Abstract
The ability of the testis to convert irreversibly androgens into estrogens is related to the presence of a microsomal enzymatic complex named aromatase, which is composed of a specific glycoprotein, the cytochrome P450 aromatase (P450arom) and an ubiquitous reductase. The aromatase gene is unique in humans and contained 18 exons, 9 of them being translated. In the rat testis we have immunolocalized the P450arom not only in Leydig cells but also in germ cells and especially in elongated spermatids. Related to the stage of germ cell maturation, we have shown that the level of P450arom mRNA transcripts decreases, it is much more abundant in pachytene spermatocytes and round spermatids than in mature germ cells whereas the aromatase activity is 2–4 fold greater in spermatozoa when compared to the younger germ cells. Using a highly specific quantitative competitive RT-PCR method we have evidenced that several factors direct the expression of the aromatase gene in Leydig cells, Sertoli cells, pachytene spermatocytes and round spermatids, and it is obvious that promoter PII is the main one but other promoters could be concerned.
In the bank-vole testis we have observed a positive correlation between a fully developed spermatogenesis and a strong immunoreactivity for both P450arom and estrogen receptor β not only in Sertoli cells but also in pachytene spermatocytes and round spermatids. Our recent data obtained from ejaculated human spermatozoa demonstrate the presence of aromatase both in terms of mRNA and protein, and in addition, we suggest that aromatase could be involved in the acquisition of sperm motility. Indeed in men the congenital aromatase deficiency is associated with severe bone maturation problems and sterility. Together with the widespread distribution of estrogen receptors in testicular cells these data clearly show that estrogens play a physiological role in the regulation of spermatogenesis in mammals.
Similar content being viewed by others
Introduction
The mammalian testis is a complex organ characterised by two main functions : synthesis of steroid hormones and production of spermatozoa. It is well known that normal testicular development and maintenance of spermatogenesis are controlled by gonadotrophins and testosterone whose effects are modulated by locally-produced factors, and among them estrogens are obviously concerned [1]. Within the reproductive tract of the male the levels of estrogens are far higher than in the general blood compartment [2], therefore favoring a testicular source of estrogens [3].
The role of estrogens in the development and physiology of male reproductive tract of mammals is still a matter of debates even though there is a growing body of evidence suggesting that estrogens are playing a role via their specific receptors (ERα and ERβ) which are distributed all along the genital tract [4–6].
Aromatase expression in male germ cells
The cytochrome P450 aromatase (P450arom) is involved in the irreversible transformation of androgens into estrogens and is present in the endoplasmic reticulum of numerous vertebrate tissues. The P450arom is a microsomal enzymatic complex composed of two proteins : a ubiquitous NADPH-cytochrome P450 reductase and a cytochrome P450 aromatase, which contains the heme and the steroid binding pocket. In humans the P450arom is the product of a single gene called Cyp19, which belongs to the cytochrome P450 gene family, containing more than 500 members [7]. The Cyp19 gene lies on more than 120 kb length with a coding region of 9 exons plus 9 untranslated exons I. The Cyp19 gene expression is regulated by tissue-specific promoters producing alternate 5'-untranslated exons I that are then spliced onto a common 3'-splice acceptor site in the exon II upstream of the translation start site [8].
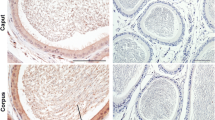
In the mammalian testis it's well known that aromatase is mainly localized in Leydig cells (for review [1]). In rodents, past efforts to determine the source of testicular estrogens have been a considerable subject of interest and led to the assertion that Leydig cells synthesize estrogen in adults, whereas Sertoli cells are the major source in immature animals. However it has been shown that germ cells from adult male rat represent a new source of estrogens [4]. Indeed, the amount of P450arom transcripts is higher in pachytene spermatocytes than in round spermatids and obviously than in testicular spermaozoa. Conversely the aromatase activity is more intense in haploid germ cells than in younger germ cells which was also confirmed by the immunolocalisation of the protein on testicular cells mainly elongated spermatids [9]. These observations have been corroborated by Janulis et al [10] who showed that the aromatase activity is localized in the cytoplasmic droplet and decreased according to the epididymal region, being higher in caput than in cauda. Moreover it has been reported the existence of P450arom transcripts in the epithelial cells of the rat epididymis [11].
In mouse [12] as well as in bank vole [13], the aromatase is present not only in Leydig cells and Sertoli cells but also in germ cells. In the bank vole, a seasonal breeder, the P450arom is much more expressed (in terms of specific transcripts, activity and immunolocalisation) in pachytene spermatocytes and spermatids of animals bred in long day-light cycle than in animals in winter rest [13, 14]. Indeed there is a synchronisation between the recrudescence of spermatogeneis and the levels of expression of aromatase and estrogen recptors beta. Recent observations made in our group showed that in seminiferous tubules of the stallion there was a positive immunoreactive signal for aromatase not only in Leydig cells but also in cytoplasm surrounding germ cells therefore suggesting strongly the presence of aromatase in Sertoli cells [15].
In the Rhesus monkey, it has been reported that testis and to a lesser extent epididymidis contained P450arom transcripts [16] and moreover, in the epididymal regions a discrepancy was observed between the amount of transcripts and the aromatase activity which was found more active in caput than in cauda although the P450arom mRNA levels were reversed. In humans the main source of estrogens is in Leydig cells ; nevertheless the Sertoli cells are able to synthesize estradiol in vitro [17]. It has been also claimed that estrogens are present in spermatozoa [1, 18]. From these observations we have examined the ability of human ejaculated spermatozoa to transform androgens into estrogens. When sperm RNA was used as template in RT-PCR we have shown the presence of P450arom transcripts; the sequences alignment from these PCR products and granulosa cells with published human P450arom gene were identical. Using Western blots and a specific monoclonal antibody against aromatase [19] we have evidenced the presence of aromatase in these sperm cells which was obviously more abundant in spermatozoa containing cytoplasmic droplets. These observations are in fitting with other recent data [20], and in addition we have demonstrated that the amount of P450arom transcripts was 30% lower in immotile than in motile spermatozoa [21].
In order to bring insights onto the role of estrogens in semiiferous tubules, it is first necessary to analyse the regulation of the Cyp19 gene transcription. Thus we have evidenced using RACE-PCR that promoter II directs the expression of aromatase gene whatever the testicular cell type studied in the rat [22]. Germ cells are potent targets for growth factors and cytokines; indeed, pachytene spermatocytes (PS) and round spermatids (RS) produce TGFβ and TNFα; but only TGFβ receptors are present in germ cells [23]. Accordingly in highly purified germ cells from adult rat (pachytene spermatocytes and round spermatids) we have demonstrated that TGFβ inhibits the expression of Cyp19 in both germ cell fractions although TNFα exerts a stimulatory role in pachytene spermatocytes only; these data have been confirmed by the estradiol outputs measured in germ cell culture media [23]. It is noteworthy that the effect of TNFα is amplified in presence of dexamethasone which is may be supported by the presence of an other promoter like promoter I.4 as shown in adipose tissue [24]. It is also obvious that other modulators direct the expression of the aromatase gene since in Leydig cells we have shown that the amount of P450arom transcripts are increased in presence of seminiferous conditioned medium [25]. Our data are likely in agreement with a recent work showing that the aromatase expression is controlled by three different promoters in mice that is to say that in the male gonad the Cyp19 gene expression is directed by a testis specific promoter [26].
Estrogens and testicular functions
In order to exert a biological role, testicular estrogens should interact with estrogen receptors (ER) which in turn modulate the transcription of specific genes. Therefore considering the presence of at least two ER (ERα and ERβ) in most of the testicular cells and the other parts of the genital tract, the physiological role of estrogens in male reproduction is now extensively revisited [4–6, 27, 28]. Spermatogenesis in rodents is in part under estrogens control, namely the stem germ cell number and the spermatid maturation [29, 30]. In the bank the expression of androgen receptors, P450arom and ERs (α and β) in testicular cells is related to the length of the photoperiod. More precisely P450arom and ERβ are much more expressed in testes (especially in spermatocytes and elongated spermatids) of long photoperiod-reared animals in which spermatogenesis is fully developed when compared to short-day length bred animals with regressed testes [13, 31]. These observations are in fitting with previous reports [32, 33] and the recent observations of Pak et al [34] demonstrating an improvement of the recrudescence of spermatogenesis in estradiol-treated hamster which have been confirmed by Bilinska et al [35] in bank vole.
Therefore estradiol is now considered as a survival factor for germ cells [36] that is consistent with data obtained in male monkeys treated with aromatase inhibitor in which a blockage of spermatid maturation has been observed [30]. In addition Shetty et al [37] have reported a negative role of androgens in the recovery of spermatogenesis in irradiated rats an in mice the sperm capacitation, acrosome reaction and fertilizing capacity are improved by estradiol and even more by phytoestrogens [38].
Today several experimental models of mice have been developed and shown helpful to clarify the estrogens role in vertebrates [39]. There is evidence from estrogen receptor gene knock out (ERα KO) mouse that estrogens are necessary for the achievement of fertility [40]. The male mice deficient in aromatase (ArKO) develop normally, the males are able to breed and to produce litters; nevertheless starting at the age of 5 months ArKO males start to have failures of spermatogenesis and by the age of one year all male mice are infertile [41].
In men it has been shown that the aromatase deficiency consecutive to a P450 aromatase gene mutation leads to sterility [42, 43] ; to our knowledge from the 6 reported (Table 1) cases 3 of them showed a low sperm counts number [44–46].
An inactivating mutation in the ERα gene (exon 2) has been reported by Smith et al [47] in an infertile man with a number of spermatozoa in the normal range although the viability was diminished. In addition a correlation between the amount of estradiol in the seminal plasma and the germ cell number has been shown [48] as well as a positive role for estradiol in stimulating the motility of human spermatozoa [49].
Durkee et al [50] have reported the existence of ER in human sperm and in addition, it has been demonstrated that the sperm membrane contains an estrogen receptor-related protein able to bind steroids (see for review [51]).
Obviously these membrane receptors would be connected with a signal transduction pathway involving quick answers such as the calcium channel and a calcium/calmoduline complex, known to be concerned for instance in sperm mobility and capacitation [52]. The demonstration of the production of nitric oxyde in spermatozoa is now considered as an alternative pathway for improving the sperm motility and capacitation [53]. Together with the existence of aromatase [21] the intracrine role of estrogens should be considered in spermatozoa.
Conclusion
Today it is clear that not only testicular somatic cells but also germ cells represent an additional source of estrogens in several species of mammals including man. Germ cells (both meiotic and post-meiotic cells) do not only produce estrogens but since they contain estrogen receptors that would explain part of the role (intracrine and / or paracrine) of estrogens in male germ cell development. The mechanisms of action of estrogens in the reproductive organs of the male (Figure 1) remain to be clarified as well as the regulation of the aromatase gene expression, not yet fully understood especially according to the testicular development. Furthermore one should kept in mind that not only rodent spermatozoa but ejaculated human spermatozoa express a functional aromatase and together with ER these data open new considerations about the role of estrogens all along the male genital tract and may be also in sperm fertilizing ability (Figure 2).
Aromatase and estrogen receptors (ER) in adult male rat gonad. PS: pachytene spermatocytes, RS: round spermatids, Spz: spermatozoa. Aromatase has been demonstrated in terms of mRNA (RT-PCR), protein (Western blots) and enzyme activity (measurements of estradiol output in culture media) in the various testicular cells. ER : estrogen receptors localisation.
References
Carreau S, Genissel C, Bilinska B, Levallet J: The oestrogen sources in the testis and the reproductive tract of the male. Inter J Androl. 1999, 22: 211-213.
Hess RA: Oestrogen in fluid transport in efferent ducts of the male reproductive tract. Rev Reprod. 2000, 5: 84-92.
Carreau S: Germ cells: a new source of estrogens in the male gonad. Mol Cell Endocrinol. 2001, 178: 65-72.
Carreau S, Bourguiba S, Lambard S, Galeraud-Denis I, Genissel C, Levallet J: Reproductive system: aromatase and estrogens. Mol Cell Endocrinol. 2002, 193: 137-143.
O'Donnell L, Robertson KM, Jones ME, Simpson ER: Estrogen and spermatogenesis. Endocr Rev. 2001, 22: 289-318.
Scobie GA, Macpherson S, Millar MR, Groome NP, Romana P, Saunders PTK: Human estrogen receptors : differential expression of ERalpha and beta and the identification of ERbeta variants. Steroids. 2002, 67: 985-992.
Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, It Y, Fisher CR, Dodson MM, Mendelson CR, Bulun SE: Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994, 15: 342-355.
Sebastian S, Bulun SE: A highly complex organization of the regulatory region of the human CYP19 (aromatase) gene as revealed by the human genome project. J Clin Endocrinol Metab. 2001, 86: 460-4602.
Levallet J, Bilinska B, Mittre H, Genissel C, Fresnel J, Carreau S: Expression and immunolocalization of functional cytochrome P450 aromatase in mature rat testicular cells. Biol Reprod. 1998, 58: 919-926.
Janulis L, Bahr JM, Hess RA, Jansen S, Osawa Y, Bunick D: Rat testicular germ cells and epididymal sperm contain active P450 aromatase. J Androl. 1998, 19: 65-71.
Wiszniewska B: Primary culture of rat epididymal epithelial cells as a source of estrogen. Andrologia. 2002, 34: 1-8.
Nitta H, Bunick D, Hess RA, Janulis L, Newton SC, Milette CF, Osawa Y, Shizuta Y, Toda K, Bahr JM: Germ cells of the mouse testis express P450 aromatase. Endocrinology. 1993, 132: 1396-1401.
Bilinska B, Schmalz-Fraczek B, Sadowska J, Carreau S: Immunolocalization of cytochrome P450 aromatase and oestrogen receptors α and β in bank vole testicular cells. Acta Histochem. 2000, 102: 167-181.
Kotula-Balak , Slomczynska M, Fraczek B, Bourguiba S, Tabarowski Z, Carreau S, Bilinska B: Complementary approaches demonstrate that cellular aromatization in the bank vole testris is related to photoperiod. Eur J Histochem. 2003, 47: 55-62.
Sipahutar H, Sourdaine P, Moslemi S, Plainfossé B, Seralini GE: Immunolocalization of aromatase in stallion leydig cells and seminiferous tubules. J Histochem Cytochem. 2003, 51: 311-318.
Pereyra-Martinez AC, Roselli CE, Stadelman HL, Resko JA: Cytochrome P450 aromatase in testis and epididymis of male rhesus monkeys. Endocrine. 2001, 16: 15-19.
Carreau S: Paracrine control of human Leydig cell and Sertoli cell functions. Folia Histochem Cytobiol. 1996, 34: 111-119.
Chew PCT, Loganath A, Peh KL, Chow WP, Gunasegaram R, Ratman SS: Concentrations of intracellular sex steroids in human spermatozoa. Arch Androl. 1993, 30: 165-170.
Turner KJ, Macpherson S, Millar MR, McNeilly AS, Williams K, Cranfield M, Groome NP, Sharpe RM, Fraser HM, Saunders PTK: Development and validation of a new monoclonal antibody to mammalian aromatase. J Endocrinol. 2002, 172: 21-30.
Rago V, Bilinska B, Palma A, Ando S, Carpino A: Evidence of aromatase localization in cytoplasmic droplet of human immature ejaculated spermatozoa. Folia Histochem & Cytobiol. 2003, 41: 23-28.
Lambard S, Galeraud-Denis I, Bouraïma H, Bourguiba S, Chocat A, Carreau S: Expression of aromatase in human ejaculated spermatozoa: a putative marker of motility. Molec Human Reprod. 2003,
Lanzino M, Catalano S, Genissel C, Ando S, Carreau S, Hamra K, McPhaul MJ: Aromatase messenger RNA is derived from the proximal promoter of the aromatase gene in Leydig, Sertoli, and germ cells of the rat testis. Biol Reprod. 2001, 64: 1439-1443.
Bourguiba S, Genissel C, Lambard S, Bouraïma H, Carreau S: Regulation of aromatase gene expression in leydig cells and germ cells. J Steroid Biochem Molec Biol. 2003,
Shozu M, Zhao Y, Simpson ER: TGFβ1 stimulates expression of the aromatase (Cyp19) gene in human osteoblast and THP-1 cells. Mol Cell Endocrinol. 2000, 160: 123-133.
Genissel C, Levallet J, Carreau S: Regulation of cytochrome P450 aromatase gene expression in adult rat Leydig cells. Comparison with estradiol production. J Endocrinol. 2001, 168: 95-105.
Golovine K, Schwerin M, Vanselow J: Three different promoters control expression of the aromatase cytochrome p450 gene (cyp 19) in mouse gonads and brain. Biol Reprod. 2003, 68: 978-984.
Mowa CN, Iwanaga T: Expression of estrogen receptor-alpha and -beta mRNAs in the male reproductive system of the rat as revaeled by in situ hybridization. J Molec Endocrinol. 2001, 26: 165-174.
Saunders PTK, Sharpe RM, Williams K, Macpherson S, Urquart H, Irvine DS, Millar M: Differential expression of oestrogen receptor α and β proteins in the testes and male reproductive system of human and non-human primates. Mol Hum Reprod. 2001, 7: 227-236.
Li H, Papadopoulos V, Vidic B, Dym M, Culty M: Regulation of rat testis gonocyte proliferation by platelet-derived growth factor and estradiol: identification of signaling mechanisms involved. Endocrinology. 1997, 138: 1289-1298.
Shetty G, Krishnamurthy H, Krishnamurthy HN, Bhatnagar S, Moudgal RN: Effect of estrogen deprivation on the male reproductive physiology of male and female primates. J Steroid Biochem Molec Biol. 1997, 61 (3–6): 26-30.
Bilinska B, Schmalz-Fraczek B, Kotula M, Carreau S: Photoperiod-dependent capability of androgen aromatization and the role of estrogens in bank vole testis visualized by means of immunohistichemistry. Mol Cell Endocrinol. 2001, 178: 189-198.
Atanassova N, McKinnell C, Turner KJ, Walker M, Fisher JS, Morley M, Millar MR, Groome NP, Sharpe RM: Comparative effects of neonatal exposure of male rats to potent and weak (environmental) estrogens on spermatogenesis at puberty and the relationship to adult testis size and fertility: evidence for stimulatory effects of low estrogen levels. Endocrinology. 2000, 141: 3898-3907.
Ebling FJP, Brooks AN, Cronin AS, Ford H, Kerr JB: Estrogenic induction of spermatogenesis in the hypogonadal mouse. Endocrinology. 2000, 141: 2861-2869.
Pak TR, Lynch R, Tsai PS: Estrogen accelerates gonadal recrudescence in photo-regressed male Siberian hamsters. Endocrinology. 2002, 143: 4131-4134.
Bilinska B, Gancarczyk , Kotula-Balak M, Slomczynska M: Effect of 17β-estradiol on testicular structure and immunoexpression of aromatase in immature bank vole testes. VIth International Aromatase Conference, Kyoto. Abs PI-8-2002, Oct 26–30
Pentakainen V, Erkkila K, Suomalainen L, Parvinen M, Dunkel L: Estradiol acts as a germ cell survival factor in the human testis. J Clin Endocrinol Metab. 2000, 85: 2057-2067.
Shetty G, Wilson G, hardy MP, Niu E, Huhtaniemi I, Meistrich ML: Inhibtion of recovery of spermatogenesis in irradiated rats by different androgens. Endocrinology. 2002, 143: 3385-3396.
Adeoya-Osiguwa SA, Markoulaki S, Pocock V, Milligan SR, Fraser LR: 17 beta-estradiol and environmental estrogens significantly affect mammalian sperm function. Human Reprod. 2003, 18: 100-107.
Cooke HJ, Saunders PTK: Mouse models of male infertility. Nature Rev Genetics. 2002, 3: 790-801.
Couse JF, Korach KS: Estrogen receptor null mice : what have we learned and where will they lead us?. Endocr Rev. 1999, 20: 358-417.
Robertson KM, O'Donnell L, Jones MEE, Meachem SJ, Boon WC, Fisher CR, Graves KH, McLachlan RI, Simpson ER: Impairment of spermatogenesis in mice lacking a functional aromatase (Cyp 19) gene. Proc Natl Acad Sc USA. 1999, 96: 7986-7997.
Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER: Effect of testosterone and estradiol in a man with aromatase deficiency. New Engl J Med. 1997, 337: 91-95.
Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K: Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995, 80: 3689-3698.
Carani C, Fabbi M, Zirilli L, Sgarbi I: Estrogen resistance and aromatase deficiency: clinical aspects in men. J Soc Bi. 2002, 196: 245-248.
Herrmann BL, Saller B, Janssen OE, Gocke P, Bockisch A, Sperling H, Mann K, Broecker M: Impact of estrogen replacement therapy in a male with congenital aromatase deficency caused by a novel mutation of the CYP19 gene. J Clin Endocrinol Metab. 2002, 87: 5476-5484.
Kottler ML, Piura M, Mittre H, Carreau S: Clinical findings in an adult man with a novel mutation in the aromatase gene. VIth International Aromatase Conference. Abs PII-2-30. 2002 Kyoto 26–30 Oct
Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS: Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. New Engl J Me. 1994, 331: 1056-1061.
Purvis K, Lndgren M, Cekan Z, Diczfalusy E: Indices of gonadal function in the human male. Seminal plasma levels of steroids in normal and pathological conditions. Clin Endocrinol. 1975, 4: 247-248.
Cheng CY, Boettcher B: The effects of steroids on the in vitro migration of washed human spermatozoa in modified tyrode's solution or in fasting human blood serum. Fertil Steril. 1979, 32: 566-570.
Durkee TJ, Mueller M, Zinaman M: Identification of estrogen receptor protein and messenger ribonucleic acid in human spermatozoa. Amer J Obst Gyn. 1998, 178: 1288-1295.
Luconi M, Forti G, Baldi E: Genomic and nongenomic effects of estrogens : molecular mechanisms of action and clinical implications for male reproduction. J Steroid Biochem Molec Biol. 2002, 80: 369-381.
Revelli A, Massobrio M, Tesarik J: Nongenomic actions of steroid hormones in reproductive tissues. Endocr Rev. 1998, 19: 3-17.
Herrero MB, Gagnon C: Nitric oxyde : a novel mediator of sperm function. J Androl. 2001, 22: 349-356.
Acknowledgments
The authors express their warm thanks for Dr Jérôme Levallet who has been a pioneer in that field in my team.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Carreau, S., Lambard, S., Delalande, C. et al. Aromatase expression and role of estrogens in male gonad : a review. Reprod Biol Endocrinol 1, 35 (2003). https://doi.org/10.1186/1477-7827-1-35
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-1-35