Abstract
Background
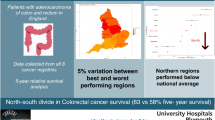
The Canadian province of Manitoba covers a large geographical area but only has one major urban center, Winnipeg. We sought to determine if regional differences existed in the quality of colorectal cancer care in a publicly funded health care system.
Methods
This was a population-based historical cohort analysis of the treatment and outcomes of Manitobans diagnosed with colorectal cancer between 2004 and 2006. Administrative databases were utilized to assess quality of care using published quality indicators.
Results
A total of 2,086 patients were diagnosed with stage I to IV colorectal cancer and 42.2% lived outside of Winnipeg. Patients from North Manitoba had a lower odds of undergoing major surgery after controlling for other confounders (odds ratio (OR): 0.48, 95% confidence interval (CI): 0.26 to 0.90). No geographic differences existed in the quality measures of 30-day operative mortality, consultations with oncologists, surveillance colonoscopy, and 5-year survival. However, there was a trend towards lower survival in North Manitoba.
Conclusion
We found minimal differences by geography. However, overall compliance with quality measures is low and there are concerning trends in North Manitoba. This study is one of the few to evaluate population-based benchmarks for colorectal cancer therapy in Canada.
Similar content being viewed by others
Background
Canadian health care is publicly administered and universal for all insured residents [1]. However, authors have raised concern over suboptimal or unequal access and quality of health care among certain Canadian populations [2, 3]. In Manitoba, colorectal cancer (CRC) is the third most common malignancy and poses a major public health issue [4]. Manitoba covers a large geographic area with 56.5% of the population living in the major urban center of Winnipeg [5, 6]. Most cancer patients in Manitoba are referred to the provincially mandated agency, CancerCare Manitoba (CCMB), for consideration of neoadjuvant and adjuvant therapies, such as chemotherapy and radiation therapy. Two tertiary locations are found in Winnipeg, while other non-tertiary affiliated sites, providing chemotherapy, are found within both Winnipeg and several communities in rural Manitoba [7]. Although surgical facilities are present in both Winnipeg and rural Manitoba, the only radiation therapy unit, during the study period, was at the tertiary location of CCMB in Winnipeg. For a large proportion of Manitobans, access to specialized medical or surgical care requires travelling great distances, personal expense, and inconvenience as medical specialists are less frequently found in remote and rural environments.
Since the quality of treatment for CRC has recently become an important area for research and quality improvement initiatives [8–10], authors have developed a set of quality indicators for CRC treatment [11]. Quality measures permit identification of suboptimal practice patterns and allow opportunities for improvement [12]. Utilization and application of quality indicators permits benchmarking between institutions.
To date, there has been no formal analysis of the quality of CRC therapy in Manitoba using published quality measures. We sought to assess whether geographic differences existed in CRC quality of care. This study has important implications for Canada and other jurisdictions around the world that face similar challenges in offering specialized health care to rural populations over great distances.
Methods
The Health Research Ethics Board (HREB) at the University of Manitoba, MB, Canada, approved this study. A population-based historical cohort analysis of all patients diagnosed with adenocarcinoma of the colon or rectum between 1 January 2004 and 31 December 2006 was undertaken to examine the presentation, treatments, and outcomes of CRC in Manitoba. Patients were identified using the population-based Manitoba Cancer Registry (MCR), maintained by CCMB. Information regarding all Manitobans diagnosed with a malignancy is collected by the MCR as cancer reporting is mandatory by Manitoba law [13]. The MCR was used to identify patients based on the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). Patients who were diagnosed with CRC at the same date as death, through autopsy or radiographic findings, were excluded (n = 3).
From the MCR, demographic and tumor-specific information, such as collaborative American Joint Committee on Cancer (AJCC) stage, were extracted. Patient-specific data from the MCR was linked to information in the Medical Claims (physician billing) database and the Hospital Discharge Abstracts database. Although health care utilization can be followed longitudinally, patient information was linked using encrypted personal health information numbers to maintain patient confidentiality. These databases are maintained by Manitoba Health, the agency responsible for providing health care to virtually all Manitobans, and contain patient-specific information about health care system contacts.
For treatment information from the MCR, the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) and the Canadian Classification of Health Interventions (CCHI) coding were used [14]. All treatment information was ascertained from within 1 year of diagnosis of CRC. Index surgical procedures, identified from the MCR, were classified in a hierarchical pattern into: major surgery, local resections, polypectomy, and none. The level of agreement for surgical procedures between the MCR and administrative records from Manitoba Health was determined using Fleiss’ kappa.
The quality of CRC care was assessed using previously published quality indicators, developed by a multidisciplinary panel using a 3-step modified Delphi approach [11]. A subset of these indicators was chosen on the ability to accrue data from administrative databases.
Perioperative total colonic examination
For patients who underwent a major surgical resection, the proportion who had a colonoscopy alone or a barium/contrast enema in conjunction with sigmoidoscopy 3 months prior or within 6 months after major surgery was determined [11, 15].
Anastomotic leak/early reoperation rate
No diagnostic code currently exists for anastomotic leaks after surgery. To estimate this rate, surrogate markers were utilized. For patients who underwent a major surgery with an anastomosis that had one of the following procedures during the same hospital stay, an anastomotic leak was assumed: percutaneous or operative drainage of an intra-abdominal abscess, laparotomy or laparoscopy post major surgery, or ostomy formation post major surgery. Although a laparotomy or laparoscopy in the postoperative period may have been indicated for other complications, such as bleeding or obstruction, these were included as early reoperation also represents a quality measure.
Extent of lymphadenectomy
Using the MCR, we assessed the median number of lymph nodes analyzed for patients undergoing a major surgical resection and the proportion of patients in which greater than 12 lymph nodes were examined [16–19].
Medical and radiation oncology consultations for patients with rectal cancer
The proportion of all patients with stage I to IV, as well as stage II and III, rectal cancer who had been seen by a medical and/or a radiation oncologist 3 months prior to major surgery was determined from the MCR. In addition, the proportion of patients with stage II or III disease seen within 8 weeks, as well as within 16 weeks, of major surgery was reported.
Medical oncology consultations for patients with colon cancer
The proportion of patients with stage III colon cancer who had been seen by a medical oncologist within 8 weeks and 16 weeks of surgery was determined.
Thirty-day mortality
The 30-day mortality rate after colon or rectal cancer major surgery was determined.
Postoperative endoscopic surveillance
For patients who underwent a major surgery, we determined the proportion of patients, who were alive, and who underwent a surveillance colonoscopy within 12 months and 14 months after the surgery date. The 1-year post surgery surveillance colonoscopy is recommended by the National Comprehensive Cancer Network (NCCN) [20, 21] and has been prioritized to be a quality measure by an expert multidisciplinary panel [11].
Five-year overall survival rate
The 5-year overall absolute Kaplan-Meier survival estimates from date of diagnosis to death, was determined using mortality data obtained up to 30 April 2011.
Geographical comparisons were made between Winnipeg and rural Manitoba, as well as between groupings of Manitoba’s regional health authorities (RHAs): Winnipeg, North Manitoba, South Manitoba, and Middle Manitoba. Manitoba’s RHAs are regional governances whose responsibility is to administer and deliver health services to specified geographic regions of the province [22].
Standard descriptive statistics and treatment frequency information were reported. Treatment information from the MCR was compared to the information taken from the Hospital Discharge Abstracts to check the validity of the data. Conventional stepwise multivariate logistic regression was used to determine variables associated with major surgery.
Survival was analyzed using Kaplan-Meier survival analysis. The Cox proportional hazards regression model was used to determine variables associated with overall survival. Significance was set at α = 0.05. SAS statistical software, versions 9.1 and 9.2 (SAS Institute Inc, Cary, NC, USA), was used for data management and statistical analyses.
Results
Between 2004 and 2006, 2,086 patients were diagnosed with stage I to IV adenocarcinoma-based CRC. Table 1 lists patient demographic information and comparisons by RHA group. There was relatively equal distribution of patients diagnosed between 2004 and 2006. A higher proportion of younger patients and a trend towards more advanced stage at presentation were noted for patients from North Manitoba.
Of all patients diagnosed with CRC, 78.04% (n = 1,628) underwent a major surgery. Fifty-eight patients (2.78%) underwent a local resection, while 70 patients (3.35%) underwent a polypectomy alone. A total of 330 patients (15.82%) did not have any surgical intervention (neither major surgery, local resection, nor polypectomy). Predictors of major surgery were analyzed using a stepwise multivariate logistic regression (Table 2). Patients diagnosed with rectal cancer, stage IV disease, and those from North Manitoba were at a lower odds of undergoing major surgery (odds ratio (OR): 0.48, 95% confidence interval (CI): 0.26 to 0.90, P = 0.037). Fleiss’ kappa level of agreement between the MCR and Hospital Discharge Abstracts was 0.788 for overall surgical categories (major surgery: 0.821, local resection: 0.615, polypectomy: 0.606, and none: 0.821).
Quality measures and geographic variations are shown in Table 3. The postoperative (30-day) mortality rate was 3.8%. Overall, 75.6% of patients (1,230/1,628) had perioperative total colonic examination. There were 217 patients (13.3%) that did not have any colonic/endoscopic imaging before or after surgery. The extent of lymphadenectomy could be determined for 96.5% of patients undergoing resection.
The 5-year overall survival for all patients diagnosed with CRC between 2004 and 2006 was 49.9%. There was no significant difference based on geography in Manitoba, although a trend towards decreased survival for patients in North Manitoba was observed (Figure 1). The multivariate Cox proportional hazards model analysis for 5-year overall survival is shown in Table 4.
Discussion
In Manitoba, between 2004 and 2006, there was minimal geographic variation of certain quality measures for CRC. However, overall compliance with many quality measures appears low. Addressing these issues is paramount in order to provide all Manitobans with coordinated, high quality care.
It was hypothesized that differences in access to cancer care could manifest with rural patients presenting with higher stage disease. This hypothesis was not confirmed. However, there was a trend towards a higher stage in patients from North Manitoba. This observation is of concern and requires further research.
Over 78% of patients underwent a major surgery, similar to findings in Ontario, Canada [23]. After controlling for stage and other confounders, living in North Manitoba at the time of diagnosis was associated with a lower chance of undergoing surgery. Both system- and patient-related factors could explain this disparity. Patient-related factors in health decision making may be numerous, including age, gender, education, emotional support, and physician trust [24]. Anxiety, personal beliefs, or aversion to postoperative complications may influence patients to not seek or receive therapy [25, 26]. Humber and Dickinson examined rural patients’ experiences of accessing surgery in British Columbia, Canada [27]. They determined that rural patients prefer individualized care in familiar environments. Transportation and financial barriers were noted to be detrimental factors. Rural patients may be viewed as a culture with their own health determinants and challenges in accessing health care [28]. The psychological effects of a cancer diagnosis compounded with the need to travel to new environments in order to access specialist care can be profound and may deter patients from seeking therapies. System-related factors, such as barriers to timely referral to surgeons, or patients being offered different treatments may also play a role.
Perioperative total colonic examination is important, since the reported incidence of synchronous colonic lesions ranges from 2.12% to 8.1% [29–33]. In Manitoba, 75.6% of patients met this quality measure, similar to a study from Nova Scotia, Canada [10]. Rates of total colonic examination were lower in North Manitoba. It can be hypothesized that this may relate to endoscopy access issues. Also, patients may present emergently and be unable to undergo full preoperative evaluation, and subsequently not undergo evaluation postoperatively. It is concerning that in our analysis, 13.3% of patients did not have any colonic investigations. The underlying reasons are unknown, but present an important area for further research. Additionally, our reported rate of surveillance colonoscopy within 1 year of surgery of 29.62% is lower than that reported by other authors [16].
Adequate lymphadenectomy is a well-established quality measure of colorectal cancer care for its vital role in appropriate staging [17, 34]. Lymph node status is a strong predictor of survival outcomes in non-metastatic CRC and implies the need for adjuvant therapy [17, 35–38]. Patients who had greater than 12 lymph nodes examined did, in fact, have a lower risk of mortality in this analysis. Adequate lymphadenectomy may provide both a direct therapeutic benefit, and improve staging accuracy and prognosis [39]. A total of 68.8% of patients had adequate nodal evaluation, as reflected by more than 12 lymph nodes examined in pathology specimens. This rate is higher than that reported by others [10, 16–18], yet still presents an opportunity for improvement.
Timely and appropriate assessment of patients diagnosed with rectal cancer by medical and radiation oncology, either preoperatively or within 8 weeks of surgery, is an important quality measure [11]. Previous studies have been unable to determine rates of consultation, and instead reported the proportion of patients that received radiation or chemotherapy [16]. Reporting rates of consultation presents a more accurate measure of quality of care. The rates of assessment by medical and radiation oncology within 8 weeks for patients with rectal cancer are quite low. Others have reported higher rates [10, 40]. When extending the timeframe to 16 weeks, the rate of consultation with medical and radiation oncology nearly doubled. This suggests that referrals are being made appropriately but system limitations may account for undue delays in patients being seen. Referral delays, wait lists, and pathology reporting delays may contribute to lower than expected adherence with the quality measures, and could explain the increased rate of patients seen within 16 weeks. During the study period, Manitoba’s radiation therapy services were only available at a single center in Winnipeg, necessitating travel for rural patients. In addition, the proportion of patients with rectal cancer seen preoperatively by radiation oncology was low, possibly because the timeframe of this study predated the general trend from adjuvant to neoadjuvant chemoradiation treatment [41].
For patients diagnosed between 2004 and 2006, Manitoba’s population-based 5-year overall survival was 49.9%. Initially, this appears to be lower than previously reported [16, 42]. However, comparisons must be made cautiously. Vergarara-Fernandez et al. reported a 5-year overall survival rate of 75% at a single, high volume tertiary center [16]. Population-based absolute survival rates are presented in our study. Most notably, no geographic differences in 5-year survival were demonstrated. However, though not statistically significant, a trend towards a lower 5-year overall Kaplan-Meier survival rate for patients from North Manitoba was noted. This trend highlights an important area for future research.
This study is limited by the retrospective collection of administrative data, which may contain incomplete records and coding errors [43–45]. However, the MCR has been demonstrated to be among the highest quality administrative cancer databases [46]. Based on the study design, patients’ preferences could not be accounted for. Additionally, we were unable to report relative survival rates to control for underlying mortality in each RHA group. This limits the ability to draw comparisons to other population-based survival analyses. Furthermore, we were unable to account for racial differences in outcomes in this analysis. However, this is the first Manitoban study to provide comprehensive CRC quality of care benchmarks, which can be used for future temporal and interprovincial comparisons. Additionally, there was a high level of agreement between the MCR and the administrative databases maintained by Manitoba Health for surgical treatment information. This finding may allow for future studies to use the MCR for surgical cancer treatment information.
We have identified concerning findings in North Manitoba, such as the lower odds of receiving major surgery, while accounting for stage and other confounders, and lower rates of total colonic examination. The trend towards lower survival and higher stage in North Manitoba is very concerning. Given that only 65 patients were from North Manitoba, this study suffered from lack of power when analyzing this subset of the population further. The MCR only began collecting detailed TNM staging information in 2004, preventing the addition of earlier years to the study data. Future analyses, including later time periods, beyond 2006, will be undertaken to better delineate the findings for patients in North Manitoba. However, these findings are important and should be shared with other jurisdictions that share similar geographical challenges in providing high quality care for CRC and other medical problems. Focus should be placed on addressing access, surgical issues, such as patients’ preferences, in addition to system-based barriers for patients living in locations remote from major urban centers. Further research on rural patients’ perspectives of surgery is warranted.
Conclusion
Although minimal geographic differences in quality measures were seen, overall adherence was less than ideal. Further research is necessary to better delineate the reasons for this. This research is important in its implication for Manitoba’s health care system, as well as for the rest of Canada, and other areas of the world that might face similar challenges in providing high quality cancer care to rural patients over significant distances.
Abbreviations
- AJCC:
-
American joint committee on cancer
- CCHI:
-
Canadian classification of health interventions
- CCI:
-
Charlson comorbidity index
- CCMB:
-
CancerCare Manitoba
- CI:
-
Confidence interval
- CRC:
-
Colorectal cancer
- HR:
-
Hazards ratio
- HREB:
-
Health research ethics board
- ICD-9-CM:
-
International classification of diseases 9th revision, clinical modification
- ICD-10:
-
International statistical classification of diseases and related health problems 10th revision
- MCR:
-
Manitoba cancer registry
- NA:
-
Not applicable
- NCCN:
-
National comprehensive cancer network
- NF:
-
Not formatted
- OR:
-
Odds ratio
- RHA:
-
Regional health authority.
References
Canada H: Canada Health Act Annual Report 2008–2009. 2009, Ottawa: Government of Canada
Maddison AR, Asada Y, Urquhart R: Inequity in access to cancer care: a review of the Canadian literature. Cancer Causes Control. 2011, 22: 359-366.
Asada Y, Kephart G: Equity in health services use and intensity of use in Canada. BMC Health Serv Res. 2007, 7: 41-
Canadian Cancer Society’s Steering Committee: Canadian Cancer Statistics 2010. 2010, Toronto: Canadian Cancer Society
Travel Manitoba.http://www.travelmanitoba.com,
Health M: Manitoba Health and Healthy Living Annual Statistics 2008–2009. 2009, Winnipeg: Health Information Management
CancerCare Manitoba.http://cancercare.mb.ca,
Hayman AV, Chang ET, Molokie RE, Kahng LS, Prystowsky JB, Bentrem DJ: Assessing compliance with national quality measures to improve colorectal cancer care at the VA. Am J Surg. 2010, 200: 572-576.
Stelzner S, Hellmich G, Haroske G, Puffer E, Jackisch T, Witzigmann H: Practicability of quality goals for the treatment of rectal cancer. Int J Colorectal Dis. 2010, 25: 1093-1102.
McConnell YJ, Inglis K, Porter GA: Timely access and quality of care in colorectal cancer: are they related?. Int J Qual Health Care. 2010, 22: 219-228.
Gagliardi AR, Simunovic M, Langer B, Stern H, Brown AD: Development of quality indicators for colorectal cancer surgery, using a 3-step modified Delphi approach. Can J Surg. 2005, 48: 441-452.
Patwardhan M, Fisher DA, Mantyh CR, McCrory DC, Morse MA, Prosnitz RG, Cline K, Samsa GP: Assessing the quality of colorectal cancer care: do we have appropriate quality measures? (A systematic review of literature). J Eval Clin Pract. 2007, 13: 831-845.
Government of Manitoba: Reporting of Diseases and Conditions Regulation, The Public Health Act. 2009, Winnipeg: Queen’s Printer,http://web2.gov.mb.ca/laws/regs/pdf/p210-037.09.pdf,
Schultz SE, Simunovic M, Urbach DR: Technical appendix (full). Cancer Surgery in Ontario: ICES Atlas. Edited by: Urbach DR, Simunovic M, Schultz SE. 2008, Toronto: Institute for Clinical Evaluation Sciences, TA1-TA19.
McGory ML, Shekelle PG, Ko CY: Development of quality indicators for patients undergoing colorectal cancer surgery. J Natl Cancer Inst. 2006, 98: 1623-1633.
Vergara-Fernandez O, Swallow CJ, Victor JC, O’Connor BI, Gryphe R, MacRae HM, Cohen Z, McLeod RS: Assessing outcomes following surgery for colorectal cancer using quality of care indicators. Can J Surg. 2010, 53: 232-240.
Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, Virnig BA: Lymph node evaluation in colorectal cancer patients: a population-based study. J Natl Cancer Inst. 2005, 97: 219-225.
Bilimoria KY, Bentrem DJ, Stewart AK, Talamonti MS, Winchester DP, Russell TR, Ko CY: Lymph node evaluation as a colon cancer quality measure: a national hospital report card. J Natl Cancer Inst. 2008, 100: 1310-1317.
Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, Miedema B, Ota D, Sargent D: Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001, 93: 583-596.
Network NCC: NCCN Clinical Practice Guidelines in Oncology Colon Cancer. 2010, Fort Washington: National Comprehensive Cancer Network
Network NCC: NCCN Clinical Practice Guidelines in Oncology Rectal Cancer. 2011, Fort Washington: National Comprehensive Cancer Network
Manitoba Centre for Health Policy: Term: Regional Health Authority (RHA). 2013, Manitoba: University of Manitoba,http://mchp-appserv.cpe.umanitoba.ca/viewDefinition.php?definitionID=103476,
Nenshi R, Baxter N, Kennedy E, Schultz SE, Gunraj N, Wilton AS, Urbach DR, Simunovic M: Surgery for colorectal cancer. Cancer Surgery in Ontario: ICES Atlas. 2008, Toronto: Institute for Clinical Evaluation Sciences
Leon-Carlyle M, Spiegle G, Schmocker S, Gagliardi A, Urbach D, Kennedy E: Using patient and physician perspectives to develop a shared decision-making framework for colorectal cancer. Implement Sci. 2009, 4: 81-
Hendren SK, O’Connor BI, Liu M, Asano T, Cohen Z, Swallow CJ, Macrae HM, Gryfe R, McLeod RS: Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg. 2005, 242: 212-223.
Peeters KC, CJ V d-V, Leer JW, Martijn H, Junggeburt JM, Kranenbarg EK, Steup WH, Wiggers T, Rutten HJ, Marijnen CA: Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients–a Dutch colorectal cancer group study. J Clin Oncol. 2005, 23: 6199-6206.
Humber N, Dickinson P: Rural patients’ experiences accessing surgery in British Columbia. Can J Surg. 2010, 53: 373-378.
Hartley D: Rural health disparities, population health, and rural culture. Am J Public Health. 2004, 94: 1675-1678.
Oya M, Takahashi S, Okuyama T, Yamaguchi M, Ueda Y: Synchronous colorectal carcinoma: clinico-pathological features and prognosis. Jpn J Clin Oncol. 2003, 33: 38-43.
Takeuchi H, Toda T, Nagasaki S, Kawano T, Minamisono Y, Maehara Y, Sugimachi K: Synchronous multiple colorectal adenocarcinomas. J Surg Oncol. 1997, 64: 304-307.
Derwinger K, Gustavsson B: A study of aspects on gender and prognosis in synchronous colorectal cancer. Clin Med Insights Oncol. 2011, 5: 259-264.
Nikoloudis N, Saliangas K, Economou A, Andreadis E, Siminou S, Manna I, Georgakis K, Chrissidis T: Synchronous colorectal cancer. Tech Coloproctol. 2004, 8 (Suppl 1): s177-s179.
Cunliffe WJ, Hasleton PS, Tweedle DE, Schofield PF: Incidence of synchronous and metachronous colorectal carcinoma. Br J Surg. 1984, 71: 941-943.
Wong SL: Lymph node evaluation in colon cancer: assessing the link between quality indicators and quality. JAMA. 2011, 306: 1139-1141.
Prandi M, Lionetto R, Bini A, Francioni G, Accarpio G, Anfossi A, Ballario E, Becchi G, Bonilauri S, Carobbi A, Cavaliere P, Garcea D, Giuliani L, Morziani E, Mosca F, Mussa A, Pasqualini M, Poddie D, Tonetti F, Zardo L, Rosso R: Prognostic evaluation of stage B colon cancer patients is improved by an adequate lymphadenectomy: results of a secondary analysis of a large scale adjuvant trial. Ann Surg. 2002, 235: 458-463.
Chau I, Cunningham D: Adjuvant therapy in colon cancer: current status and future directions. Cancer Treat Rev. 2002, 28: 223-236.
Shimomura M, Ikeda S, Takakura Y, Kawaguchi Y, Tokunaga M, Egi H, Hinoi T, Okajima M, Ohdan H: Adequate lymph node examination is essential to ensure the prognostic value of the lymph node ratio in patients with stage III colorectal cancer. Surg Today. 2011, 41: 1370-1379.
Beattie GC, McAdam TK, Elliott S, Sloan JM, Irwin ST: Improvement in quality of colorectal cancer pathology reporting with a standardized proforma–a comparative study. Colorectal Dis. 2003, 5: 558-562.
Le-Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG: Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003, 21: 2912-2919.
Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL: Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States?. J Clin Oncol. 2006, 24: 626-634.
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R, German Rectal Cancer Study Group: Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004, 351: 1731-1740.
Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, Nur U, Tracey E, Coory M, Hatcher J, McGahan CE, Turner D, Marrett L, Gjerstorff ML, Johannesen TB, Adolfsson J, Lambe M, Lawrence G, Meechan D, Morris EJ, Middleton R, Steward J, Richards MA, ICBP Module 1 Working Group: Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011, 377: 127-138.
Malin JL, Kahn KL, Adams J, Kwan L, Laouri M, Ganz PA: Validity of cancer registry data for measuring the quality of breast cancer care. J Natl Cancer Inst. 2002, 94: 835-844.
Heaman MI, Newburn-Cook CV, Green CG, Elliott LJ, Helewa ME: Inadequate prenatal care and its association with adverse pregnancy outcomes: a comparison of indices. BMC Pregnancy Childbirth. 2008, 8: 15-
Vlasschaert ME, Bejaimal SA, Hackam DG, Quinn R, Cuerden MS, Oliver MJ, Iansavichus A, Sultan N, Mills A, Garg AX: Validity of administrative database coding for kidney disease: a systematic review. Am J Kidney Dis. 2011, 57: 29-43.
Copeland G, Lake A, Firth R, Bayakly R, Wu XC, Stroup A, Russell C, Kimberley B, Niu X, Schymura M, Hofferkamp J, Kohler B: Cancer in North America: 2003–2007 Volume 2 Registry-specific Cancer Incidence in the United States and Canada. 2010, Springfield: North American Association of Central Cancer Registries, Inc
Acknowledgements
We acknowledge the financial support of the following granting agencies: the Manitoba Medical Service Foundation, the Winnipeg Foundation, and the Department of Surgery Geographic Full Time (GFT) Group Research Fund, University of Manitoba. No official endorsement by Manitoba Health is intended or should be inferred. The results and conclusions are those of the authors.
Synopsis
Minimal geographic differences exist in most colorectal cancer quality measures in Manitoba, Canada. However, overall compliance rates are low, and we have identified trends towards higher stage at presentation and lower rates of surgery in North Manitoba.
Helewa RM, Turner D, Wirtzfeld D, Park J, Shu E, Xue L, McKay A. Does geography influence the treatment and outcomes of colorectal cancer in the province of manitoba? Presented at Society of Surgical Oncology, 65th Annual Cancer Symposium. Rosemont: Society of Surgical Oncology; March 2012.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RMH and AM made substantial contributions to study conception, design, acquisition, analysis and interpretation of data, as well as drafting and critically revising the manuscript. DT made substantial contributions to study conception, design, analysis and interpretation of data, as well as drafting and revising the manuscript. DW, JP, DH, PC, and HS made substantial contributions to study conception, design, analysis and interpretation of data, as well as critically revising the manuscript. ES and LX made substantial contributions to acquisition, analysis and interpretation of data, as well as critically revising the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Helewa, R.M., Turner, D., Wirtzfeld, D. et al. Does geography influence the treatment and outcomes of colorectal cancer? A population-based analysis. World J Surg Onc 11, 140 (2013). https://doi.org/10.1186/1477-7819-11-140
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7819-11-140