Abstract
Background
The association of ureterosigmoidostomy with colonic cancer is well established. A 100-fold increased risk of malignancy has been proposed in association with ureterosigmoidostomy. Characteristically there is a latent period of around 20–30 years before the occurrence of cancer.
Case presentation
An unusual case of adenocarcinoma of the colon in a 36-year-old patient is presented. The patient underwent three operations in his infancy for exstrophy but after failure to close bladder, ureterosigmoidostomy was attempted at the age of 5 years and was converted to an ileal conduit after 8 months. At the age of 36 years, 30 years following ileal conduit urinary diversion for exstrophy, he presented in emergency with large bowel obstruction due to adenocarcinoma of the sigmoid colon.
Conclusion
Patients who undergo urinary diversion for exstrophy may be kept on a regular follow-up surveillance colonoscopy as most of these young adults may later present with vague abdominal symptoms which may not be taken seriously until they increase to an extent as to present with intestinal obstruction as in the present case.
Similar content being viewed by others
Background
The ureterosigmoidostomy is reported to increase the risk of malignancy by 100-fold increased risk of malignancy [1–3]. Characteristically this occurs after a latent period of 20–30 years.
Colonic mucosa has been shown to be at increased risk of cancer development after prolonged exposure to urine [4], a term urocolonic tumors was coined by Gittes to explain this phenomenon [5]. The pathogenesis of these tumors is not clear. One theory suggests production of nitrite and N-nitroso compounds from nitrate by the nitrate reducing bacterial flora in the presence of neutral colonic pH [6]. Although this was challenged by Stribling [7] and Sahands et al [8], who documented very low levels of N-nitroso compounds production in the rat models treated with ascorbic acid as compared to controls, yet both groups of rats produced urocolonic tumors with same frequency. To answer the question another theory of phagocytic activation response was proposed by Dell and colleagues [9]. Excessive production of oxygen derived free radical form uncontrolled inflammatory response, leading to DNA damage forms the basis of this [10]. We report here a case of colonic carcinoma occurring 36 years after uretrosigmoidstomy for exstrophy.
Case presentation
A 36-years-old male presented in emergency department with constipation for 5 days followed by colicky lower abdominal pain. There were no vomiting or bowel symptoms. He was born with exstrophy and at the age of 5 years he had underwent an ureterosigmoidostomy, after the attempts to do a primary closure of the bladder failed. However, due to continence problems, this was converted to an ileal conduit eight months later.
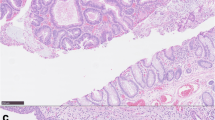
On examination he was apyrexial, hemodynamically stable but looked pale. Long midline and para-median scars were visible and abdomen was slightly distended. Urostomy bag was present in the right iliac fossa. He had minimal tenderness in the left half of the abdomen. Bowel sounds were audible and digital examination of the rectum was unremarkable. Routine blood tests showed mildly deranged renal functions (urea of 9.2 mmol/L and creatinine of 139 mmol/L). Plain abdominal x-ray showed faecal loading of the left colon. A couple of enemas were prescribed but they failed to relieve the symptoms. Over the next two days he gradually deteriorated with increasing abdominal distension and vomiting. Repeat abdominal film showed dilated small and large bowel loops. An unprepared gastrograffin enema was organized which showed a stricture at the recto-sigmoid junction. An emergency laparotomy was carried out which revealed an annular stenosing lesion at the recto-sigmoid junction with enlarged mesenteric lymph nodes and dilated proximal bowel. A loop of ileum was adherent to the surface of colon at the site of tumor. A Hartman's procedure was carried out and the adherent loop of ileum was also excised (figure 1). Histopathology showed it to be Dukes C, poorly differentiated adenocarcinoma of the sigmoid colon. Proximity of the tumor to the ectopic urothelium in the sigmoid colon was also demonstrated, which was the site of previous ureterosigmoidostomy. Further staging investigations did not show any evidence of metastasis. The Hartman's procedure was reversed 2 months later and currently he is receiving chemotherapy.
Discussion
Development of a malignant tumor in colon at the age of 36 years without having any familial predisposition to bowel cancer highlights the possible role of urinary diversion as the predisposing factor. The occurrence of large bowel tumors after ureterosigmoidostomy (USM) is well established and backed by several case reports [1]. Some studies have shown a 100 fold risk of malignancy associated with USM [2]. A recent study showed 11% risk in those having USM for 15 years or more [3].
Latent period between USM and the development of the tumor is another interesting phenomenon. The average age at diagnosis is reported to be 33 years with a median interval of 26 years [3]. Due to well established link between USM and colorectal tumors, other forms of urinary diversion have been tried, ileal conduit formation being one of them. Following this procedure no case of urocolonic tumor has been reported till date, although adenocarcinoma occurring in enteric augmentations is reported.
Due to the high-risk of malignant tumors following USM a large number of USM were converted to other forms of diversions, mostly ileal conduit formation. But the initial exposure of the colonic mucosa to the urine was enough to start the chain of events leading to development of tumors. Strachan and colleagues reported a case of epispadias treated with USM initially and converted to ileal conduit 4 years later, and he developed adenocarcinoma at the site of ureteric stump 24 years later [11]. In the present case the patient had USM only for 8 months and was converted into ileal conduit, yet after 28 years, he developed adenocarcinoma of the sigmoid colon. This is so far the shortest possible exposure of colonic mucosa to urine, reported in literature leading to the development of tumor.
Conclusion
The link between USM and colonic carcinogenesis is well established. The risk is there even with short term exposure of the colonic mucosa to urine and this risk is not abolished by converting USM to ileal conduits. USM being a very popular method of urinary diversion, many general surgeons and colorectal surgeons may be required to deal with such patients presenting to emergency with bowel related symptoms later in their life. It is important to be familiar with the reconstituted anatomy of the pelvis, to appreciate the possible risks and to intervene earlier.
After the relevant literature review, the recommendations for such cases can be summarized as follows
-
Avoid USM as the form of diversion in patients having benign conditions. Primary closure and staged reconstruction should be attempted first in cases of exstrophy and in cases with persistent incontinence use of artificial sphincter should be considered [12].
-
Patients having USM should be on regular follow-up with surveillance colonoscopy annually. And if they show dysplasia, polyps or tumor, USM should be changed to other forms of diversion [13].
-
Obstructive urinary symptoms, bleeding per rectum and/or change in bowel habits after USM should be taken seriously and promptly investigated by colonoscopy and CT-scan.
-
If USM is converted to other forms of diversion, the site of implantations of the ureters into sigmoid should be excised.
References
Zabbo A, Kay R: Ureterosigmoidostomy and bladder exstrophy a long term follow up. J Urol. 1986, 136: 396-398.
Stewart M, Macrae FA, Williams CB: Neoplasia and ureterosigmoidostomy: a colonoscopy survey. Br J Surg. 1982, 69: 414-416.
Strachan JR, Woodhouse CR: Malignancy following ureterosigmoidostomy in patients with exstrophy. Br J Surg. 1991, 78: 1216-1218.
Kotanagi H, Ito M, Koyama K, Sato K, Kato T: Colon cancer in rectal bladder. J Gastroenterol. 2001, 36: 718-722. 10.1007/s005350170037.
Gittes RF: Carcinogenesis in ureterosigmoidostomy. Urol Clin North Am. 1986, 13: 201-205.
Kalble T, Tricker AR, Mohring K, Berger MR, Geiss H, Staehler G: The role of nitrate, nitrite and N-nitrosamines in carcinogenesis of colon tumors following ureterosigmoidostomy. Urol Res. 1990, 18: 123-129.
Stribling D, Cohen S, Fagan D, Sanchez L, Anderson D, Davis P, Warren M: The effect of ascorbic acid on urinary nitrosamines and tumor development in rat animal model for ureterosigmoidostomy. J Urol. 1989, 141: 304A [abstract 540]-
Shands C, McDougal WS, Wright EP: Prevention of cancer at the urothelial enteric anastomosis site. J Urol. 1989, 141: 178-181.
Dull BJ, Gittes RF, Goldman P: Nitrate production and phagocytic activation: difference among Sprauge-Dawley, Wistar-Furth and Lewis rats. Carcinogenesis. 1988, 9: 625-627.
Weitzman SA, Weitberg AB, Clark EP, Stossel TP: Phagocytes as carcinogens: malignant transformation produced by human neutrophils. Science. 1985, 227: 1231-1233.
Strachan JR, Rees HC, Cox R, Woodhouse CR: Mucin changes adjacent to carcinoma following ureterosigmoidosotmy. Eur Urol. 1987, 13: 419-
Husmann DA, Spence HM: Current status of the tumors of the bowel following ureterosigmoidostomy: a review. J Urol. 1990, 144: 607-610.
Azimuddin K, Khubchandani IT, Stasik JJ, Rosen L, Riether RD: Neoplasia after ureterosigmoidostomy. Dis Colon Rectum. 1999, 42: 1632-1638.
Acknowledgement
Patient consent was obtained for publication of his case record and specimen photograph.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Authors' contributions
KM – conceived the study, did literature search and coordinated preparation of the manuscript for submission.
NA – helped in literature search and in preparation of the manuscript.
LR – reviewed and edited the manuscript for final submission.
All authors read and approved the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Khan, M.N., Naqvi, A.H. & Lee, R.E. Carcinoma of sigmoid colon following urinary diversion: a case report and review of literature. World J Surg Onc 2, 20 (2004). https://doi.org/10.1186/1477-7819-2-20
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7819-2-20