Abstract
Transthoracic Doppler echocardiographic-derived coronary flow reserve is an useful hemodynamic index to assess dysfunction of coronary microcirculation. Isolated coronary microvascular abnormalities are overt by reduced coronary flow reserve despite normal epicardial coronary arteries. These abnormalities may occur in several diseases (arterial hypertension, diabetes mellitus, hypercholesterolemia, syndrome X, aortic valve disease, hypertrophic cardiomyopathy and idiopathic dilated cardiomyopathy). The prognostic role of impaired microvascular coronary flow reserve has been shown unfavourable especially in hypertrophic or idiopathic dilated cardiomyopathies. Coronary flow reserve reduction may be reversible, for instance after regression of left ventricular hypertrophy subsequent to valve replacement in patients with aortic stenosis, after anti-hypertensive treatment or using cholesterol lowering drugs. Coronary flow reserve may increase by 30% or more after pharmacological therapy and achieve normal level >3.0. In contrast to other non invasive tools as positron emission tomography, very expensive and associated with radiation exposure, transthoracic Doppler-derived coronary flow reserve is equally non invasive but cheaper, very accessible and prone to a reliable exploration of coronary microvascular territories, otherwise not detectable by invasive coronary angiography, able to visualize only large epicardial arteries.
Similar content being viewed by others
The concept of coronary flow reserve (CFR) and transmural reduction of CFR
The coronary arterial tree consists of four basic segments. Epicardial coronary arteries give off small transmural penetrating arteries, which have branches in the myocardial layers. These branches are defined arterioles, terminating in capillary vessels, directly supplying myocardial cells. Each of these coronary segments produces different level and degree of resistance to coronary blood flow. Normal (non-stenosed) large epicardial coronary arteries play a minor role in the regulation of coronary vascular resistance and act mainly as conductance (conduit) vessels. Most of the resistance, which opposes coronary blood flow, arises from resistance arterioles. The resistance is manifest by decreased coronary perfusion pressure. The percent distribution of the length and resistance of individual segments of the coronary vascular tree is summarised in Table 1 and depicted in Figure 1.
The difference between coronary blood flow corresponding to flow autoregulation plateau at rest and coronary blood flow after maximal vasodilatation is traditionally defined as coronary flow reserve (CFR) [1–6]. CFR is usually calculated as the ratio of maximal (hyperemic) to resting coronary blood flow (Figure 2). CFR is an important functional parameter to understand the pathophysiology of coronary circulation and can be used to examine the integrity of microvascular circulation.
The assessment of regional coronary flow reveals marked spatial heterogeneity of CFR across the myocardial wall. According to the model of Hoffman [2], the highest CFR is that measurable in the subepicardial layer of myocardium. In relation to a transmural flow reduction, CFR is significantly lower in the subendocardial layer, also due to elevated left ventricular (LV) diastolic pressure increasing extravascular compressive forces. As a result of this transmural coronary flow distribution, CFR is exhausted firstly in the subendocardial layer [2] (Figure 2). The lower limit of coronary flow autoregulation is unfavourably shifted to the higher value of coronary perfusion pressure in the subendocardial layer as compared with the subepicardial layer (i.e. 55–65 mm Hg versus 30–40 mmHg, respectively) [5] (Figure 2). According to Hoffman [2] the reduction of global CFR from 4.0 to 2.0 could be associated with the loss of flow reserve in a part or all of the subendocardial layer of the myocardium.
In human studies, due to limited spatial resolution of positron emission tomography (PET), the demonstration of subendocardial hypoperfusion has been made possible only in the hypertrophied myocardium [7]. In patients with aortic valve stenosis and normal epicardial coronary arteries [7], subendocardial CFR (1.43 ± 0.33) was lower than subepicardial CFR (1.78 ± 0.35; P = 0.01). In the subgroup of severe aortic stenosis (aortic valve area <0.8 cm2) CFR was <2.0 both at subendocardial and subepicardial levels and in two of these patients subendocardial CFR was <1.0 (lack of CFR).
An alternative approach to document transmural steal phenomenon is the calculation of subendocardial/subepicardial flow ratio [8, 9]. A hyperemic value of subendocardial / subepicardial flow ratio <0.8 (i.e., subendocardial flow is at least 20% lower than subepicardial) has been proposed as marker of subendocardial hypoperfusion. In some patients with hypertrophic cardiomyopathy (HCM), subendocardial flow was 40% lower than subepicardial flow (ratio = 0.6) after infusion of a vasodilator agent [8, 9].
Magnetic resonance imaging (MRI) with gadolinium contrast agent, has higher resolution and appears more sensitive providing the opportunity to assess even patients without left ventricular hypertrophy, i.e. with normal wall thickness [10]. Accordingly, cardiac MRI demonstrates subendocardial hypoperfusion in patients with syndrome X during the intravenous administration of adenosine when compared with healthy control subjects who showed increase of subendocardial perfusion after induced hyperemia [10].
The lower limit of normal CFR and criteria for normal reference values
An important practical issue is to confirm in clinical studies that the lower limit of normal CFR is >3.0. This aspect is summarised in Table 2.
Before defining the normal value of CFR, we should identify appropriately reference groups of healthy subjects. In order to achieve this aim, non invasive studies present a particularly useful approach because we may recruit any subject who gives informed consent to examination. The perfect candidate is a healthy volunteer without cardiovascular signs/symptoms and/or risk factors for both vascular dysfunction and coronary artery disease. In contrast, invasive studies are performed in subjects who complain about chest pain or other cardiac symptoms, implying that coronary microvascular vasodilator dysfunction may limit coronary blood flow and determines angina despite normal coronary epicardial arteries. Two studies [18, 19] have shown that such a coincidence is quite frequent. Bearing in mind this limitation it has been proposed by Baumgart et al [20] that in invasive measurements normal limits of CFR may be derived only from highly selected subjects according to the following restricted criteria:
- truly normal epicardial arteries confirmed by intracoronary ultrasound examination
- age <50 years
- absence of symptoms (in addition, we propose an obligatory absence of risk factors for vascular/endothelial dysfunction).
These highly-selected subjects (table 2) exhibit CFR markedly exceeding the cut-off point of 3.0, this value is near to the highest value obtained in an athletes' populations (CFR > 5.0).
The recruitment of reference controls subjects with normal epicardial coronary arteries verified by intracoronary ultrasound is crucial as indicated by the following example. Positive exercise myocardial scintigraphy, primarily considered as false positive in relation to angiographically normal coronary vessels, may frequently turn out to be true positive when control intracoronary ultrasound reveals vascular lesions [21]. In this clinical setting, CFR is a fairly good predictor of "soft lesions", non-visualizable even by coronary angiography [21].
Baumgart [20] proposed a range of age <50 years since PET revealed a significantly higher CFR in younger than in the elderly subjects (Table 2). Other investigators [13] demonstrated that aging-induced reduction of CFR is a result of increased coronary blood flow at rest, whereas maximal blood flow remained relatively unchanged during the years. Only over 70 years, maximal coronary blood flow is reduced. According to these findings [20], the lower limit of normal CFR should be reasonably fixed to 3.0 for subjects up to the age of 50.
Factors limiting coronary flow reserve
In general, the reduction of CFR may be associated with three main kinds of abnormalities [2, 3, 22, 23] (Table 3, Figure 3) and it is even possible for two factors to co-operate simultaneously. However, it has to be taken into account that epicardial coronary artery stenosis, the most visible factor of patients with angina pectoris (examined alone by coronary angiography in daily practice), is only one possible determinant in contrast to several other multi-factorial mechanisms involving coronary microvascular dysfunction. Structural changes (remodelling) in coronary microcirculation can themselves be responsible of CFR reduction. By using myocardial biopsy, some studies have provided the opportunity to compare pathomorphological changes of coronary microcirculation and CFR reduction, documenting the relationship between morphological and hemodynamic abnormalities. In patients with hypertrophic cardiomyopathy, a reduced arteriolar lumen was associated to a reduced CFR [24, 25]. Also in hypertensive patients [26] reduced CFR correlated with increased coronary arteriolar wall thickness, i.e, arteriolar remodelling. A decreased arteriolar wall/lumen ratio correlates with reduction of CFR as well as with abnormalities of derived parameters as coronary resistance reserve. All together, these studies strongly support the hypothesis that the microvascular factor is a further important contributor (as extravascular, myocardial factor, i.e. LV hypertrophy, excessive intramyocardial pressure) of CFR reduction. From a pathophysiological point of view, coronary microvascular disease is associated with alternative ischemic cascade where stress test induces chest pain, ECG ST-segment depression and myocardial scintigraphic perfusion defect despite the lack of changes in echocardiographic-derived regional myocardial wall motion [27].
How much coronary microvascular disease may reduce CFR?
In a recent study Voci et al. [28] minimised the role of reduced microvascular CFR, probably underestimating the unfavourable influence of impaired coronary microcirculation on prognosis. These authors stated that patients die of epicardial coronary artery disease, not of microvascular disease. In other studies [29–35], however, patients with no or minimal coronary stenosis of epicardial coronary arteries exhibited a significantly blunted CFR in relation to microvascular abnormalities induced by various cardiovascular diseases (Table 4). Several patients had markedly reduced CFR < 2.0 and in some pediatric patients with hypertrophic cardiomyopathy CFR was <1.0 [29]. On these grounds, the authors [29] postulated that non-hypertrophic free wall steals the blood flow from the hypertrophied septum after a vasodilator infusion. A gradual decrease of CFR, parallel to more advanced stages of microvascular disease, was observed in diabetic patients, patients with syndrome X and also hypertensive patients without overt coronary artery stenosis (Table 4). In this view it is notable the experience of Rigo [36], who collected CFR values measured by transthoracic Doppler echocardiography in large population samples affected by various cardiac diseases (Table 5). It is also of interest that the reduction of CFR is reversible in some cases, for instance after regression of LV hypertrophy subsequent to aortic valve replacement in patients with aortic valve stenosis, after anti-hypertensive treatment or even after cholesterol treatment [37–42]. (Table 6). Importantly, even drugs of the same group (angiotensin converting enzyme inhibitors [perindopril versus enalapril] and statins [simvastatin versus pravastatin]) exhibited different influence on CFR (Table 6). After simvastatin treatment [39], the percent increase of CFR correlated with percent decrease of cholesterol and triglycerides levels.
Interestingly, in patients with epicardial coronary artery stenosis and severe hypercholesterolemia, single LDL-apheresis improved microcirculation by increasing CFR from 1.91 ± 0,68 to 2.48 ± 0.68 [43]. This finding demonstrates that, even after single LDL-lowering intervention, some patients can move quickly from a group where PTCA appears to be required (corresponding to CFR < 2.0) to a group with CFR >2.0, where PTCA may not be needed anymore.
Vasodilators inducing hyperemia
Two main pharmacological vasodilators, adenosine and dipyridamole, are used in humans to recruit CFR. The characteristics of these agents are compared in Table 7. These agents have an advantage over exercise and dobutamine, which represent submaximal stimuli for coronary flow reserve and are much more technically demanding for ultrasound imaging of CFR [44]. Either adenosine or dipyridamole were used in referred studies of Tables 4, 5, where only studies with control group are reported. The control group provides an opportunity to compare how much CFR is reduced (sometimes more than 50%) in patients with different involvement of coronary microvascular disease.
Prognostic value of impaired microvascular CFR
The prognostic role of impaired microvascular CFR has been found unfavourable. In a study by Marks at al. [45], reduced CFR due to unspecified microvascular disease predicted increased mortality – 20% vs. 7% in a group with normal CFR (p < 0.016). The relationship between unfavourable prognosis and reduced CFR in patients with hypertrophic or idiopathic dilated cardiomyopathies was recently reported [46, 47]. In other two studies where patients were divided according to CFR tercentyle [48] or maximally stimulated coronary blood flow tercentyle [49], the subgroup defined as the lowest tercentyle had the worst outcome. The markedly blunted maximal blood flow was related with poor prognosis not only in HCM patients [49] but also in patients with idiopathic dilated cardiomyopathy (DCM) [50]. In this clinical setting, strongly depressed dipyridamole-stimulated maximal coronary blood flow was associated, with a 3.5 relative risk of death or development or progression of heart failure. These results support the hypothesis that chronic myocardial hypoperfusion or repetitive myocardial ischemia attributable to abnormal coronary microcirculatory flow could exert a detrimental role in the evolution of idiopathic LV dysfunction toward overt DCM. Cecchi et al. [49] hypothesised that coronary microvascular dysfunction may represent a common pathway leading to a disease progression in different cardiomyopathies, including conditions as aortic valve stenosis and hypertensive heart disease.
Limitations and hypothesis
In several of the referred reports, dipyridamole was used to produce vasodilating hyperemia. Adenosine (140 ug/kg/min) has been shown to be either similar [51] or more potent vasodilator agent [52] than high-dose dipyridamole (0.84 mg/Kg). In relation to the possibility that dipyridamole-recruited CFR could be submaximal, we can not be absolutely sure about the appropriateness of the lower limit of normal CFR = 3.0. To resolve this problem, apart from vasodilator selection, the choice of appropriate control groups is also due, by excluding smokers and patients with arterial hypertension, hyperlipidemia, obesity, diabetes mellitus, and, possibly, those affected by hyperhomocysteinemia. These highly selecting criteria probably was not fulfilled in previous studies, in particular the oldest, where the newest recognized factors limiting CFR were not yet known. Recently, new evidence has been given that in healthy subjects even single exposition to passive smoking [53], fat meal inducing hypertriglyceridemia [54], hyperhomocysteinemia [55], and estrogen decrease in menstrual phase of cycle [56] can reduce CFR by approximately 30%. Thus, we can not be certain that normal CFR starts from 3.0 because CFR values higher than 5.0 were recorded in humans. Interestingly, in a transplanted heart which can not be equivalent of intact heart in healthy volunteers, CFR may also achieved more 5.0 (see Table 2). This high value is probably the result of intracoronary papaverine, which is not used currently. However, these findings strongly supports the hypothesis that CFR may achieve much higher level than 3.0.
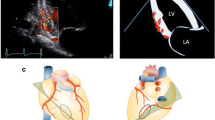
As regard validation of a non-invasive methods, transthoracic Doppler echocardiography closely agrees with intracoronary Doppler flow wire results in assessing CFR. Good correlation of non-invasive and invasive Doppler assessment of CFR has been shown both in LAD [57] and RCA [58]. The feasibility of transthoracic Doppler echocardiography to detect coronary flow is 80–98% in LAD, 50–87% in RCA and 43–72% in Cx [6, 36, 59].
Conclusion
Isolated coronary microvascular abnormalities are overt by reduced CFR despite normal epicardial coronary arteries. These abnormalities may occur in several diseases (arterial hypertension, diabetes mellitus, hypercholesterolemia, syndrome X, aortic valve disease, hypertrophic cardiomyopathy and idiopathic dilated cardiomyopathy). The prognostic role of impaired microvascular CFR has been shown unfavourable, in particular in patients with hypertrophic or idiopathic dilated cardiomyopathies. CFR reduction may be reversible, for instance after regression of left ventricular hypertrophy subsequent to valve replacement in patients with aortic stenosis, after anti-hypertensive treatment or using cholesterol lowering drugs. CFR may increase by 30% or more after pharmacological therapy and achieve level >3.0. In contrast to other non invasive tools as PET, very expensive and associated with radiation exposure, transthoracic Doppler-derived CFR is equally non invasive but cheaper, very accessible [60] and prone to a reliable exploration of coronary microvascular territories, not detectable by invasive coronary angiography, able to visualize only large epicardial arteries.
References
Ganz P, Ganz W: Coronary blood flow and myocardial ischemia. In Heart Disease. Edited by: Braunwald E, Zipes DP, Libby P. New York: W.B.Sauders; 2001:1087-1113.
Hoffman JI: Problems of coronary flow reserve. Ann Biomed Eng 2000, 28: 884-896. 10.1114/1.1308503
Klocke FJ: Measurements of coronary flow reserve: defining pathophysiology versus making decisions about patient care. Circulation 1987, 76: 1183-1189.
Mosher PJ, Ross J Jr, Mcfate PA, Shaw RF: Control of coronary blood flow by an autoregulatory mechanism. Circ Res 1964, 14: 250-259.
Dimitrow PP: Coronary flow reserve- measurement and application: focus on transthoracic Doppler echocardiography. Boston/Dordrecht/London: Kluwer Academic Publishers; 2002.
Dimitrow PP: Transthoracic Doppler echocardiography- noninvasive diagnostic window for coronary flow reserve assessment. Cardiovasc Ultrasound 2003, 1: 4. 10.1186/1476-7120-1-4
Rajappan K, Rimoldi O, Dutka DP, Ariff B, Pennell DJ, Sheridan DJ, Camici PG: Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation 2002, 105: 470-476. 10.1161/hc0402.102931
Choudhury L, Elliott P, Rimoldi O, Ryan M, Lammertsma AA, Boyd H, McKenna WJ, Camici PG: Transmural myocardial blood flow distribution in hypertrophic cardiomyopathy and effect of treatment. Basic Res Cardiol 1999, 94: 49-59. 10.1007/s003950050126
Lorenzoni R, Gistri R, Cecchi F, Olivotto I, Chiriatti G, Elliott P, McKenna WJ, Camici PG: Coronary vasodilator reserve is impaired in patients with hypertrophic cardiomyopathy and left ventricular dysfunction. Am Heart J 1998, 136: 972-981.
Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, Pennell DJ: Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med 2002, 346: 1948-1953. 10.1056/NEJMoa012369
McGinn AL, White CW, Wilson RF: Interstudy variability of coronary flow reserve. Influence of heart rate, arterial pressure, and ventricular preload. Circulation 1990, 81: 1319-1330.
Chauhan A, Mullins PA, Petch MC, Schofield PM: Is coronary flow reserve in response to papaverine really normal in syndrome X? Circulation 1994, 89: 1998-2004.
Czernin J, Muller P, Chan S, Brunken RC, Porenta G, Krivokapich J, Chen K, Chan A, Phelps ME, Schelbert HR: Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation 1993, 88: 62-69.
Hirata K, Shimada K, Watanabe H, Otsuka R, Tokai K, Yoshiyama M, Homma S, Yoshikawa J: Black tea increases coronary flow velocity reserve in healthy male subjects. Am J Cardiol 2004, 93: 1384-1388. 10.1016/j.amjcard.2004.02.035
Uren NG, Marraccini P, Gistri R, de Silva R, Camici PG: Altered coronary vasodilator reserve and metabolism in myocardium subtended by normal arteries in patients with coronary artery disease. J Am Coll Cardiol 1993, 22: 650-658.
Kubo S, Tadamura E, Toyoda H, Mamede M, Yamamuro A, Magata Y, Mukai T, Kitano H, Tamaki N, Konishi J: Effect of caffeine intake on myocardial hyperemic flow induced by adenosine triphosphate and dipyridamole. J Nucl Med 2004, 45: 730-738.
Hildick-Smith DJ, Johnson PJ, Wisbey CR, Winter EM, Shapiro LM: Coronary flow reserve is supranormal in endurance athletes: an adenosine transthoracic echocardiographic study. Heart 2000, 84: 383-389. 10.1136/heart.84.4.383
Hasdai D, Holmes DR Jr, Higano ST, Burnett JC Jr, Lerman A: Prevalence of coronary blood flow reserve abnormalities among patients with nonobstructive coronary artery disease and chest pain. Mayo Clin Proc 1998, 73: 1133-1140.
Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, Rogers WJ, Walsh EG, Fuisz AR, Kerensky R, Detre KM, Sopko G, Pepine CJ: Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol 1999, 33: 1469-1475. 10.1016/S0735-1097(99)00072-8
Baumgart D, Haude M, Liu F, Ge J, Goerge G, Erbel R: Current concepts of coronary flow reserve for clinical decision making during cardiac catheterization. Am Heart J 1998, 136: 136-149.
Verna E, Ceriani L, Giovanella L, Binaghi G, Garancini S: "False-positive" myocardial perfusion scintigraphy findings in patients with angiographically normal coronary arteries: insights from intravascular sonography studies. J Nucl Med 2000, 41: 1935-1940.
Gould KL, Lipscomb K, Hamilto GW: Physiology basis for assessing critical coronary stenosis: instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol 1974, 33: 87-94. 10.1016/0002-9149(74)90743-7
Gould KL, Kirkeeide RL, Buchi M: Coronary flow reserve as a physiologic measure of stenosis severity. J Am Coll Cardiol 1990, 15: 459-474.
Krams R, Kofflard MJ, Duncker DJ, Von Birgelen C, Carlier S, Kliffen M, ten Cate FJ, Serruys PW: Decreased coronary flow reserve in hypertrophic cardiomyopathy is related to remodeling of the coronary microcirculation. Circulation 1998, 97: 230-233.
Schwartzkopff B, Mundhenke M, Strauer BE: Alterations of the architecture of subendocardial arterioles in patients with hypertrophic cardiomyopathy and impaired coronary vasodilator reserve: a possible cause for myocardial ischemia. J Am Coll Cardiol 1998, 31: 1089-1096. 10.1016/S0735-1097(98)00036-9
Schwartzkopff B, Motz W, Frenzel H, Vogt M, Knauer S, Strauer BE: Structural and functional alterations of the intramyocardial coronary arterioles in patients with arterial hypertension. Circulation 1993, 88: 993-1003.
Picano E, Palinkas A, Amyot R: Diagnosis of myocardial ischemia in hypertensive patients. J Hypertens 2001, 19: 1177-1183. 10.1097/00004872-200107000-00001
Voci P, Pizzuto F, Romeo F: Coronary flow: a new asset for the echo lab? Eur Heart J 2004, 25: 1867-1879. 10.1016/j.ehj.2004.07.029
Tadamura E, Yoshbayashi M, Yonemura T, Kudoh T, Kubo S, Motooka M: Significant regional heterogeneity of coronary flow reserve in paediatric hypertrophic cardiomyopathy. Eur J Nucl Med 2000, 27: 1340-1348. 10.1007/s002590000300
Nitenberg A, Foult JM, Antony I, Blanchet F, Rahali M: Coronary flow and resistance reserve in patients with chronic aortic regurgitation, angina pectoris and normal coronary arteries. J Am Coll Cardiol 1988, 11: 478-486.
Canetti M, Akhter MW, Lerman A, Karaalp IS, Zell JA, Singh H, Mehra A, Elkayam U: Evaluation of myocardial blood flow reserve in patients with chronic congestive heart failure due to idiopathic dilated cardiomyopathy. Am J Cardiol 2003, 92: 1245-1249. 10.1016/j.amjcard.2003.08.002
Santagata P, Rigo F, Gherardi S, Pratali L, Drozdz J, Varga A, Picano E: Clinical and functional determinants of coronary flow reserve in non-ischemic dilated cardiomyopathy An echocardiographic study. Int J Cardiol 2005, in press.
Akasaka T, Yoshida K, Hozumi T, Takagi T, Kaji S, Kawamoto T, Morioka S, Yoshikawa J: Retinopathy identifies marked restriction of coronary flow reserve in patients with diabetes mellitus. J Am Coll Cardiol 1997, 30: 935-941. 10.1016/S0735-1097(97)00242-8
Youn HJ, Park SJ, Cho EJ, Jung HO, Jeon HK, Lee JM, Oh YS, Chung WS, Kim JH, Choi KB, Hong SJ, Choi K: Pattern of exercise-induced ST change is related to coronary flow reserve in patients with chest pain and normal coronary angiogram. Int J Cardiol 2005, 101: 299-304. 10.1016/j.ijcard.2004.03.037
Schafer S, Kelm M, Mingers S, Strauer BE: Left ventricular remodeling impairs coronary flow reserve in hypertensive patients. J Hypertens 2002, 20: 31-37. 10.1097/00004872-200207000-00031
Rigo F: Coronary flow reserve in stress-echo lab. From pathophysiologic toy to diagnostic tool. Cardiovasc Ultrasound 2005, 3: 8. 10.1186/1476-7120-3-8
Hildick-Smith DJ, Shapiro LM: Coronary flow reserve improves after aortic valve replacement for aortic stenosis: an adenosine transthoracic echocardiography study. J Am Coll Cardiol 2000, 36: 1889-1896. 10.1016/S0735-1097(00)00947-5
Yokoyama I, Yonekura K, Inoue T, Ohtomo K, Nagai R: Long-term effect of simvastatin on the improvement of impaired myocardial flow reserve in patients with familial hypercholesterolemia without gender variance. J Nucl Cardiol 2001, 8: 445-451. 10.1067/mnc.2001.115517
Yokoyama I, Inoue Y, Moritan T, Ohtomo K, Nagai R: Impaired myocardial vasodilatation during hyperaemic stress is improved by simvastatin but not by pravastatin in patients with hypercholesterolaemia. Eur Heart J 2004, 25: 671-679. 10.1016/j.ehj.2004.02.017
Galderisi M, Cicala S, D'Errico A, de Divitiis O, de Simeone G: Nebivolol improves coronary flow reserve in hypertensive patients without coronary heart disease. J Hypertens 2004, 22: 2201-2208. 10.1097/00004872-200411000-00024
Schwartzkopff B, Brehm M, Mundhenke M, Strauer BE: Repair of coronary arterioles after treatment with perindopril in hypertensive heart disease. Hypertension 2000, 36: 220-225.
Parodi O, Neglia D, Palombo C, Sambuceti G, Giorgetti A, Marabotti C, Gallopin M, Simonetti I, L'Abbate A: Comparative effects of enalapril and verapamil on myocardial blood flow in systemic hypertension. Circulation 1997, 96: 864-873.
Mellwig KP, Baller D, Gleichmann U, Moll D, Betker S, Weise R, Notohamiprodjo G: Improvement of coronary vasodilatation capacity through single LDL apheresis. Atherosclerosis 1998, 139: 173-178. 10.1016/S0021-9150(98)00055-0
Takeuchi M, Miyazaki C, Yoshitani H, Sakamoto K, Yoshikawa J: Assessment of coronary flow velocity with transthoracic Doppler echocardiography during dobutamine stress echocardiography. J Am Coll Cardiol 2001, 38: 117-123. 10.1016/S0735-1097(01)01322-5
Marks DS, Gudapati S, Prisant LM, Leir B, Donato-Gonzalez C, Baller JL, Houghton JL: Mortality in patients with microvascular disease. J Clin Hypertens 2004, 6: 304-309.
Rigo F, Cortigiani L, Gherardi S, Zanella C, Di Pede F, Pasanisi E, Raviele A, Picano E: The prognostic meaning of coronary flow reserve assessed by Doppler echocardiography in nonischemic dilated cardiomyopathy. [Abstract]. Circulation 2004, 110: III-511.
Rigo F, Pasanisi E, Gherardi S, Zanella C, Cutaia V, Cortigiani L, Raviele A, Picano E: Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy: a echocardiographic study. [Abstract]. Eur Heart J 2004, 25: 16.
Britten MB, Zeiher AM, Schachinger V: Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis 2004, 15: 259-264. 10.1097/01.mca.0000134590.99841.81
Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG: Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med 2003, 349: 1027-1035. 10.1056/NEJMoa025050
Neglia D, Michelassi C, Trivieri MG, Sambuceti G, Giorgetti A, Pratali L, Gallopin M, Salvadori PA, Sorace O, Carpeggiani C, Poddighe R, L'Abbate A, Parodi O: Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation 2002, 105: 186-193. 10.1161/hc0202.102119
Lim HE, Shim WJ, Rhee H, Kim SM, Hwang GS, Kim YH, Seo HS, Oh DJ, Ro YM: Assessment of coronary flow reserve with transthoracic Doppler echocardiography: comparison among adenosine, standard-dose dipyridamole, and high-dose dipyridamole. J Am Soc Echocardiogr 2000, 13: 264-270. 10.1067/mje.2000.103508
Holdright DR, Lindsay DC, Clarke D, Fox K, Poole-Wilson PA, Collins P: Coronary flow reserve in patients with chest pain and normal coronary arteries. Br Heart J 1993, 70: 513-519.
Otsuka R, Watanabe H, Hirata K, Tokai K, Muro T, Yoshiyama M, Takeuchi K: Acute effects of passive smoking on the coronary circulation in healthy young adults. JAMA 2001, 286: 436-441. 10.1001/jama.286.4.436
Hozumi T, Eisenberg M, Sugioka K, Kokkirala A: Change in coronary flow reserve on transthoracic Doppler echocardiography after a single high-fat meal in young healthy men. Ann Inter Med 2002, 136: 523-528.
Ascione L, De Michele M, Accadia M, Rumolo S, Sacra C, Alberta Ortali V, Inserviente L, Petti M, Russo G, Tuccillo B: Effect of acute hyperhomocysteinemia on coronary flow reserve in healthy adults. J Am Soc Echocardiogr 2004, 17: 1281-1285. 10.1016/j.echo.2004.07.011
Hirata K, Shimada K, Watanabe H, Muro A: Modulation of coronary flow velocity reserve by gender, menstrual cycle and hormone replacement therapy. J Am Coll Cardiol 2001, 38: 1879-1884. 10.1016/S0735-1097(01)01658-8
Caiati C, Montaldo C, Zedda N, Montisci R, Ruscazio M, Lai G, Cadeddu M, Meloni L, Iliceto S: Validation of a new noninvasive method (contrast-enhanced transthoracic second harmonic echo Doppler) for the evaluation of coronary flow reserve: comparison with intracoronary Doppler flow wire. J Am Coll Cardiol 1999, 34: 1193-1200. 10.1016/S0735-1097(99)00342-3
Lethen H, Tries HP, Kersting S, Lambertz H: Validation of noninvasive assessment of coronary flow velocity reserve in the right coronary artery. A comparison of transthoracic echocardiographic results with intracoronary Doppler flow wire measurements. Eur Heart J 2003, 24: 1567-1575. 10.1016/S0195-668X(03)00284-7
Fujimoto K, Watanabe H, Hozumi T, Otsuka R, Hirata K, Yoshiyama M, Yoshikawa J: New noninvasive diagnosis of myocardial ischemia of the left circumflex coronary artery using coronary flow reserve measurement by transthoracic Doppler echocardiography: comparison with thallium-201 single photon emission computed tomography. J Cardiol 2004, 43: 109-16.
Krzanowski M, Bodzon W, Dimitrow PP: Imaging of all three coronary arteries by transthoracic echocardiography: an illustrated guide. Cardiovasc Ultrasound 2003, 1: 16. 10.1186/1476-7120-1-16
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Dimitrow, P.P., Galderisi, M. & Rigo, F. The non-invasive documentation of coronary microcirculation impairment: role of transthoracic echocardiography. Cardiovasc Ultrasound 3, 18 (2005). https://doi.org/10.1186/1476-7120-3-18
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-7120-3-18