Abstract
Introduction
Mitral annulus calcification (MAC) is a common finding on echocardiographic examination. The goal of this study was to evaluate associations between MAC and cardiac abnormalities using a large echocardiographic database.
Methods
For this study we retrospectively reviewed 24,380 echocardiograms performed for clinical reasons between the years 1984 and 1998.
Results
MAC was reported in 1,494 (6.1%) subjects. Using multivariate analysis, age, left ventricular hypertrophy (LVH), mitral regurgitation (MR), tricuspid regurgitation (TR), aortic stenosis (AS), left atrial (LA) enlargement and reversed E/A ratio were independently associated with MAC.)MAC was noted in 11.7 % of patients with MR vs. 4.3% without MR (OR: 2.0, CI 1.6–2.6, p < 0.0001), in 13.9% of those with TR vs. 4.5% without TR (OR: 3.8, CI 2.9–4.8, p < 0.0001), in 10.6% with LVH vs. 4.2% without LVH (OR: 1.9, CI 1.5–2.4, p < 0.0001), in 14.8% with AS vs. 5.5% without AS (OR: 1.4, CI 1.08–1.9, p = 0.01), in 9.4% with reversed E/A ratio vs. 3.8% without reversed E/A ratio (OR: 1.7, CI 1.4–2.2, p < 0.0001) and in 8.2% with LA enlargement vs. 4.8% without LA enlargement (OR: 1.3, CI 1.06–1.7, p = 0.02).
Conclusion
In our study, MAC independently correlated with significant structural heart abnormalities. This suggests that identification of MAC may serve as a marker for other cardiac structural disorders.
Similar content being viewed by others
Background
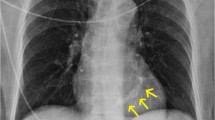
Idiopathic (degenerative) mitral annular calcification (MAC) is one of the most common cardiac abnormalities found upon autopsy. Four examples of normal, mild, moderate and severe MAC can be seen in figure 1. Although the development of degenerative calcification of the mitral annulus is functionally of little consequence in most hearts, it shares common risk factors with atherosclerosis; including systemic hypertension, hypercholesterolemia, and diabetes [1]. Therefore it is important to risk-stratify patients with MAC because of its association with other important disorders such as coronary and carotid atherosclerosis and increased risk for cardiovascular morbidity and mortality [1–3].
It has also been suggested that MAC might become an important cause of mitral regurgitation (MR) when calcification is severe [4, 5]. Previous observational studies suggest that MAC might be associated with several other cardiovascular disorders such as atherosclerosis, MR, stroke, atrioventricular conduction defects and hypertrophic cardiomyopathy [4–7]. In this study we analyzed a large echocardiographic database of over 24,000 patients. We performed comprehensive uni- and multivariate analyses to evaluate associations between MAC and cardiac abnormalities including valvular abnormalities, left ventricular hypertrophy (LVH) and left atrial (LA) enlargement.
Methods
We retrospectively reviewed 24,380 echocardiograms performed between the years 1984 and 1998, which were ordered by clinicians for various indications. MAC and other echocardiographic findings were diagnosed based on the interpretation of echocardiograms by various cardiologists using echocardiographic parameters on a complete echocardiogram, including pulse and continuous wave Doppler, Doppler color flow imaging and spectral Doppler velocimetry studies. The documented final diagnoses were available and used for our study. The reports were generated by clinical cardiologists trained in the interpretation of echocardiograms. The diagnosis of regurgitations included mild to severe, but trace regurgitations that are commonly observed in healthy population were not included in the diagnosis of regurgitations. Using uni- and multivariate analysis, we evaluated the association between age, gender, valvular abnormalities, pericardial effusion, decreased fractional shortening (FS defined as FS < 25%), left ventricular hypertrophy (LVH defined as left ventricular wall thickness >11 mm measured in standard parasternal long axis view involving at least one of the ventricular walls), aortic thickening, aortic root enlargement (defined as aortic root over 35 mm measured in parasternal long axis), abnormal early over late mitral flow reversal (reversed E/A ratio), body mass index (BMI>25) and enlarged left atrial (LA defined as left atrial size > 40 mm measured in standard parasternal long axis view), tricuspid regurgitation (TR), aortic regurgitation (AR) and aortic stenosis (AS). The statistical analysis was performed using SPSS software. A p-value of less than 0.05 was interpreted as statistically significant. Variables were analyzed using χ2 test. Quantitative variables were specified as mean ± standard deviation. In the multivariate analysis, a logistic regression model was utilized to calculate the odds ratio and their 95% confidence interval to indicate the strength of influence. We used SPSS statistical program version 13 for the statistical analysis. Baseline clinical characteristics of the patients were not available in the database. The institutional review board approved this study.
Results
MAC was diagnosed in 1,494 (6.1%) of the 24,380 echocardiograms. Using univariate analysis, MAC was significantly associated with age, gender, MR, TR, AR AS, thickened aortic valve; abnormal early over late mitral flow reversal (reversed E/A ratio; dilated aortic root; LA enlargement; BMI >25 and LVH. The association between MAC and valvular regurgitations was independent of the severity of regurgitation. Using multivariate analysis, age, LVH, MR, TR, AS, LA enlargement and reversed E/A ratio were independently associated with MAC. Mean age of patients with MAC was 68 years vs. 50 years in those without MAC (OR: 1.059, CI 1.054–1.062, p < 0.0001).)MAC was noted in 11.7 % of patients with MR vs. 4.3% without MR (OR: 2.0, CI 1.6–2.6, p < 0.0001), in 13.9% of those with TR vs. 4.5% without TR (OR: 3.8, CI 2.9–4.8, p < 0.0001), in 10.6% with LVH vs. 4.2% without LVH (OR: 1.9, CI 1.5–2.4, p < 0.0001), in 14.8% with AS vs. 5.5% without AS (OR: 1.4, CI 1.08–1.9, p = 0.01), in 9.4% with reversed E/A ratio vs. 3.8% without reversed E/A ratio (OR: 1.7, CI 1.4–2.2, p < 0.0001) and in 8.2% with LA enlargement vs. 4.8% without LA enlargement (OR: 1.3, CI 1.06–1.7, p = 0.02). There was no association between MAC and mitral stenosis, decreased fractional shortening or pericardial effusion (Table 1).
Discussion
Calcification within the mitral annulus results from a degenerative process in the cardiovascular fibrous skeleton, which is reported to be accelerated by advanced age, systemic hypertension, hypercholesterolemia, diabetes mellitus, chronic renal failure with secondary hyperparathyroidism and genetic abnormalities of the fibrous skeleton; such as, Marfan and Hurler syndromes [1, 8, 9]. The initial pathologic event is considered to be fibrillar alteration of the collagen ultrastructure [10]. This change triggers lipid deposition and the subsequent development of small foci of calcification deep within the annulus and at points of interdigitation between the annulus and ventricular muscle fibers.
Our study was conducted in order to evaluate association between MAC and age, gender and other echocardiographic cardiac abnormalities using a large number of echocardiograms. We demonstrated that MAC was significantly associated with advanced age, valvular regurgitations such as MR, TR and aortic stenosis, but not AR or MS. It is suggested that when the mitral valve annulus becomes thick, rigid and calcified, it may interfere with valve closure, causing MR. In this setting, age is considered to play a significant role in mitral valve degeneration, triggering MAC. This association also explains the association between advanced age and the occurrence of MAC. The reason for MAC association with TR is not known. A secondary increase in the pulmonary arterial pressure due to associated MR or AS may explain this association. Furthermore, patients with MAC are also at risk for calcification in other vascular systems as a marker of atherosclerosis and aging [3, 6, 8, 11, 12]. However, the independent association of MAC with AS in our study suggests that MAC may play a direct role in the pathogenesis of AS or that patients with MAC may represent a group of individuals with increased susceptibility to generalized calcium deposition in the cardiac and extra cardiac tissue. Fulkerson et al., on the other hand, reported that conditions associated with chronically increased left ventricular pressures, such as aortic stenosis and systemic hypertension, enhance stress on the mitral valve and apparatus or promote abnormal valve motion. These effects are considered to accelerate the degenerative process, leading to premature calcium deposition as a possible explanation of our findings [10]. Furthermore, our large population study confirms Fulkerson et al.'s observation that MAC was significantly associated with LVH and AS. Coronary artery disease and systemic calcified atherosclerosis have been found to be strongly associated with MAC suggesting similar pathogenesis in calcium deposition involving cardiovascular system [13, 14]. Our study failed to show any significant association between MAC and AR. It appears that MAC does not decrease the integrity of aortic valve that could lead to AR. We have a small number of patients with mitral stenosis in our database limiting the assessment of the association of MAC and mitral stenosis. Finaly, MAC has been found to be associated with significant cardiovascular morbidity and mortality [15] confirming our finding that the presence of MAC could be marker for underlying cardiovascular pathology.
Conclusion
This is the first large-scale study demonstrating that MAC is independently associated with advanced age, MR, TR, AS, diastolic mitral flow reversal, left atrial enlargement and LVH. This suggests that identification of MAC may serve as a marker for other cardiac structural disorders and that patients with MAC may require further evaluation.
Limitations
This is a retrospective study that relied on previous reports, resulting in non-uniform assessment of the echocardiographic abnormalities. The diagnoses of cardiac abnormalities were not standardized. Data regarding criteria for diagnosis and grading of MAC was not available. The sensitivity of the older equipments for detection of MAC is probably less than the newer equipments. We scored MAC in our study as absent or present. However, a semiquantitative assessment of MAC severity could have improved the study interpretation.
References
Fox CS, Vasan RS, Parise H, Levy D, O'Donnell CJ, D'Agostino RB, Benjamin EJ: Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation 2003,107(11):1492-1496. 10.1161/01.CIR.0000058168.26163.BC
Jeon DS, Atar S, Brasch AV, Luo H, Mirocha J, Naqvi TZ, Kraus R, Berman DS, Siegel RJ: Association of mitral annulus calcification, aortic valve sclerosis and aortic root calcification with abnormal myocardial perfusion single photon emission tomography in subjects age < or =65 years old. J Am Coll Cardiol 2001,38(7):1988-1993. 10.1016/S0735-1097(01)01678-3
Adler Y, Koren A, Fink N, Tanne D, Fusman R, Assali A, Yahav J, Zelikovski A, Sagie A: Association between mitral annulus calcification and carotid atherosclerotic disease. Stroke 1998,29(9):1833-1837.
Carabello BA: Mitral valve regurgitation. Curr Probl Cardiol 1998,23(4):202-241. 10.1016/S0146-2806(98)80005-4
Mann JM: Mitral Valve Diesease. London, Butterworths; 1996:16-27.
Nair CK, Thomson W, Ryschon K, Cook C, Hee TT, Sketch MH: Long-term follow-up of patients with echocardiographically detected mitral anular calcium and comparison with age- and sex-matched control subjects. Am J Cardiol 1989,63(7):465-470. 10.1016/0002-9149(89)90321-4
Aronow WS, Kronzon I: Prevalence of hypertrophic cardiomyopathy and its association with mitral anular calcium in elderly patients. Chest 1988,94(6):1295-1296.
Merjanian R, Budoff M, Adler S, Berman N, Mehrotra R: Coronary artery, aortic wall, and valvular calcification in nondialyzed individuals with type 2 diabetes and renal disease. Kidney Int 2003,64(1):263-271. 10.1046/j.1523-1755.2003.00068.x
Roberts WC, Perloff JK: Mitral valvular disease. A clinicopathologic survey of the conditions causing the mitral valve to function abnormally. Ann Intern Med 1972,77(6):939-975.
Fulkerson PK, Beaver BM, Auseon JC, Graber HL: Calcification of the mitral annulus: etiology, clinical associations, complications and therapy. Am J Med 1979,66(6):967-977. 10.1016/0002-9343(79)90452-2
Cacciaputi F DR Davino M, lama D, Coppola F, Bianchi U, varricchio M: Calicifications of the mitral annulus as a marker of atherosclerosis in the elderly. Arch of Gerontology and geriatrics 1991, suppl.2: 339-343.
Adler Y, Levinger U, Koren A, Gabbay R, Shapira Y, Vaturi M, Fink N, Herz I, Zelikovski A, Sagie A: Association between mitral annulus calcification and peripheral arterial atherosclerotic disease. Angiology 2000,51(8):639-646.
Allison MA, Cheung P, Criqui MH, Langer RD, Wright CM: Mitral and aortic annular calcification are highly associated with systemic calcified atherosclerosis. Circulation 2006,113(6):861-866. 10.1161/CIRCULATIONAHA.105.552844
Barasch E, Gottdiener JS, Larsen EK, Chaves PH, Newman AB, Manolio TA: Clinical significance of calcification of the fibrous skeleton of the heart and aortosclerosis in community dwelling elderly. The Cardiovascular Health Study (CHS). Am Heart J 2006,151(1):39-47. 10.1016/j.ahj.2005.03.052
Barasch E, Gottdiener JS, Marino Larsen EK, Chaves PH, Newman AB: Cardiovascular morbidity and mortality in community-dwelling elderly individuals with calcification of the fibrous skeleton of the base of the heart and aortosclerosis (The Cardiovascular Health Study). Am J Cardiol 2006,97(9):1281-1286. 10.1016/j.amjcard.2005.11.065
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Movahed, MR., Saito, Y., Ahmadi-Kashani, M. et al. Mitral Annulus Calcification is associated with valvular and cardiac structural abnormalities. Cardiovasc Ultrasound 5, 14 (2007). https://doi.org/10.1186/1476-7120-5-14
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-7120-5-14