Abstract
Enteric duplications are rare, but can occur anywhere along the digestive tract. Most of the patients become symptomatic in early childhood and only a few cases of adult patients have been reported in literature. Here we report a unique case of an adenocarcinoma arising in a coincidentally found cystic duplication of the small bowel.
Similar content being viewed by others
Background
The term 'alimentary tract duplication' was characterized by W.E. Ladd to describe those congenital malformations that involve the mesenteric side of the associated alimentary tract and share a common blood supply with the native bowel [1]. Enteric duplications are unusual, but can occur anywhere along the digestive tract [2–7], most frequently found around the ileocoecal region [2–7]. Most patients become symptomatic within the first year of life [2–5]. Reports of enteric duplications in adulthood are extremely scarce in English language literature [8]. Although rare, malignant change can occur within the intestinal duplication [9]. In this report we present a case of an adenocarcinoma arising in a coincidentally found cystic duplication of the small bowel.
Case presentation
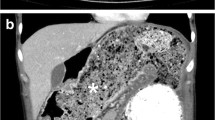
A 51-year-old man with no abdominal symptoms was admitted to our hospital with an external computed tomography (CT) scan showing a cystic mass in the mid-abdomen (Figure 1). The cystic mass was low-density and had enhanced margins. The size of the structure was measured at 4 × 10 cm and it was located in the ileal mesenterium. The differential diagnosis contained a mesenteric cyst, a Meckel's diverticulum, and an enteric duplication.
Physical examination was unremarkable but laboratory tumor marker levels were slightly elevated: carcinoembryonic antigen (CEA) 13.2 μg/l (standard value < 5 μg/l) and CA19-9 55 kU/l (standard value < 37 kU/l).
During explorative laparotomy a cystic mass was found in the mesenterium which looked similar to the small bowel but had no connection to the alimentary tract (Figure 2). The surface was smooth and without deposits. An en-bloc resection of the cystic mass could be performed without the necessity of a small bowel resection. At the end of the operation the specimen was opened (Figure 3). It contained an odorless, cloudy liquid. The luminal surface showed partly brownish deposits, the surface was irregular but smooth and the walls were uniformly 3 mm thick.
Histological examination revealed a duplication of the small bowel in the mesenterium with nearby physiological architecture. The inner lining mucosa showed indicated villi and crypts and numerous mucous cells. The epithelium showed partly dysplastic areas. At one point it contained a high-grade intraepithelial neoplasia with transition into a poorly differentiated invasive adenocarcinoma infiltrating the muscularis propria (Figure 4).
Immunohistochemistry revealed a high expression of CK20 and spot-like CK7. Analogue to the TNM-classification of the small bowel, the tumor was classified as pT2, pN0 (0/25), L0, V0, G2.
Postoperative recovery was unremarkable and the patient was discharged six days after surgery. Regular oncologic follow-up in an outpatient setting for one year after surgery showed no hints of tumor recurrence with inconspicuous physical examination and normal tumor marker levels, ultrasound, and CT findings.
Discussion
Enteric duplications (EDs) are rare but can occur anywhere along the digestive tract from the oral cavity to the rectum [2–7, 10]. The majority of ED occurs intra-abdominally and over half of them are ileal duplications [4–7]. According to W.E. Ladd, those congenital malformations involve the mesenteric side of the associated alimentary tract and share a common blood supply with the native bowel [1]. The etiology of ED still remains unknown. Several theories have been postulated such as an abnormal recanalization after the solid epithelial stage of embryonic bowel development [11]. Other theories consider persisting embryologic diverticula or 'aborted Gemini' [12]. The most accepted theory, however, is the 'intrauterine vascular accident theory' [13, 14], but no single theory can explain all the known duplications [7].
EDs usually become symptomatic within the first year of life [2–7]. Reports of ED in adulthood are extremely scarce in English language literature [8]. Most frequently, the patients present vague symptoms mimicking other more common pathologies such as volvulus, appendicitis, intussusception, pelvic abscess, diverticulitis, achalasia, and Hirschsprung's disease [4, 6, 7, 15–17].
EDs are most commonly diagnosed when complications like bowel obstruction, perforation, or bleeding occur. Prior to surgery it is difficult to diagnose EDs because of the non-specificity of symptoms and presentation. However, ultrasound, CT scan, and magnetic resonance imaging (MRI) have been useful. Ultrasound can depict the characteristic location adjacent to the bowel and the two-layered wall of EDs [15, 18–20]. Bowel duplication cysts present with heterogeneous signal intensity on T1- and homogeneous signal intensity on T2-weighted images on MRI [21, 22]. Latter modalities can even assist in prenatal diagnosis [22]. Where duplication is tubular, barium examination may be diagnostic if not contraindicated [7]. Technetium scanning can also be used to diagnose EDs [3]. The majority of EDs are isolated and cystic in structure. Reports of tubular duplications are less common. However, both could be associated with other malformations like intestinal malrotation and genitourinary or spinal malformations [23–25].
Heterotopic mucosa of gastric or pancreatic origin is a common finding in histological examination of ED [7]. In the current case the specimen had a similar physiological architecture to the small bowel with indicated villi, crypts, and a two-layered muscular wall. The epithelium contained many mucous cells. Gastric or pancreatic origin was not confirmed.
Carcinomas arising in duplication cysts are extremely rare complications and only few cases have been reported in literature including carcinoid tumors, squamous cell carcinomas, and common adenocarcinomas [26–50].
Malignant change in small bowel duplications is described most frequently [14, 20, 22, 26–35], followed by colonic [36–43] and rectal [44–46] duplications. There are also reports about carcinomas arising in duplications of the duodenum [47, 48] and the stomach [49, 50]. Due to the rare presentation with unspecific symptoms the tumors are commonly diagnosed at advanced tumor stage with metastatic disease [26–28, 35]. If malignant change is found in small bowel duplications, the high rate of lymph node metastases should be considered [26]. The mode of metastasis is similar to that of primary small bowel cancer [26, 27]. Curative resections could hardly be performed [26, 28]. Thus, the prognosis is generally poor once malignant change has occurred. Fortunately the suspicion of ED was a coincidental finding in an abdominal CT scan in the present case. This led to a timely operative exploration and malignant change was diagnosed at an early stage. A curative en-bloc resection of the duplication including the tumor could be performed and all of the resected 25 lymph nodes were free of metastasis.
Histological examination depicted dysplastic areas in the epithelium with an area of a high-grade intraepithelial neoplasia and transition into a poorly differentiated invasive adenocarcinoma. This indicates a tendency to undergo malignant change, which was also reported by Orr and Edwards [9]. Moreover, all cases of malignant change in duplication cysts that have been reported have occurred in adults aged 26 to 88 years. This is in contrast to the presentation of benign cysts that are diagnosed in childhood [2, 4–7].
Conclusion
The experience of this case and other reports about malignant transformation shows that whenever intestinal duplication is suspected, an immediate operative resection should be performed.
Consent
Written consent was obtained from the patient for the use and publication of this case report and the accompanying images. A copy of the written consent is available for review from the Editor-in-Chief of this journal.
References
Ladd WE, Gross RE: Surgical treatment of duplications of the alimentary tract. Surg Gynecol Obstet. 1940, 70: 295-307.
Bower RJ, Sieber WK, Kiesewetter WB: Alimentary tract duplications in children. Ann Surg. 1978, 188: 669-674. 10.1097/00000658-197811000-00015.
Hocking M, Young DG: Duplications of the alimentary tract. Br J Surg. 1981, 68: 92-96. 10.1002/bjs.1800680210.
Schalamon J, Schleef J, Höllwarth ME: Experience with gastro-intestinal duplications in childhood. Langenbecks Arch Surg. 2000, 385: 402-405. 10.1007/s004230000170.
Karnak I, Ocal T, Senocak ME, Tanyel FC, Büyükpamukcu N: Alimentary duplications in children: report of 26 years' experience. Turk J Pediatr. 2000, 42: 118-125.
Kuo HC, Lee HC, Shin CH, Sheu JC, Chang PY, Wang NL: Clinical spectrum of alimentary tract duplication in children. Acta Paediatr Taiwan. 2004, 45: 85-88.
Olajide ARL, Yisau A, Abdulraseed NA, Kashim IO, Olaniyi AJ, Morohunfade AO: Gastrointestinal duplications: experience in seven children and a review of the literature. Saudi J Gastroenterol. 2010, 16: 105-109. 10.4103/1319-3767.61237.
Johnson JA, Poole GV: Ileal duplications in adults. Presentation and treatment. Arch Surg. 1994, 129: 659-661. 10.1001/archsurg.1994.01420300103018.
Orr MM, Edwards AJ: Neoplastic change in duplications of the alimentary tract. Br J Surg. 1975, 62: 269-274. 10.1002/bjs.1800620405.
Chen MK, Gross E, Lobe TE: Perinatal management of enteric duplication cysts of the tongue. Am J Perinatol. 1997, 14: 161-163. 10.1055/s-2007-994119.
Bremer JL: Diverticula and duplications of the intestinal tract. Arch Pathol. 1944, 38: 132-140.
Letelier AM, Barría CM, Beltran MA, Moreno CCH: Duplicación intestinal: Diagnóstico y tratamiento de una condición inusual. Rev Chil Cir. 2009, 61: 171-175.
Favara BE, Franciosi RA, Akers DR: Enteric duplications. Thirty-seven cases: a vascular theory of pathogenesis. Am J Dis Child. 1971, 122: 501-506.
Beltrán MA, Barría C, Contreras MA, Wison CS, Cruces KS: Adenocarcinoma and intestinal duplication of the ileum. Report of one case. Rev Méd Chile. 2009, 137: 1341-1345.
Chou YH, Tiu CM, Pan HB, Yeh CJ, Wei CF, Chang TE: Ultrasonographic demonstration of duplication cyst of the ileum. Zhonghua Yi Xue Za Zhi (Taipei). 1990, 46: 237-239.
Otter MI, Marks CG, Cook MG: An unusual presentation of intestinal duplication with a literature review. Dig Dis Sci. 1996, 41: 627-629. 10.1007/BF02282353.
Ameh EA, Jimoh AO, Rafindadi AH, Shehu SM: Sublingual gastric duplication cyst causing respiratory obstruction: case report. East Afr Med J. 2000, 77: 394-395.
Kangarloo H, Sample WF, Hansen G, Robinson JS, Sarti D: Ultrasonic evaluation of abdominal gastrointestinal tract duplication in children. Radiology. 1979, 131: 191-194.
Rice CA, Anderson TM, Sepahdari S: Computed tomography and ultrasonography of carcinoma in duplication cyst. J Comput Assist Tomogr. 1986, 10: 233-235. 10.1097/00004728-198603000-00012.
Tew K, Soans BK, Millar EA: Adenocarcinoma in an ileal duplication cyst: ultrasound and computed tomography findings. Australas Radiol. 2000, 44: 228-231. 10.1046/j.1440-1673.2000.00791.x.
Berrocal T, Lamas M, Gutieèrez J, Torres I, Prieto C, del Hoyo ML: Congenital anomalies of the small intestine, colon and rectum. Radiographics. 1999, 19: 1219-1236.
Radich GA, Altinook D, Adsay NV, Soulen RL: Papilarry adenocarcinoma in a small-bowel duplication in a pregnant woman. AJR Am J Roentgenol. 2006, 186: 895-897. 10.2214/AJR.04.1488.
Somuncu S, Cakmak M, Caglayan E, Unal B: Intestinal duplication cyst associated with intestinal malrotation anomaly: report of a case. Acta Chir Belg. 2006, 106: 611-612.
Shah KR, Joshi A: Complete genitourinary and colonic duplication: a rare presentation in an adult patient. J Ultrasound Med. 2006, 25: 407-411.
Chaiyasate K, Bruch S: Colonic duplication associated with anterior spinal bar and left-sided inferior vena cava. Surgery. 2007, 141: 823-825. 10.1016/j.surg.2006.06.024.
Kusunoki N, Shimada Y, Fukumoto S, Iwatani Y, Ohshima T, Arahi E, Miyazaki N, Maeda S: Adenocarcinoma arising in a tubular duplication of the jejunum. J Gastroenterol. 2003, 38: 781-785. 10.1007/s00535-002-1146-8.
Kim TH, Kim JK, Jang EH, Lee JH, Kim YB: Papillary adenocarcinoma arising in a tubular duplication of the jejunum. Br J Radiol. 2010, 83: e61-e64. 10.1259/bjr/68269826.
Devos B, Schreurs L, Duponselle E, Hendrix T, Van Dijck H, Van Vuchelen J: Adenocarcinoma optredend in een cystische duplicatie van het ileum. Acta Chir Belg. 1987, 87: 235-238.
De Tullio D, Rinaldi R, Pellegrini D, Stano R, Messina F, Cavazzini L, Azzena G, Occhionorelli S: Adenocarcinoma arising in an elderly patient's large ileal duplication. Int J Surg Pathol. 2008, 19: 681-684.
Babu MS, Raza M: Adenocarcinoma in an ileal duplication. J Assoc Physicians India. 2008, 56: 119-120.
Micolonghi T, Meissner GF: Gastric-type carinoma arising in duplication of the small intestine. Ann Surg. 1958, 147: 124-127. 10.1097/00000658-195801000-00021.
Adair HM, Trowell JE: Squamous cell carcinoma arising in duplication of the small bowel. J Pathol. 1981, 133: 25-31. 10.1002/path.1711330104.
Smith JHF, Hope PG: Carcinoid tumor arising in a cystic duplication of the small bowel. Arch Pathol Lab Med. 1985, 109: 95-96.
Ribaux C, Meyer P: Adenocarcinome dans une duplication intestinale grele. Ann Pathol. 1995, 15: 443-445.
Fletcher DJ, Goodfellow PB, Bardsley D: Metastatic adenocarcinoma arising from a small bowel duplication cyst. Eur J Surg Oncol. 2002, 28: 93-94. 10.1053/ejso.2001.1154.
Lee J, Jeon YH, Lee S: Papillary adenocarcinoma arising in a duplication of the coecum. Abdom Imaging. 2008, 33: 601-603. 10.1007/s00261-007-9330-1.
Heiberg ML, Marshall KG, Himal HS: Adenocarcinoma arising in a duplicated colon. Case report and review of literature. Br J Surg. 1973, 60: 981-982. 10.1002/bjs.1800601218.
Arkema KK, Calendoff L: Adenocarcinoma in tubular duplication of the sigmoid colon. Gastrointest Radiol. 1977, 2: 137-139. 10.1007/BF02256486.
Hickey WF, Corson JM: Squamous cell carcinoma arising in a duplication of the colon: case report and literature review of squamous cell carcinoma of the colon and of malignancy complicating colonic duplication. Cancer. 1981, 47: 602-609. 10.1002/1097-0142(19810201)47:3<602::AID-CNCR2820470330>3.0.CO;2-8.
Neal JW, Zuk RJ, Baithun SI: Squamous cell carcinoma in a duplicate large intestine. A case report. Virchows Arch A Pathol Anat Histopathol. 1989, 415: 383-385. 10.1007/BF00718641.
Delladetsima J, Papachristodoulou A, Zografos G: Carcinoma arising in duplicated colon. Am Surg. 1992, 58: 782-783.
Inoue Y, Nakamura H: Adenocarcinoma arising in colonic duplication cysts with calcification: CT findings of two cases. Abdom Imaging. 1998, 23: 135-137. 10.1007/s002619900305.
Hattori H: Adenocarcinoma occurring just at the attached site of colonic duplication in an adult man. Dig Dis Sci. 2005, 50: 1754-10.1007/s10620-005-2930-0.
Weitzel RA, Breed JR: Carcinoma arising in a rectal duplication (enterocystoma). Ann Surg. 1963, 157: 476-480. 10.1097/00000658-196303000-00021.
Downing R, Thompson H, Alexander-Williams J: Adenocarcinoma arising in a duplication of the rectum. Br J Surg. 1978, 65: 572-574. 10.1002/bjs.1800650813.
Gibson TC, Edwards JM, Shafig S: Carcinoma arising in a rectal duplication cyst. Br J Surg. 1986, 73: 377-10.1002/bjs.1800730520.
Falk GL, Young CJ, Parer J: Adenocarcinoma arising in a duodenal duplication cyst: a case report. Aust NZ J Surg. 1991, 61: 551-553. 10.1111/j.1445-2197.1991.tb00289.x.
Hata H, Hiraoka N, Ojima H, Shimada K, Kosuge T, Shimoda T: Carcinoid tumor arising in a duplication cyst of the duodenum. Pathol Int. 2006, 56: 272-278. 10.1111/j.1440-1827.2006.01957.x.
Coit DG, Mies C: Adenocarcinoma arising within a gastric duplication cyst. J Surg Oncol. 1992, 50: 274-277. 10.1002/jso.2930500417.
Kuraoka K, Nakayama H, Kagawa T, Ichikawa T, Yasui W: Adenocarcinoma arising from a gastric duplication cyst with invasion to the stomach: a case report with literature review. J Clin Pathol. 2004, 57: 428-431. 10.1136/jcp.2003.013946.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
GB collected the information, researched the literature, and wrote the article. AK helped with literature research and in preparing the manuscript. BS performed the histological examination and helped prepare the manuscript. RL helped in literature research and edited the final version of the manuscript. All authors read and approved the final version of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Blank, G., Königsrainer, A., Sipos, B. et al. Adenocarcinoma arising in a cystic duplication of the small bowel: case report and review of literature. World J Surg Onc 10, 55 (2012). https://doi.org/10.1186/1477-7819-10-55
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7819-10-55