of p161INK4A protein. Cell Death Differ. 2000, 7: 706-711. 10.1038/sj.cdd.4400703.
Shaulian E, Karin M: AP-1 in cell proliferation and survival. Oncogene. 2001, 20: 2390-2400. 10.1038/sj.onc.1204383.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Bayrak, A., Oktay, K. The expression of cyclin-dependent kinase inhibitors p15, p16, p21, and p27 during ovarian follicle growth initiation in the mouse. Reprod Biol Endocrinol 1, 41 (2003). https://doi.org/10.1186/1477-7827-1-41
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-1-41