Abstract
Left ventricular (LV) diastolic dysfunction (DD) and diastolic heart failure (HF), that is symptomatic DD, are due to alterations of myocardial diastolic properties. These alterations involve relaxation and/or filling and/or distensibility. Arterial hypertension associated to LV concentric remodelling is the main determinant of DD but several other cardiac diseases, including myocardial ischemia, and extra-cardiac pathologies involving the heart are other possible causes. In the majority of the studies, isolated diastolic HF has been made equal to HF with preserved systolic function (= normal ejection fraction) but the true definition of this condition needs a quantitative estimation of LV diastolic properties. According to the position of the European Society of Cardiology and subsequent research refinements the use of Doppler echocardiography (transmitral inflow and pulmonary venous flow) and the new ultrasound tools has to be encouraged for diagnosis of DD. In relation to uncertain definitions, both prevalence and prognosis of diastolic heart failure are very variable. Despite an apparent lower death rate in comparison with LV systolic HF, long-term follow-up (more than 5 years) show similar mortality between the two kinds of HF. Recent studies performed by Doppler diastolic indexes have identified the prognostic power of both transmitral E/A ratio < 1 (pattern of abnormal relaxation) and > 1.5 (restrictive patterns). The therapy of LV DD and HF is not well established but ACE-inhibitors, angiotensin inhibitors, aldosterone antagonists and β-blockers show potential beneficial effect on diastolic properties. Several trials, completed or ongoing, have been planned to treat DD and diastolic HF.
Similar content being viewed by others
Introduction
Heart failure (HF) is a clinical syndrome whose symptoms and signs are due to increased extravascular water and decreased tissue / organ perfusion. The definition of the mechanisms inducing HF needs the measurement of both left ventricular (LV) systolic and diastolic function since HF may occur in patients with either normal or abnormal LV ejection fraction (EF) [1].
Arterial hypertension is the most common risk factor for HF in the general population and myocardial infarction, LV hypertrophy (LVH) and valve heart disease represent predictors of subsequent HF in hypertensive patients of both genders [2]. The progression of hypertensive cardiomyopathy towards HF includes serial LV changes – LV concentric remodelling and LVH – whose prognostic role is recognized [3–5]. In presence of these LV geometric abnormalities, deep modifications of LV diastolic properties occur. These modification are globally defined as LV diastolic dysfunction (DD) and include alterations of both relaxation and filling [6, 7] which can precede alterations of LV systolic function and be per se main determinants of symptoms and signs of HF. Several other cardiac pathologies as well as extra-cardiac diseases involving secondarily the left ventricle can also affect myocardial diastolic properties and determine LV DD.
LV DD and diastolic HF, that is the symptomatic DD, represent clinical entities which can be described at different levels, from the hystologic and ultrastructural features to the clinic manifestations and diagnostic instrumental findings, until the prognostic and therapeutic aspects. The growing interest for DD and for diastolic HF has been developed gradually in the last 10–15 years. It rises mainly from the advancement of non invasive imaging tools, above all Doppler echocardiography, which, to date, allows easy and repeatable identification of LV diastolic abnormalities, and by the growing impulse of pharmaceutical industry, at constant search of new therapeutic applications. In relation to the increase of the average life and the future projections which suggest HF as the most important pathology of the new millennium, particularly in the elderly population, it has to be understood how diagnosis, prognosis and therapeutic management of DD represent very attractive perspectives.
Physiology of diastole
Although in normal hearts the transition from contraction to relaxation begins much more before LV end-systole, i.e., at 16% to 20% of the ejection period [8, 9] and even prior to aortic valve opening when LV contractility is severely impaired (9), the traditional definition of diastole (in ancient Greek language the term διαστολε means "expansion"), includes the part of the cardiac cycle starting at the aortic valve closure – when LV pressure falls below aortic pressure – and finishing at the mitral valve closure. A normal LV diastolic function may be clinically defined as the capacity of the left ventricle to receive a LV filling volume able in its turn to guarantee an adequate stroke volume, operating at a low pressure regimen.
In merely descriptive terms, diastole can be divided in 4 phases [10]:
1. Isovolumetric relaxation, period occurring between the end of LV systolic ejection (= aortic valve closure) and the opening of the mitral valve, when LV pressure keeps going its rapid fall while LV volume remains constant. This period Is mainly attributed to the active LV relaxation, with a lower, variable contribution of elastic recoil of the contracted fibers;
2. LV rapid filling, which begins when LV pressure falls below left atrial pressure and the mitral valve opens. During this period the blood has an acceleration which achieves a maximal velocity, direct related to the magnitude of atrio-ventricular pressure, and stops when this gradient ends. This period represents a complex interaction between LV suction (= active relaxation) and visco-elastic properties of the myocardium (= compliance);
3. diastasis, when left atrial and LV pressures are almost equal and LV filling is essentially maintained by the flow coming from pulmonary veins – with left atrium representing a passive conduit – with an amount depending of LV pressure, function of LV "compliance".
4. atrial systole, which corresponds to left atrial contraction and ends at the mitral valve closure. This period is mainly influenced by LV compliance, but depends also by the pericardial resistance, by the atrial force and by the atrio-ventricular synchronicity (= ECG PR interval).
Cardiac catheterization allows to assess the pressure-volume relation along the overall cardiac cycle. Among the various hemodynamic measurements, τ (= time constant of the isovolumic-pressure decline) and DP/DV ratio, expression of LV end-diastolic myocardial stiffness, are the main invasive measurements of LV diastolic function [10]. On the other hand, Doppler recording of transmitral and pulmonary venous flow measure flow velocities and time intervals, whose variations occur in relation to analogous variations of left atrial and LV pressures [11, 12]. Thus, Doppler parameters provide important information about dynamics of LV filling and LV diastolic properties during disease evolution or improvement [13].
Ultrastructural features of diastolic dysfunction
The extracellular matrix (ECM), corresponding to fibrillar collagen, is an important structure for processes of both myocardial contraction and relaxation. It facilities the arrangement of the cardiomyocites into the most suitable allocation for the development of force and shortening, giving a substantial support to the maintenance of an effective myocardial performance [14]. The myocardial remodelling is accompanied by changes of myocardial cell factors but also of the ECM where fibroblast proliferation, alteration of the collagen network and increase in interstitial and perivascular collagen are strongly promoted by renin-angiotensin-aldosterone system [15]. ECM has, therefore, to be considered a dynamic entity playing a fundamental role into the myocardial adaptation to physiologic and pathologic stress [14]. ECM undergoes an intense turnover, due to balanced action of metalloproteases, proteolytic enzymes activated by several factors including also BNP, and tissue inhibitors counterbalancing the activity of metalloproteases [16]. Thus, if the collagen destruction alters both geometry and function of contractile myocardium throughout an up-regulation of metalloproteases, on the other hand myocardial fibrosis occurs because of an imbalance where collagen deposition prevails over its degradation. According to the ultrastructural view, we can hypothesize two opposite pathologic conditions: the first one, when the collagen loss, e.g, after acute myocardial infarction, deprives myocardium of its indispensable support structure, thus inducing a reduction of myocardial systolic function; the second one, when the accumulation of the same collagen, main component of myocardial fibrosis, determines both systolic and diastolic myocardial dysfunction. In this context not only the total amount of collagen is main determinant of LV diastolic stiffness but also distribution, configuration, disorganization of collagen fibers (cross-hatching), and ratio of collagen type I to collagen type III play an important role [14].
Clinical, hemodynamic and diagnostic and aspects of diastolic dysfunction
In the clinical setting the coexistence of systolic and diastolic dysfunction in patients with symptomatic HF occurs very often. In fact, LV stiffness (or compliance) is related to the length of myocardial fibers, reflecting in its turn on LV end-diastolic dimensions. LV diastolic function, through the influence on left atrial and capillary wedge pressures, determines the onset of symptom in patients with prevalent LV systolic dysfunction too.
In parallel to the ultra-structural level, the clinical progression of HF may follow two different routes. In the first one, as it happens after acute myocardial infarction, post-infarction LV dilation (= remodelling) leads to systolic dysfunction and/or systolic heart failure. In the second one, LV structural abnormalities (= LV concentric geometry) induce functional alterations of DD. When diastolic dysfunction becomes symptomatic – that is, when dyspnoea occurs – diastolic heart failure rises.
The majority of patients affected by isolated diastolic HF show symptoms not at rest but in relation to stress conditions (II NYHA class). Symptoms can be induced or worsened by, firstly, physical exercise but also by events as anaemia, fever, tachycardia and some systemic pathologies. In particular, tachycardia reduces the time needed for global LV filling, thus inducing an increase of left atrial pressure and consequent appearance of dyspnoea, because of accumulation of pulmonary extravascular water.
The diagnosis of HF can be performed obviously by the simple clinical examination but the identification of the diastolic origin needs an instrumental assessment. In fact, the objective examination of patients with diastolic HF allows to notice the same signs occurring for systolic HF and even the thoracic X-ray can not be useful to distinguish the two entities. ECG can show signs of LVH, due to hypertensive cardiomyopathy or other causes. DD may be asymptomatic and, therefore, identified occasionally during a Doppler echocardiographic examination (Figure 1). The diagnostic importance of this tool rises from the high feasibility of transmitral Doppler indexes of diastolic function, shown even in studies on population [17], such to be suitable and accurate also for serial evaluations over time. To date, standard Doppler indexes may be efficaciously supported by the evaluation of pulmonary venous flow [18] (Figure 2) and by new ultrasound technologies as Tissue Doppler [19] (Figure 3) and color M-mode derived flow propagation rate [20]. The application of maneuvers (Valsalva, leg lifting) [21, 22] to Doppler transmitral pattern and/or different combination of standard transmitral Doppler with the new tools (ratio between atrial reverse velocity duration and transmitral A velocity duration, ratio between transmitral E peak velocity and Tissue Doppler derived Em of the mitral annulus or flow propagation velocity [Vp]) are sufficiently reliable to predict capillary wedge pressure and to distinguish accurately variations of LV end-diastolic pressure [23, 24]. Some of these tools are effective even in particular situations as sinus tachycardia [25] and atrial fibrillation [26] while the pulmonary venous flow or the Valsalva maneuver applied to transmitral inflow has to be preferred in the case of mitral valve prosthesis and aortic valve regurgitation [27]. In addition, Tissue Doppler is also able to "read" the percentage of myocardial fibrosis [28], primum movens of DD. Alone or, better, combined together, these tools permits to recognize normal diastole as well as to diagnose and follow the progression of DD from the pattern of abnormal relaxation (grade I of DD) until pseudonormal (grade II) and restrictive (grade III-IV) patterns (Table 1).
In the left screen, methodological outline for the measurement of Doppler transmitral indexes of diastolic function. In the right screen, normal diastolic pattern (upper part) and pattern of abnormal relaxation (lower part). A = atrial velocity (m/s), DT = deceleration time of E velocity (ms), E = early diastolic velocity (cm/s), IVRT = isovolumic relaxation time (ms)
In the left screen, methodological outline for the measurement of Tissue Doppler indexes. In the right screen, normal myocardial diastolic pattern (upper part) and pattern of abnormal myocardial relaxation (lower part). Am = myocardial atrial velocity (cm/s), CTm = myocardial contraction time (ms), DTm = myocardial deceleration time of Em(ms), Em = myocardial early-diastolic velocity (cm/s), PCTm = myocardial pre-contraction time (ms), RTm = myocardial relaxation time (ms).
By the hemodynamic point of view, the differences between diastolic and systolic HF are expressed by the pressure-volume loop (Figure 4) [29]. When systolic HF occurs, increased LV filling pressures correspond to increased LV volumes, with a displacement of the loop upon and at right. In the case of diastolic HF, the increase of LV filling pressures occur in the presence of normal or even reduced LV volumes, thus moving the loop up and to the left. It is obvious that in the more advanced stages of HF, diastolic and systolic dysfunction coexist.
Determinants of diastolic dysfunction
LV DD develops in several cardiac diseases [30] as well as in extra-cardiac pathologies involving the heart (accumulation diseases as amyloidosis, thyroid disorders, acromegaly and others) [31, 32] and in myocardial ischemia due to coronary artery stenosis or even to isolated dysfunction of coronary microcirculation [33]. However, the main cause of DD is arterial hypertension [5–7]. Overweight and obesity, often coexisting with the same hypertension, deeply affects LV diastolic function, forcing the left ventricle to a working overload [34]. In this view, DD represents one of the cardiac consequences of pluri-metabolic syndrome, where arterial hypertension, obesity, glucose intolerance and hypertrygliceridemia cohabit in the same subject, having their common matrix in the insulin resistance. High levels of insulin resistance, often evident in arterial hypertension [35], are positively associated with the prolongation of isovolumic relaxation time, independent of LV geometric changes and of increased afterload [36]. The alteration of diastolic isovolumic relaxation is probably due to an increment of intracellular calcium, which has been observed in insulin resistant hypertensives and is induced in its turn by an abnormal re-uptake of calcium by sarcoplasmic reticulum [37]. Also the hormones produced by adipose tissue, as leptin – involved into the control of body weight throughout food absorption and energy-giving cost – negatively affects LV diastolic function [38]. The association of arterial hypertension and diabetes mellitus worsens furher Doppler indexes of LV diastolic function as shown into the population of the Strong Heart Study [39].
It is controversial whether LV DD is necessarily accompanied on the development of LVH or rises up independent of it [5–7, 40–43]. It is true that DD is a direct sequence of pressure overload, associated to elevated 24-hour blood pressure [40] and even more to the increment of night-time diastolic blood pressure [43]. Recent studies point out that the diastolic abnormalities of hypertensive patients are related to inappropriately high levels of LV mass, disproportionate to the hemodynamic load predicted by the individual body size and cardiac load, more than to the values of LV mass which traditionally define LVH [44]. Inappropriately high LV mass is a potent predictor of cardiovascular risk in hypertensive patients, in presence as in absence of clear cut LVH [45]. The concept of DD onset preceding the appearance of LVH is consistent with the observation that BNP, whose levels grow gradually with the progression of DD grading (from abnormal relaxation until restrictive Doppler patterns) [46], are increased in patients with diastolic HF independent of the magnitude of LV mass [47]. Even a new ultrasound technology as Tissue Doppler supports the hypothesis of an early evidence of DD in hypertensive heart: myocardial DD (= Em/Am ratio < 1 at the level of multiple LV walls in the apical views) is detectable before the appearance of the abnormalities involving LV transmitral inflow and is uniform in non hypertrophic patients while it becomes prominent at the septum in presence of overt LVH [48]. Table 2 reports the differential characteristics involving in the meantime the myocardial ultra-structure and LV geometry in systolic and diastolic HF: it is clear that diastolic HF is associated to both increase of collagen amount and LV concentric geometry [49]. This concept is further supported by the HyperGEN study where delayed LV relaxation is independently associated with concentric LV geometry in 1384 hypertensive participants including obese and diabetic patients [50].
Definition and classification criteria for diastolic HF
The evidence of acute HF in absence of overt LV systolic dysfunction rises by the experience of Gandhi and coworkers [51]: thirty-height hypertensive patients affected by pulmonary oedema, undergoing echocardiographic examination during the acute episode and after clinical stabilization respectively (1–3 day after), did not show significant variations of LV EF (50 ± 15% and 50 ± 13% respectively, NS) and of wall motion score index between the two examinations. This clinical condition, defined as heart failure with preserved systolic function or, better, with normal EF, has been made equal to isolated diastolic heart failure. A truly correct definition of this clinical entity should, however, be done on the grounds of direct estimation of LV diastolic function and establishment of reference normal values. Strong controversy has been developed in the previous years about this issue, with opposite scientific positions. The American point of view, corresponding to the Framingham Heart Study investigators, has sustained the concept that diastolic HF is "definite" only when an invasive hemodynamic assessment shows diastolic alterations in the temporal proximity of the acute episode [52]. On the other hand, the European point of view (European Group on Diastolic Heart Failure) has defined diastolic HF according to criteria including clinical examination, echocardiographic assessment (normal EF) and Doppler indexes (derived by both transmitral inflow e pulmonary veins flow), whose normal partition values are referred for age ranges [53] (Table 3). Despite the obvious superiority of the invasive technique [54], it has to be taken into account that the need of cardiac catheterization for establishing a definite diagnosis of diastolic HF raises practical and even ethical issues. Practical issues are related to the low priority such examination would have in a cath-lab overloaded by coronary procedures and to the poor interest of hemodynamists in the assessment of indexes of LV diastolic function. Ethical concern lies upon the fact that the present reliance on echo-Doppler examination of LV diastolic function makes cardiac catheterization an useless invasive procedure to this end, except very particular cases. Moreover, if it is true that the prevalence of abnormal Doppler indexes (from 38% of isovolumic relaxation time to 64% for deceleration time) is much lower to that showed by the more reliable invasive measurements (92 % for LV end-diastolic pressure and 79 % for τ) [55], is also true that this can be, at least partially, due to the confounding influence of physiologic variables as age [56] and heart rate [57]. In this view, reference normal values of Doppler indexes of LV diastolic function should be done considering ranges of both age and heart rate. It is now current opinion that the diagnosis of diastolic HF can be made even without measurement of diastolic function if three criteria are present: 1) symptoms and signs of HF (Framingham criteria), 2) LV EF> 50%, and 3) ability to rule out mitral stenosis, pericardial disease, and non cardiac causes of dyspnoea, oedema and fatigue [58]. Recent evidences further sustain the definite role of Doppler echocardiography to diagnose diastolic HF [59, 60].
To date, however, no certain definition of diastolic HF exists and the recognition of its existence is not unanimously accepted [49]. Studies performed by both standard Doppler echocardiography [61] and Tissue Doppler [62, 63] demonstrated how sub-clinic alterations of myocardial systolic function are already overt in diastolic HF. Because of the use of LV EF is a rather insensitive indicator of true LV myocardial contractility, the assessment of LV long-axis function by the simple M-mode of the mitral lateral annulus could help to identify initial LV systolic dysfunction [64]. Finally, it has also to be taken into account how concomitant variables, including obesity, chronic obstructive lung disease and even myocardial ischemia, can be confounding factors leading to "false" diagnosis of diastolic HF, particularly in the elderly population [65].
Prevalence of diastolic HF
The studies performed until now have assessed above all the prevalence of HF with normal EF, using standard echocardiography without Doppler. In a first meta-analysis of 1995, the investigators of the Framingham Heart Study [66] showed wide variability in the prevalence of this kind of HF (range = 13–74%) while a subsequent study involving the Framingham offspring cohort pointed out a 51% prevalence of overall HF [67]. Very recently, Hogg et al collected ten "cross-sectional" studies on population, in the United States as in several European countries, and found very high variability of HF with normal EF. The explanation of this variability is related mostly to different age and gender of participants. It has to be considered that this kind of HF is particularly frequent in the elderly population, occurs more often in the female gender and is associated much more with arterial hypertension and atrial fibrillation than to coronary heart disease [68]. The data collected between 1995 and 1999 from Italian Network on Congestive Heart Failure (IN-CHF) are strongly consistent with these results [69]. The choice of different cut-off points for normal LV EF can be an additional reason of variability for the prevalence of diastolic HF in the above mentioned studies.
Prognosis of diastolic heart failure
Great heterogeneity exists also for results in prognosis of diastolic HF. By the Framingham meta-analysis the annual mortality varies from 1.3% to 17.5% [66]. This wide variability depends by several factors including first of all, the modality used to classify this kind of HF – mostly according to the evidence of normal EF – but also age and follow-up duration. In a study by registry on 1291 hospitalized patients (70) the mortality was lower in patients with EF ≥ 50% than in those with EF ≤ 39% (OR = 0.69 95% CI 0.49–0.98, p = 0.04) . The Framingham offspring cohort informed that the rate of death after 5 years is 68% in patients with HF and normal EF in comparison with 82% of systolic HF, with a mortality, however, four times greater than that presented by healthy subjects [67]. Although Senni et al (71) did not find difference of mortality between the two kinds of HF in a 4-year follow-up of a population with mean age of 78 years, the analysis of Hogg and coworkers, assembling the results of recent cohort studies performed on patients hospitalized for HF, noticed how the percentage of mortality for patients with HF and normal EF, mild during the first year and half, becomes similar to that of systolic HF after 5–6 years of follow-up (68). It is worthy of note the recent study of Badano and coworkers who, using the ESC criteria to identify diastolic HF in 179 patients hospitalized with HF, do not observe significant difference in 6-month mortality in comparison with patients having prevalent LV systolic dysfunction [72].
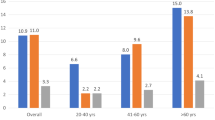
Two important studied have finally pointed out the prognostic value of Doppler indexes of LV diastolic function and in particular of transmitral E/A ratio [73, 74]. The first one, the PIUMA study [73], evidenced that the pattern of abnormal relaxation (= E/A ratio lower than that predicted individually by age and heart rate) increases the risk of cardiovascular events (odds ratio 1.57, 95% CI 1.1-2,18, p < 0.01) in a population of 1839 hypertensive patients during a 11 years follow-up. This prognostic value is independent of the effect of LV mass and even of ambulatory 24-hour blood pressure. In the second one, the Strong Heart Study [74], by a 3-year follow-up on a population of 3008 American Indians, a transmitral E/A ratio < 0.6 (= likewise pattern of abnormal relaxation) is associated to a doubled increase of mortality risk – despite not independent of other covariates – and an E/A ratio > 1.5 (= likewise pattern pseudonormal / restrictive) is associated to an threefold increase of cardiac mortality, which is also independent of several confounders including LVH. This result is consistent with the findings of the Framingham Heart Study, where an "U" relation between transmitral A velocity and risk of atrial fibrillation is detectable, and the arrhythmia appears independently associated with both A velocity increase (= abnormal relaxation) and E/A ratio increase (pattern pseudonormal / restrictive) [75]. These two studies, in particular the Strong Heart Study by data on mortality, are very consistent with the physiopathologic point of view of the Mayo Clinic investigators, who created an ingenious classification of Doppler-.derived DD some years ago [21]. In this classification, the pattern of abnormal relaxation (grade I of DD) and both reversible and non reversible restrictive patterns (grade III and IV respectively) are at opposite sides in the clinical progression towards the end stages of HF while the pseudo-normal pattern has an intermediate, but clinically crucial, position (Figure 5). In view of these findings and combining the value of the prognostic studies, we can suppose that the relatively long time (5–6 years) needed to assimilate the prognosis of diastolic HF to that of systolic HF depends mainly by the transition from the initial grade of DD, when the pattern of abnormal relaxation prevails and dyspnoea is overt only during exercise, to the more advanced stages, when the high LV end-diastolic pressure is associated to "end-stage" HF.
Results of overall and cardiac mortality in relation to transmitral E/A ratio in the Strong Heart Study [74] (upper panel)) and classification of DD grades (I-IV) according to Mayo Clinic suggestions [21] (lower panel). It can be observed a parallel behaviour between clinic progression and prognostic value of different grade of DD: the increment of mortality in Strong Heart Study has an "U" behavior, where E/A ratio <0.6 (grade I of DD) and >1.5 (grades II, III, IV) are both main predictors of mortality DD = diastolic dysfunction, NYHA = New York Heart Association, MAP = mean atrial pressure
Therapy of DD and diastolic HF
The objectives of the therapy for LV DD include the improvement of hemodynamic conditions, concerning both preload and afterload. The volume overload, such to induce episodes of acute HF, can be prevented or reduced by hypo-saline diet or also by a moderate diuretic administration.
Conceptually, both ACE-inhibitors and angiotensin-inhibitors can exert a beneficial effect on DD, since they reduce both afterload and preload, induce regression of LVH and decrease of myocardial interstitial fibrosis [70]. Also the aldosterone antagonists, as sprironalattone [76] and canrenone [77], able to reduce the myocardial fibrosis, can be suitable to this aim.
When DD is overt, it is also important to control heart rate and avoid tachycardia. β-blockers, and, with a lower extent, calcium antagonist verapamile, can be particularly useful. Lower heart rate induces prolongation of LV filling time, allowing to counterbalance the resistance to the diastolic inflow of a stiffened left ventricle. Last generation β-blockers (carvedilol, nebivolol), provided of vasodilation activity, could be particularly indicated for the management of DD. A recent study has tested the ability of nebivolol on 26 patients affected by HF and normal EF, in comparison with the traditional atenolol, combining both invasive hemodynamic and Doppler echocardiographic assessment [78]. After six-month therapy, nebivolol much more than atenolol induced increase of both E/A ratio (from 0.79 ± 0.13 a 0.91 ± 0.11) from 0.84 ± 0.12 a 0.89 ± 0.15) (p < 0.004) and cardiac index and reduction of "wedge" pressures, both at rest and during exercise.
On these grounds, pharmaceutical industry has planned clinical trias to evaluate the prognostic impact of several drugs on diastolic HF. Indeed, the trials completed to date have been disappointing. The CHARM-2 (= Candesartan in Heart Failure – Assessment of Reduction in Mortality) [79] did not evidenced significant improvement of all-cause mortality, of cardiovascular mortality and of hospitalization rate for HF in the sub-set of patients with preserved systolic function, but the follow-up (37.7 months) was probably too short to verify the effects. Into SWEDIC (= Swedish Doppler-Echocardiographic study) [80], carvedilol, inducing a positive influence on transmitral E/A ratio in patients with heart rate > 71 bpm but not in those with HR < 71 bpm, did not exert any effects on the events. Among ongoing trials, the analysis of SENIORS (= Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with heart failure) [81] has not yet performed in the sub-set of patients with normal EF. PEP-CHF (perindopril versus placebo), I-Preserve (Irbesartan versus placebo) study and Hong Kong (rampiril, irbesartan, placebo) study have not completed to date [82].
New therapeutic fields for HF will be opened in view of the associations observed between the state of coronary microcirculation and LV diastolic function [33]. The beneficial effect of ACE-inhibitors on coronary flow reserve, reliable marker of coronary microcirculation function when stenosis of epicardial coronary arteries are not detectable , has been documented in relation to both blood pressure fall and reduction of LV mass. It has recently shown an improvement of transthoracic Doppler-derived coronary flow reserve after only 4-week anti-hypertensive nebivolol therapy, in relation to its endothelium-mediated vasodilation activity [85]. It has to hypothesize that the a restored function of the coronary microcirculation could be useful even for the improvement of DD in hypertensive heart [83, 84].
Conclusive implications
DD and diastolic HF are common entities in the clinical practice, particularly in hypertensive population. The diagnosis of diastolic HF can be considered in the presence of the signs of HF and normal EF (50% or more) but it should be usually supported by a Doppler examination. Diastolic HF is associated to four-fold increase mortality. If it is true that the mortality expectation is lower than in patients with systolic HF, it is even true that this difference has a trend to be blunted during long-term follow-up, with possible overlapping after 5.5 years or more. The therapeutic management of diastolic HF is, at least partially, empirical and several studies, ongoing or completed, have been planned to test the effects of ACE-inhibitors, angiotensin-inhibitors and β-blockers. The prevention of diastolic HF may be obtained by a better control of blood pressure values and of concomitant risk factors in hypertensive patients.
References
Jessup M, Brozena S: Heart failure. N Engl J Med 2003, 348: 2007-2018. 10.1056/NEJMra021498
Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK: The progression from hypertension to congestive heart failure. JAMA 1996, 275: 1557-1762. 10.1001/jama.275.20.1557
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP: Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990, 322: 1561-1566.
Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH: Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 1991, 114: 345-352.
Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Bartoccini C, Santucci A, Santucci C, Reboldi G, Porcellati C: Adverse prognostic significance of concentric remodeling of the left ventricle in hypertensive patients with normal left ventricular mass. J Am Coll Cardiol 1995, 25: 871-878. 10.1016/0735-1097(94)00424-O
Inouye I, Massie B, Loge D, Simpson P, Tubau JF: Abnormal left ventricular filling: an early finding in mild to moderate systemic hypertension. Am J Cardiol 1984, 53: 120-126. 10.1016/0002-9149(84)90695-7
Fouad FM, Slominsky JM, Tarazi RC: Left ventricular diastolic function in hypertension: relation to left ventricular mass and systolic function. J Am Coll Cardiol 1984, 3: 1500-1506.
Solomon SB, Nikolic SD, Fraser RWM, Yellin EL: Contraction-relaxation coupling: determination of the onset of diastole. Am J Physiol 1999, 277: H23-H27.
Gillebert TC, Leite-Moreira AF, De Hert SG: The hemodynamic manifestations of normal myocardial relaxation. A framework from experimental and clinical evaluation. Acta Cardiol 1997, 52: 223-246.
Zile MR: Diastolic dysfunction: Detection, consequences and treatment. Part I: Definition and determinants of diastolic function. Mod Concepts Cardiovasc Dis 1989, 58: 67-71.
Rokey R, Kuo LC, Zoghbi WA, Limacher MC, Quinones MA: Determination of parameters of left ventricular diastolic filling with pulsed Doppler echocardiography: comparison with cineangiography. Circulation 1985, 71: 543-550.
Stoddard MF, Pearson AC, Kern MJ, Ratcliff J, Mrosek DG, Labovitz AJ: Left ventricular diastolic function: comparison of pulsed Doppler echocardiographic and hemodynamic indexes in subjects with and without coronary artery disease. J Am Coll Cardiol 1989, 13: 327-336.
Appleton CP, Galloway JM, Gonzalez MS, Gaballa M, Basnight MA: Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease: additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol 1993, 22: 1972-1982.
Spinale FG: Bioactive peptide signaling within the myocardial interstitium and the matrix metalloproteinases. Circ Res 2002, 91: 1082-1084. 10.1161/01.RES.0000047874.80576.5A
Unger T, Li J: The role of the renin-angiotensin-aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst 2004,5(Suppl 1):S7-10.
MacKenna D, Summerour SR, Villarreal FJ: Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res 2000, 46: 257-263. 10.1016/S0008-6363(00)00030-4
Galderisi M, Benjamin EJ, Evans JC, D'Agostino RB, Fuller DL, Lehman B, Wolf PA, Levy D: Intra- and inter-observer reproducibility of Doppler assessed indexes of left ventricular diastolic function in a population based study (the Framingham Heart Study). Am J Cardiol 1992, 70: 1341-1346. 10.1016/0002-9149(92)90772-Q
Masuyama T, Nagano R, Nariyama K, Lee JM, Yamamoto K, Naito J, Mano T, Kondo H, Hori M, Kamada T: Transthoracic Doppler echocardiographic measurements of pulmonary venous flow patterns: comparison with transesophageal echocardiography. J Am Soc Echocardiogr 1995, 8: 61-69.
Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA: Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 1997, 30: 1527-1533. 10.1016/S0735-1097(97)00344-6
Garcia MJ, Smedira NG, Greenberg NL, Main M, Firstenberg MS, Odabashian J, Thomas JD: Color M-mode Doppler flow propagation velocity is a preload insensitive index of left ventricular relaxation: animal and human validation. J Am Coll Cardiol 2000, 35: 201-208. 10.1016/S0735-1097(99)00503-3
Nishimura RA, Tajik J: Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician's Rosetta Stone. J Am Coll Cardiol 1997, 30: 8-18. 10.1016/S0735-1097(97)00144-7
Pozzoli M, Traversi S, Cioffi G, Stenner R, Sanarico M, Tavazzi L: Loading manipulations improve the prognostic value of Doppler evaluation of mitral flow in patients with chronic heart failure. Circulation 1997, 5: 1222-1230.
Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ: Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler catheterization study. Circulation 2000, 102: 1788-1794.
Garcia MJ, Ares MA, Asher C, Rodriguez L, Vandervoort P, Thomas JD: An index of early left ventricular filling that combined with pulsed Doppler peak E velocity may estimate capillary wedge pressure. J Am Coll Cardiol 1997, 29: 448-454. 10.1016/S0735-1097(96)00496-2
Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quinones MA, Zoghbi WA: Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of Tissue Doppler Imaging. Circulation 1998, 98: 1644-1650.
Nagueh SF, Kopelen HA, Quinones MA: Assessment of left ventricular filling pressures by Doppler in presence of atrial fibrillation. Circulation 1996, 94: 2138-2145.
Vilacosta I, San Roman JA, Castillo JA, Arganda L, Rollan MJ, Peral V, Sanchez-Harguindey L, Zarco P: Retrograde atrial kick in acute aortic regurgitation. Study of mitral and pulmonary venous flow velocities by transthoracic and transesophageal echocardiography. Clin Cardiol 1997, 20: 35-40.
Shan K, Bick RJ, Poindexter BJ, Shimoni S, Letsou GV, Reardon MJ, Howell JF, Zoghbi WA, Nagueh SF: Relation of tissue Doppler derived myocardial velocities to myocardial structure and beta-adrenergic receptor density in humans. J Am Coll Cardiol 2000, 36: 891-896. 10.1016/S0735-1097(00)00786-5
Zile MR, Brutsaert DI: New concepts in diastolic dysfunction and diastolic heart failure. Circulation 2002, 105: 1387-1393. 10.1161/hc1102.105289
Spirito P, Maron BJ: Relation between extent of left ventricular hypertrophy and diastolic filling abnormalities in hypertrophic cardiomyopathy. J Am Coll Cardiol 1990, 15: 808-813.
Klein AL, Hatle LK, Taliercio CP, Taylor CL, Kyle RA, Bailey KR, Seward JB, Tajik AJ: Serial Doppler echocardiographic follow-up of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol 1990, 16: 1135-1141.
Biondi B, Fazio S, Palmieri EA, Carella C, Panza N, Cittadini A, Bone F, Lombardi G, Sacca : Left ventricular diastolic dysfunction in patients with subclinical hypothyroidism. J Clin Endocrinol Metab 1999, 84: 2064-2067. 10.1210/jc.84.6.2064
Galderisi M, Cicala S, Caso P, De Simone L, D'Errico A, Petrocelli A, de Divitiis O: Coronary flow reserve and myocardial diastolic dysfunction in arterial hypertension. Am J Cardiol 2002, 90: 860-864. 10.1016/S0002-9149(02)02708-X
Mureddu GF, de Simone G, Greco R, Rosato GF, Contaldo F: Left ventricular filling in arterial hypertension. Influence of obesity and hemodynamic and structural confounders. Hypertension 1997, 29: 544-550.
Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S: Insulin resistance in essential hypertension. N Engl J Med 1987, 317: 350-357.
Galderisi M, Paolisso G, Tagliamonte MR, Alfieri A, Petrocelli A, de Divitiis M, Varricchio M, de Divitiis O: Is insulin action a determinant of left ventricular relaxation in uncomplicated essential hypertension? J Hypertens 1997, 15: 745-50. 10.1097/00004872-199715070-00006
Draznin B, Sussman KE, Eckel RH, Kao M, Yost T, Sherman NA: Possible role of cytosolic free calcium concentrations in mediating insulin resistance of obesity and hyperinsulinemia. J Clin invest 1988, 82: 1848-1852.
Galderisi M, Tagliamone MR, D'Errico A, Carella C, Varricchio G, Mondillo S, de Divitiis O, Paolisso G: Independent association of plasma leptin levels and left ventricular isovolumic relaxation in uncomplicated hypertension. Am J Hypertens 2001, 14: 1019-1024. 10.1016/S0895-7061(01)02137-9
Liu JE, Palmieri V, Roman MJ, Bella JN, Fabsitz R, Howard BV, Welty TK, Lee ET, Devereux RB: The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: the Strong Heart Study. J Am Coll Cardiol 2001, 37: 1943-1949. 10.1016/S0735-1097(01)01230-X
White WB, Schulman P, Dey HM, Katz AM: Effects of age and 24-hour ambulatory blood pressure on rapid left ventricular filling. Am J Cardiol 1989, 63: 1343-1347. 10.1016/0002-9149(89)91046-1
Verdecchia P, Schillaci G, Guerrieri M, Boldrini F, Gatteschi C, Benemio G, Porcellati C: Prevalence and determinants of left ventricular diastolic filling abnormalities in an unselected hypertensive population. Eur Heart J 1990, 11: 679-691.
Aeschbacher BC, Hutter D, Fuhrer J, Weidmann P, Delacretaz E, Allemann Y: Diastolic dysfunction precedes myocardial hypertrophy in the development of hypertension. Am J Hypertens 2001, 14: 106-113. 10.1016/S0895-7061(00)01245-0
Galderisi M, Petrocelli A, Alfieri A, Garofalo M, de Divitiis O: Impact of ambulatory blood pressure on left ventricular diastolic dysfunction in uncomplicated arterial systemic hypertension. Am J Cardiol 1996, 77: 597-601. 10.1016/S0002-9149(97)89313-7
Palmieri V, Wachtell K, Gerdts E, Bella JN, Papademetriou V, Tuxen C, Nieminen MS, Dahlof B, de Simone G, Devereux RB: Left ventricular function and hemodynamic features of inappropriate left ventricular hypertrophy in patients with systemic hypertension: the LIFE study. Am Heart J 2001, 141: 784-791. 10.1067/mhj.2001.114803
de Simone G, Verdecchia P, Pede S, Gorini M, Maggioni AP: Prognosis of inappropriate left ventricular mass in hypertension: the MAVI Study. Hypertension 2002, 40: 470-476. 10.1161/01.HYP.0000034740.99323.8A
Lubien E, DeMaria A, Krishnaswamy P, Clopton P, Koon J, Kazanegra R, Gardetto N, Wanner E, Maisel AS: Utility of B-natriuretic peptide in detecting diastolic dysfunction: comparison with Doppler velocity recordings. Circulation 2002, 105: 595-601. 10.1161/hc0502.103010
Yamaguchi H, Yoshida J, Yamamoto K, Sakata Y, Mano T, Akehi N, Hori M, Lim YJ, Mishima M, Masuyama T: Elevation of plasma brain natriuretic peptide is a hallmark of diastolic heart failure independent of ventricular hypertrophy. J Am Coll Cardiol 2004, 43: 55-60. 10.1016/j.jacc.2003.07.037
Galderisi M, Caso P, Severino S, Petrocelli A, De Simone L, Izzo A, Mininni N, de Divitiis O: Myocardial diastolic impairment caused by left ventricular hypertrophy involves basal septum more than other walls: analysis by pulsed Doppler tissue imaging. J Hypertens 1999, 17: 685-693. 10.1097/00004872-199917050-00013
Zile MR: Heart failure with preserved ejection fraction: Is this diastolic heart failure? J Am Coll Cardiol 2003, 41: 1519-1522. 10.1016/S0735-1097(03)00186-4
de Simone G, Kitzman DW, Chinali M, Oberman A, Hopkins PN, Rao DC, Arnett DK, Devereux RB: Left ventricular concentric geometry is associated with impaired relaxation in hypertension: the HyperGEN study. Eur J Heart 2004, 30: 1-7.
Gandhi SK, Powers JC, Nomeir AM, Fowle K, Kitzman DW, Rankin KM, Little WC: The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 2001, 344: 17-22. 10.1056/NEJM200101043440103
Vasan RS, Levy D: Defining diastolic heart failure. A call for standardized diagnostic criteria. Circulation 2000, 101: 2118-2121.
European Study Group on Diastolic Heart Failure: How to diagnose diastolic heart failure. Eur Heart J 1998, 19: 990-1003. 10.1053/euhj.1998.1057
Zile MR, Baicu CF, Gaasch WH: Diastolic heart failure. Abnormalities in acrive relaxation and passive stiffness of the left ventricle. N Engl J Med 2004, 350: 1953-1959. 10.1056/NEJMoa032566
Zile MR, Gaasch WH, Carroll JD, Feldman MD, Aurigemma GP, Schaer GL, Ghali JK, Liebson PR: Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation 2001, 104: 779-782.
Benjamin EJ, Levy D, Anderson KM, Wolf PA, Plehn JF, Evans JC, Comai K, Fuller DL, Sutton MS: Determinants of Doppler indexes of left ventricular diastolic function in normal subjects (the Framingham Heart Study). Am J Cardiol 1992, 70: 508-515. 10.1016/0002-9149(92)91199-E
Galderisi M, Benjamin EJ, Evans JC, D'Agostino RB, Fuller DL, Lehman B, Levy D: Impact of heart rate and PR interval on Doppler indexes of left ventricular diastolic filling in an elderly cohort (the Framingham Heart Study). Am J Cardiol 1993, 72: 1183-1187. 10.1016/0002-9149(93)90991-K
Zile MR, Baicu CF: Alterations in ventricular function: diastolic heart failure. In "Heart Failure, A Companion to Braunwald's Heart Disease". Edited by: Mann D. Saunders; 2004.
Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ: Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003, 289: 194-202. 10.1001/jama.289.2.194
Khouri SJ, Maly GT, Suh DD, Walsh TE: A practical approach to the echocardiographic evaluation of diastolic function. J Am Soc Echocardiogr 2004,17(3):290-297.
de Simone G, Greco R, Mureddu GF, Romano C, Guida R, Celentano A, Contaldo F: Relation of left ventricular diastolic properties to systolic function in arterial hypertension. Circulation 2001, 101: 152-157.
Yp G, Wang M, Zhang Y, Fung JW, Ho PY, Sanderson JE: Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart 2002, 87: 121-125. 10.1136/heart.87.2.121
Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW: Progression of systolic abnormalities in patients with "isolated" diastolic heart failure and diastolic dysfunction. Circulation 2002, 105: 1195-1201. 10.1161/hc1002.105185
Chen QM, Li W, O'Sullivan C, Francis DP, Gibson D, Henein MY: Clinical in vivo calibration of pulse wave tissue Doppler velocities in the assessment of ventricular wall motion. A comparison study with M-mode echocardiography. Int J Cardiol 2004, 97: 289-295. 10.1016/j.ijcard.2004.03.048
Caruana L, Petrie MC, Davie AP, McMurray JJ: Do patients with suspected heart failure and preserved left ventricular systolic function suffer from "diastolic heart failure" or from misdiagnosis? A prospective descriptive study. BMJ 2000, 321: 215-218. 10.1136/bmj.321.7255.215
Vasan RS, Benjamin EJ, Levy D: Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol 1995, 26: 1565-1574. 10.1016/0735-1097(95)00381-9
Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D: Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 1999, 33: 1948-1955. 10.1016/S0735-1097(99)00118-7
Hogg K, Swedberg K, McMurray J: Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol 2004, 43: 317-327. 10.1016/j.jacc.2003.07.046
Tarantini L, Faggiano P, Senni M, Lucci D, Bertoli D, Porcu M, Opasich C, Tavazzi L, Maggioni AP: Clinical features and prognosis associated with a preserved left ventricular systolic function in a large cohort of congestive heart failure outpatients managed by cardiologists. Data from the Italian Network on Congestive Heart Failure. Ital Heart J 2002, 3: 656-664.
Philbin EF, Rocco TA Jr, Lindenmuth NW, Ulrich K, Jenkins PL: Systolic versus diastolic heart failure in community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med 2000, 109: 605-613. 10.1016/S0002-9343(00)00601-X
Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM: Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation 1998, 98: 2282-2289.
Badano LP, Albanese MC, De Biaggio P, Rozbowsky P, Miani D, Fresco C, Fioretti PM: Prevalence, clinical characteristics, quality of life, and prognosis of patients with congestive heart failure and isolated left ventricular diastolic dysfunction. J Am Soc Echocardiogr 2004, 17: 253-261.
Schillaci G, Pasqualini L, Verdecchia P, Vaudo G, Marchesi S, Porcellati C, de Simone G, Mannarino E: Prognostic significance of left ventricular diastolic dysfunction in essential hypertension. J Am Coll Cardiol 2002, 39: 2005-2011. 10.1016/S0735-1097(02)01896-X
Bella JN, Palmieri V, Roman MJ, Liu JE, Welty TK, Lee ET, Fabsitz RR, Howard BV, Devereux RB: Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults. The Strong Heart Study. Circulation 2002, 105: 1928-1933. 10.1161/01.CIR.0000015076.37047.D9
Vasan RS, Larson MG, Levy D, Galderisi M, Wolf PA, Benjamin EJ: Doppler transmitral flow indexes and risk of atrial fibrillation (The Framingham Heart Study). Am J Cardiol 2003, 91: 1079-1083. 10.1016/S0002-9149(03)00152-8
Brilla CG, Matsubara LS, Weber KT: Antifibrotic effects of spironolactone in preventing myocardial fibrosis in systemic arterial hypertension. Am J Cardiol 1993, 71: 12A-16A. 10.1016/0002-9149(93)90239-9
Grandi AM, Imperiale D, Santillo R, Barlocco E, Bertolini A, Guasti L, Venco A: Aldosterone antagonist improves diastolic function in essential hypertension. Hypertension 2002, 40: 647-652. 10.1161/01.HYP.0000036399.80194.D8
Nodari S, Metra M, Dei Cas L: Beta-blocker treatment of patients with diastolic heart failure and arterial hypertension. A prospective, randomized, comparison of the long-term effects of atenolol vs. nebivolol. Eur J Heart Fail 2003, 5: 621-627. 10.1016/S1388-9842(03)00054-0
Solomon SD, Wang D, Finn P, Skali H, Zornoff L, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Pocock S, Pfeffer MA: Effect of candesartan on cause-specific mortality in heart failure patients: the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2004, 110: 2180-2183. 10.1161/01.CIR.0000144474.65922.AA
Bergstrom A, Andersson B, Edner M, Nylander E, Persson H, Dahlstrom U: Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler-echocardiographic study (SWEDIC). Eur J Heart Fail 2004, 6: 453-461. 10.1016/j.ejheart.2004.02.003
Shibata MC, Flather MD, Bohm M, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Parkhomenko A, Soler-Soler J, Tavazzi L, Toman J, Van Veldhuisen DJ, Coats AJ, Poole-Wilson P: Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure. Rehospitalisation in Seniors with Heart Failure (SENIORS). Rationale and design. Int J Cardiol 2002, 86: 77-85. 10.1016/S0167-5273(02)00321-2
Banerjee P, Banerjee T, Khand A, C lark AL, Cleland JG: Diastolic heart failure: neglected or misdiagnosed? J Am Coll Cardiol 2002, 39: 138-141. 10.1016/S0735-1097(01)01704-1
Motz W, Strauer BE: Improvement of coronary flow reserve after long-term therapy with enalapril. Hypertension 1996, 27: 1031-1038.
Schwartzkopff B, Brehm M, Mundhenke M, Strauer BE: Repair of coronary arterioles after treatment with perindopril in hypertensive heart disease. Hypertension 2000, 36: 220-225.
Galderisi M, Cicala S, D'Errico A, de Divitiis O, de Simone G: Nebivolol improves coronary flow reserve in hypertensive patients without coronary heart disease. J Hypertens 2004, 22: 2201-2208. 10.1097/00004872-200411000-00024
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Financial competing interests
None.
Non-financial competing interests
None.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Galderisi, M. Diastolic dysfunction and diastolic heart failure: diagnostic, prognostic and therapeutic aspects. Cardiovasc Ultrasound 3, 9 (2005). https://doi.org/10.1186/1476-7120-3-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-7120-3-9