Abstract
Background
Omega-3 poly-unsaturated fatty acids (ω-3 PUFAs) have demonstrated to be beneficial in the prevention of cardiovascular disease, however, the mechanisms by which they perform their cardiovascular protection have not been clarified. Intriguingly, some of these protective effects have also been linked to HDL. The hypothesis of this study was that ω-3 PUFAs could modify the protein cargo of HDL particle in a triglyceride non-dependent mode. The objective of the study was to compare the proteome of HDL before and after ω-3 PUFAs supplemented diet.
Methods
A comparative proteomic analysis in 6 smoker subjects HDL before and after a 5 weeks ω-3 PUFAs enriched diet has been performed.
Results
Among the altered proteins, clusterin, paraoxonase, and apoAI were found to increase, while fibronectin, α-1-antitrypsin, complement C1r subcomponent and complement factor H decreased after diet supplementation with ω-3 PUFAs. Immunodetection assays confirmed these results. The up-regulated proteins are related to anti-oxidant, anti-inflammatory and anti-atherosclerotic properties of HDL, while the down-regulated proteins are related to regulation of complement activation and acute phase response.
Conclusions
Despite the low number of subjects included in the study, our findings demonstrate that ω-3 PUFAs supplementation modifies lipoprotein containing apoAI (LpAI) proteome and suggest that these protein changes improve the functionality of the particle.
Similar content being viewed by others
Background
In the last decades, different observational and intervention studies have demonstrated a beneficial effect of diets enriched in omega-3 poly-unsaturated fatty acids (ω-3 PUFAs) in the prevention of cardiovascular disease (CVD). Most common ω-3 PUFAs are α-linolenic acid C18:3 n-3, present in plants, and eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), found in fish oil. The dose–response (3–4 g/day) hypotriglyceridemic effect (fasting and postprandial) is the best defined metabolic effect of ω-3 PUFAs [1]. The mechanism for this lipid lowering effect seems to be due to activation of peroxisome proliferator-activated receptors (PPAR) [2].
Other potential beneficial effects of ω-3 PUFAs include reduction of susceptibility to ventricular arrhythmia [3]; antithrombogenic and antioxidant effect [4]; retardation of the atherosclerotic plaque growth by reduced expression of adhesion molecules and platelet-derived growth factor [4] and anti-inflammatory effect [5]; promotion of endothelial relaxation by induction of nitric-oxide production, and mild hypotensive effect [6]. All these effect are explained because diet EPA and DHA are rapidly incorporated into the cellular membrane phospholipids where carry out their potential attributed actions [7].
Interestingly, several of the beneficial effects attributable to ω-3 PUFAs have also been linked to HDL. HDL has been involved in anti-thrombosis and endothelial dysfunction [8], anti-inflammatory effect [9], inhibition of lipoprotein oxidation [10, 11], regulation of the complement system, inhibition of proteolysis and regulation of acute phase response [12]. Omega-3 PUFAs do not substantially modify the cholesterol transported by the HDL but, it is now assumed that HDL cholesterol is not a good marker of the functional capacities of the particle. Recent studies have confirmed that protein content of HDL is complex, and it is more related with certain anti-atherogenic properties of HDL than HDL cholesterol [13].
The hypothesis of this study was that ω-3 PUFAs could modify the protein cargo of HDL particle in a triglyceride non-dependent mode. Regarding this, the effect of ω-3 PUFAs would be directly related, at least in part, to cardioprotective actions of HDL particle. In order to elucidate this hypothesis, a comparative proteomic analysis of HDL particle before and after a ω-3 PUFAs enriched diet has been performed in a smoking healthy male volunteers group, a population characterised by dysfunctional HDL particles [14] in which high fish consumption reduces the CVD risk associated with smoking [15].
Results and dicussion
Results
Clinical, biochemical and dietary characteristics
The study group was composed of 6 male smokers, all of whom completed the experiment. Tobacco consumption was used as a model to study dysfunctional HDL particle. Their mean age was 43.0 (28.5-51.0) (median (interquartile range)) and their mean tobacco consumption was 25.5 (20.0-31.3) cigarettes per day. Table 1 shows the main clinical variables at baseline and at the end of the intervention. As expected, due to the low dose of ω-3 PUFAs supplementation, there were not changes in the lipids parameters. Baseline diet assessment showed a mean energy intake of 2847 (2671–2991) kcal with a high consumption of total fat 37.6 (35.8-40.4)%, a low intake of carbohydrates 42.8 (39.0-47.6)%, and a protein intake of 14.8 (14.3-15.8)%. Marine and non-marine omega-3 fatty acids intake was 0.60 (0.25-1.07) g/day and 1.36 (1.19-1.98) g/day, respectively. Physical activity questionnaire results showed a mean of 64.2 (36.5-81.3) METs/week. Dietary parameters and exercise level were stable throughout the study as FFQ and physical activity questionnaire performed at the end of the study revealed (data not shown).
Omega-3 PUFAs compliance
Omega-3 PUFAs supplements were well tolerated by all subjects who did not show any side effect. According to participant’s reports and recounts of empty packages, compliance with the supplements was 100%.
Proteomic results
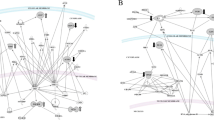
Proteomic analysis revealed that 28 spots were differentially expressed (p < 0,05) between both study periods. By mass spectrometry, 16 out of the 28 spots were identified: 10 spots were identified up-regulated after ω-3 PUFAs treatment and 6 spots were down-regulated. The up-regulated spots corresponded to apoAI, clusterin, paraoxonase (PON1), fibrinogen β, haptoglobin-related protein (HPTR) and immunoglobulin kappa chain C region; and the down-regulated spots corresponded to fibronectin, α-1-antitrypsin (A1AT), serum albumin, immunoglobulin mu chain C region, complement C1r subcomponent and complement factor H. Mass spectrometry identification results are presented in Table 2 and a representative 2D image is shown in Figure 1. Moreover, in Figure 2 a representative section of the gel labelled with the DIGE dyes is presented. In Additional file 1: Table S1 the normalized volume of each spot in every gel is detailed. To better understand the differences existing between basal and after-treatment HDL proteome, proteins identified were classified by biological function (Table 3).
Differentially expressed protein spots identified by DIGE analysis. Proteins were extracted as described and separated in pH 3–10 IPG strips for the first dimension and 10% polyacrylamide for the second dimension. The image was acquired on a Typhoon 9400 scanner at 633/670-nm excitation/emission wavelengths. Spots detected by the analysis software are indicated. Ten protein spot-features were found to be significantly up-regulated (red) after the ω-3 PUFAs supplementation and six were significantly down-regulated (blue) in Lp-AI of smoker participants.
Representative section of the 2D-DIGE proteome map of basal and after the ω-3 PUFAs supplementation. Proteins were labeled with Cy3 (basal situation) and Cy5 (after the ω-3 PUFAs supplementation). An internal standard comprised of equal amount of proteins from all samples (basal and after the treatment) was labeled with Cy2 and included in all gels. The green spots indicate downregulated proteins, while the red spots indicate upregulated proteins after the ω-3 PUFAs supplementation. Some of the most representative identified proteins that showed significantly altered expression after ω-3 PUFAs supplementation are indicated with arrows and labeled with the respectives protein entry name.
Confirmation of proteomic results by immunodetection methods
Clusterin increase observed by proteomics was confirmed by immunodetection (p < 0.05). The fold change observed in clusterin measured by ELISA was 2.18 (basal situation: 3.81E-7 (3.72E-7-3.90E-7); after the supplementation: 8.29E-7 (5.06E-7-1.17E-6). Comparison of HDL PON1 between the study situations showed an increased in enzyme activity (fold change: 1.22) after ω-3 PUFAs supplementation, but it did not achieve statistical significance (basal situation: 4.47 (3.10-5.30); after the supplementation: 6.68 (4.24-11.5)).
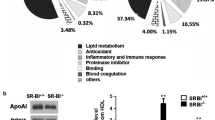
Moreover, Milliplex MAP assays were performed to measure apoAI, apoE and apoCIII, proteins known to be constituents of HDL and to be related to cardiovascular disease. Significant results are shown in Figure 3.
Expression levels of apoAI, apoCIII and clusterin. Protein expression levels were divided by total protein concentration in order to normalize values. Expression level of apoAI and apoCIII were measured by MILLIPLEX MAP assays and clusterin levels was measured by ELISA in participants Lp-AI particles. Changes in protein expression level were all of them statistically significant (p < 0,05).
Discussion
A recent meta-analysis of 29 clinical trials including more than 35,000 high risk cardiovascular patients has explored the effects of ω-3 PUFAs and it has shown a reduction in total mortality associated with the use of these fatty acids [16]. Similar results were obtained from the meta-analysis of 12 studies including 32,779 patients from randomised controlled trials of fish oil as dietary supplements [17]. Both independent studies have concluded that fish oil supplementation was associated with a reduction in deaths from cardiac causes. However, the mechanisms of these pleiotropic effects of fish oil remain mostly unknown [18]. In spite of evident similarities between the protective cardiovascular action of ω-3 PUFAs and HDL, no previous studies had explored the effects of ω-3 PUFAs on protein composition, other than apoAI, on HDL.
Proteomic analysis revealed that some proteins associated to HDL particle were significantly up- or down-regulated in smoking healthy male subjects after 5 weeks consuming a ω-3 PUFAs enriched diet. The down-regulated proteins were mainly related to acute-phase response, complement system regulation and regulation of platelet activation, while proteins up-regulated after the treatment were proteins implicated in lipid metabolism, coagulation, signal transduction, proteolysis regulation, and protection against oxidation.
In this study, we observed an increase in PON1 produced by the ω-3 PUFAs supplementation. PON1 activity was also measured and, although it did not reach statistical significance, activity was higher after the treatment. It is clearly demonstrated that the higher the level of paraoxonase in HDL, higher is the protective effect of the particle [19]. Taken together, this suggests that HDL cardiovascular functions could be improved by PON1 increase. However, it must be further studied.
Interestingly, clusterin, one of the most abundant proteins in HDL, was also up-regulated after ω-3 PUFAs enriched diet. Although the role of clusterin is not yet fully understood, it is known that administration of an oral clusterin-peptide significantly improves HDL anti-inflammatory properties in animal models [20]. The clusterin increase observed in this study supports the cardioprotective effect of ω-3 PUFAs.
Three well-known proteins related to lipid metabolism and transport, apoAI, apoE and apoCIII, were also increased by the ω-3 PUFAs addition. Apolipoprotein AI is the major protein component of HDL. ApoAI protects from atherosclerosis development by its participation in cholesterol efflux and through its antiinflamatory and antioxidant properties. ApoAI by itself is able to reduce oxidized lipids and its inflammatory effects [21–23]. Many lines of evidence indicate that apoE plays a role in modulating atherogenesis promoting cholesterol efflux from macrophages [24]. Studies made in transgenic mice have shown that apoE-deficient animals, which develop hypercholesterolemia and are prone to spontaneous atherosclerosis, show decreased lesion size when overexpressing human apoAI [21], suggesting that apoE and apoAI operate together to optimize mobilization of macrophage cholesterol, a process critical to limiting plaque development. The observation of apoE increasing levels after ω-3 PUFAs in this study is also in agreement with an improvement in the protective properties of the particle. In this study, an increase in apoCIII protein level has been observed after ω-3 PUFAs supplementation. ApoCIII is present in HDL, but it is also a major protein in VLDL and LDL. ApoCIII has been related to elevated VLDL, LDL cholesterol, TG levels and cardiovascular risk [25]. It was demonstrated that the presence of ApoCIII in HDL is related to diminished cholesterol efflux [26]. One possible explanation is that, although no changes in the lipid profile were observed, changes or redistribution of proteins between proatherogenic lipoproteins, VLDL and LDL, to cardioprotective HDL were actually possible.
The complement system is activated by tobacco consumption [27] and in vitro studies indicate that HDL blocks the assembly of the terminal complement attack complex on endothelial cells [28]. Supporting this observation, our data probably indicate that HDL is involved in the regulation of complement activation by tobacco. Decrease in fibronectin and A1AT, two proteins related to acute phase response [12] and induced by smoking [29, 30], is also another sign of the improvement in HDL protective capabilities recovered by ω-3 PUFAs supplementation.
As far as we know, this is the first HDL proteomic study carried out with smokers and one of the few proteomic analysis made with ω-3 PUFAs [31–33]. This study suggests that ω-3 PUFAs modify the particle composition in some proteins that have been clearly associated with cardiovascular protection. However, the sample size is small and further analysis is necessary to extract final conclusions. These results uncover a potential mechanism that could, at least partially, explain some of the benefits of ω-3 PUFAs.
Conclusions
In conclusion, as expected, low doses of omega-3 fatty acid supplementation do not have effect in the lipid profile. However, they modify HDL proteome, suggesting a positive change in the functionality of the particle in smoking men. Functional studies and in-deep proteomic studies will be important for the best knowledge of the particle. If the observed changes in the protein cargo of HDL have clinical implications must be further studied.
Methods
Subjects
The study group was composed by 6 unrelated smoking (≥20 cigarettes/day in the last three months) healthy male volunteers who underwent a routine medical examination in the Hospital Universitario Miguel Servet (Zaragoza, Spain). The study was approved by the local ethical committee and informed consents were obtained from all participant. Clinical examination and blood tests were performed. Inclusion criteria were: age 16–65 years old and normolipidemia defined as cholesterol LDL < 190 mg/dL and triglycerides (TG) < 150 mg/dL. Exclusion criteria were: current use of drugs that modify lipid or glucose metabolism; anti-inflammatory drugs, included statins, fibrates, ezetimibe, resins, aspirin, nonsteroidal drugs, corticoids, immunodepressors, and vitamin complexes; current acute illness (including hepatic illness, diabetes mellitus, kidney illness, cancer and thyroid illness non controlled); parental history of dyslipidemia and ω-3 PUFAs allergy; alcohol consumption (> 30 g/day of ethanol) and any condition that, in the researcher opinion, could interfere with the study.
Study design
This study was designed as a 5 weeks open study in which 2 g/day (1 g/capsule twice a day) of ω-3 PUFAs, in the commercial format of Omacor© (Ferrer Internacional), were administrated to participants. Basal and after the 5 weeks of supplementation, the lipid profile and the HDL proteome were analyzed.
Plasma samples
Ten mL of blood before and after ω-3 PUFAs enriched diet were collected on Vacutainer tubes with EDTA as anticoagulant from fasted (≥ 12 hours) volunteers. Sodium azide and the protease inhibitor Pefabloc SC (Roche) were added to plasma at a concentration of 1.5 and 0.5 mM, respectively. All samples were stored at −80°C until they were processed.
Quantification of lipids and lipoproteins
Total serum cholesterol and triglyceride levels were quantified enzymatically with a Beckman Synchron CX7 analyzer (Boehringer Mannheim, Ingelheim am Rhein, Germany). HDL cholesterol was measured after precipitation of apolipoprotein B-containing lipoproteins with Mg++ phosphotungstate (Boehringer Mannheim, Ingelheim am Rhein, Germany). LDL cholesterol was calculated by the Friedewald formula [34].
Dietary and physical activity assessment
Before the study, participants were instructed to maintain their usual dietary and physical activity patterns throughout all study to avoid changes that could alter the HDL composition. At baseline and final visits, a Spanish validated 137-item food frequency questionnaire (FFQ) and The Minnesota Leisure Time Physical Activity Questionnaire were performed [35, 36]. The FFQ included the consumption frequency of each of the 137-item food by choosing between nine possibilities (from never or less than once per month to six or more times per day) and the portion size. The total energy and nutrient intake were calculated based on previously validated Spanish food composition tables [35].
Isolation of HDL by Fast Protein Liquid Chromatography-affinity cromatography (FPLC-AC)
FPLC-AC analysis was carried out on an ÄKTA FPLC system (GE Healthcare, Waukesha, WI, USA) equipped with a fraction collector. Briefly, fractions of 2 mL of plasma from a single sample were applied to the anTi-ApoAI affinity column made with a 5 mL HiTrapTM NHS-activated HP (GE Healthcare) coupled to 5 mg of antibody against human apoAI (BioDesign International). Firstly, 10 mL of PBS were used to ensure the equilibration of the column. The binding reaction was performed in a saline buffer (0,1 M NaHCO3, 0,5 M NaCl, 1 mM EDTA, pH 8,0) at a flow rate of 1 mL/min with a maximum pressure of 0,5 MPa. When the non-binding fraction was washed, buffer was changed to elution buffer (0,1 M glycine, 0,5 M NaCl, 10% dioxane, pH 2,8) at the same flow rate. HDL was collected using a Frac 900 fraction collector (GE Healthcare) at 4°C as a single 5 mL fraction over 1 mL of equilibration buffer (0,5 M Tris–HCl, pH 8) to quickly neutralize the acid pH and to prevent protein degradation. HDL fraction was then concentrated to 200 μL using Amicon Ultra 10 KDa filters (Millipore, Billerica, MA, USA) at 3000 rpm for 35–40 minutes at 4°C. Total protein concentration was calculated by RC/DC Protein assay (BioRad). Finally, samples were treated with the 2-D Clean-Up Kit (GE Healthcare).
Fluorescence labelling
Three groups were defined for labelling: pooled internal standard, before ω-3 PUFAs and after ω-3 PUFAs enriched diet. Labelling was made following the protocol provided in the kit. A total of 50 μg of each type of HDL sample was labelled with one of the three CyDye DIGE Fluors (GE Healthcare). CyDyes were reconstituted in anhydrous dimethylformamide and combined with samples at a ratio of 400 pmol of CyDye to 50 μg of protein. Labelling was performed on ice and in the dark for 30 min. The reaction was then quenched by incubating with 1 μL of 10 mM lysine on ice and in the dark for 10 min.
2-D Gel Electrophoresis
Firstly, immobilized pH gradient (IPG) strips were equilibrated in Destreak rehydratation solution (GE Healthcare) in which anfolites were added. The three labelled protein samples were combined and were focused on 24 cm, 3–10 IPG strips (GE Healthcare) using an IPGphor focusing apparatus (GE Healthcare, Waukesha, WI, USA). Once first dimension electrophoresis had finished, IPG strips were equilibrated with dithiothreitol (DTT) and iodoacetamine buffers. Then, proteins were separated by electrophoresis on 10% polyacrylamide Tris-glycine gels using an Ettan DALT II System (GE Healthcare, Waukesha, WI, USA).
DIGE Analysis
The gels were scanned with a Typhoon 9400 scanner (GE Healthcare, Waukesha, WI, USA). Spot detection, quantification and image matching were performed with Progenesis SameSpots software (Nonlinear Dinamics, Newcastle upon Tyne, UK). Finally, 2-D gels were fixed in 30% methanol, 7.5% acetic acid, and stained with a silver nitrate protocol compatible with mass spectrometry for protein identification. Relative protein quantification of HDL samples before and after ω-3 enriched diet was performed using Progenesis SameSpots software (Nonlinear Dinamics, Newcastle upon Tyne, UK). The Cy2-labelled pooled internal standard on every gel allowed accurate relative quantification of protein spot features across different gels. Student's t-test was used to identify differences in relative abundances of protein spot-features.
In-gel digestion
Protein spots were excised manually from 2-D gels. Briefly, spots were washed with water, ammonium bicarbonate (25 mM NH4HCO3), acetonitrile (ACN) and a freshly made mix of potassium ferricyanide 30 mM and sodium thiosulfate 100 mM to eliminate silver. Next, samples were reduced and alquilated by incubation with DTT (10 mM) at 60°C during 45 min followed by incubation with iodoacetamide (50 mM) at room temperature during 30 minutes. Finally, proteins were trypsin digested overnight at 37°C (2,5 ng/μl, ratio enzyme:protein 1:20, Trypsin gold, Promega). Digestion was stopped by addition of 0.5% trifluoroacetic acid (TFA) and tryptic peptides were extracted sequentially with increasing concentrations of ACN in H2O. Peptides were concentrated and desalted by passing them through ZipTip C18 columns (Millipore) following the manufacturer’s instructions and eluting with 50%ACN/0,1%TFA/H2O.
Mass spectrometry analyses
Sample (0,4 μl) and matrix (0,8 μl saturated solution of alpha-Cyano-4-hydroxycinnamic acid in 50% ACN/0.1% TFA/H2O) were spotted in duplicate onto a Opti-Tof 384 well insert plate (Applied Biosystems, Carlsbad, CA, USA). MALDI-TOF MS was performed using a 4800plus MALDI-TOFTOF (Applied Biosystems, Carlsbad, CA, USA) in the reflector mode with accelerating voltage of 20 kV, mass range of 800 to 4000 Da, 1000 shots/spectrum and laser intensity of 2832. MSMS spectra were performed automatically on twenty of most intense precursors, with 1000 shots/spectrum and laser intensity of 3700. Spectra were calibrated externally using a standard protein mixture (4700 Calmix, Applied Biosystems).
Alternatively, samples were dried and resuspended in 0.1% formic acid and analysed by LC-MSMS in a nano Acquity (Waters, Milford, MA, USA) coupled to an OrbitrapVelos (ThermoScientific, Waltham, MA, USA). Sample was injected in a C18 phase reverse column (75 μm Øi, 10 cm, nano Acquity, 1.7 μm BEH column, Waters) in a gradient of 40-60% buffer B during 5 minutes at a flow rate of 250 nl/min (A: 0.1% formic acid; B: ACN/0.1% acid formic). Eluted peptides were ionized by ESI (PicoTipTM, New Objective 2000 V). Peptide mases were analized in the Orbitrap in full scan (m/z 350–1700) and the 5 most abundant peptides were selected for collision-induced dissociation fragmentation using helium as collision gas. Data were extracted with software Thermo Xcalibur (v.2.1.0.1140).
Protein identification
Proteins were identified using the search engine Mascot and the Uniprot database. Search parameters used were: human, missed cleavage 1, fixed modifications carbamidomethyl (cysteines) and peptide tolerance 0.2 Da (MS) 0.3 Da (MSMS). Proteins with a score above 61 were considered a positive hit.
Human paraoxonase and complement C3 component ELISA assays
Two commercial ELISA assays against paraoxonase (Uscn Life Science Inc.) and complement C3 component (AssayPro) were performed following manufacter’s instructions. Assays were performed by duplicate in each sample used in 2D-DIGE proteomic analysis. Results were normalized by the total protein concentration. Data are presented as μg (studied protein)/μg (total protein).
Milliplex MAP assays
Two MILLIPLEXTM MAP assays (Millipore) were done. Apolipoproteins apoAI, apoCIII, apoE and the acute-phase protein serum amyloid A (SAA) were measured. Analyses were performed as manufacturer suggested and HDL dilutions were done in function of the sensibility of the panel. Assays were performed by duplicate in each sample used in 2D-DIGE proteomic analysis. Results were normalized by the total protein concentration. Data are presented as μg (studied protein)/μg (total protein).
Statistical analysis
Continuous clinic and biochemical variables and protein levels of ELISA and MILLIPLEX MAP assays were expressed as median (interquartile range). Differences in median values were assessed using Wilcoxon test. All statistical analyses were performed with SPSS software (version 15.0), with significance set at p < 0.05.
References
Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ: Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008, 197: 12-24. 10.1016/j.atherosclerosis.2007.11.008
Berger J, Moller DM: The mechanisms of action of PPARs. Annu Rev Med. 2002, 53: 409-435. 10.1146/annurev.med.53.082901.104018
Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J: Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995, 274: 1363-1367. 10.1001/jama.1995.03530170043030
Calabresi L, Villa B, Canavesi M, Sirtori CR, James RW, Bernini F: An omega-3 polyunsaturated fatty acid concentrate increases plasma high-density lipoprotein 2 cholesterol and paraoxonase levels in patients with familial combined hyperlipidemia. Metabolism. 2004, 53: 153-158. 10.1016/j.metabol.2003.09.007
De Caterina R, Liao JK, Libby P: Fatty acid modulation of endothelial activation. Am J Clin Nutr. 2000, 71: 213S-223S.
Connor WE: Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000, 71: 171S-175S.
Harris WS: Omega-3 fatty acids and cardiovascular disease: a case for omega-3 index as a new risk factor. Pharmacol Res. 2007, 55: 217-223. 10.1016/j.phrs.2007.01.013
Mineo C, Deguchi H, Griffin J, Shaul P: Endothelial and antithrombotic actions of HDL. Circ Res. 2006, 98: 1352-1364. 10.1161/01.RES.0000225982.01988.93
Barter P, Nicholls S, Rye K, Anantharamaiah G, Navab M, Fogelman A: Antiinflammatory properties of HDL. Circ Res. 2004, 95: 764-772. 10.1161/01.RES.0000146094.59640.13
Navab M, Hama S, Cooke C, Anantharamaiah G, Chaddha M, Jin L: Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J Lipid Res. 2000, 41: 1481-1494.
Navab M, Hama S, Anantharamaiah G, Hassan K, Hough G, Watson A: Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000, 41: 1495-1508.
Vaisar T, Pennathur S, Green P, Gharib S, Hoofnagle A, Cheung M: Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007, 117: 746-756. 10.1172/JCI26206
Rader D: Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006, 116: 3090-3100. 10.1172/JCI30163
Tselepis AD, Panagiotakos DB, Pitsavos C, Tellis CC, Chrysohoou C, Stefanadis C: Smoking induces lipoprotein-associated phospholipase A2 in cardiovascular disease free adults: the ATTICA Study. Atherosclerosis. 2009, 206: 303-308. 10.1016/j.atherosclerosis.2009.02.016
Rodriguez BL, Sharp DS, Abbott RD, Burchfiel CM, Masaki K, Chyou PH: Fish intake may limit the increase in risk of coronary heart disease morbidity and mortality among heavy smokers. The Honolulu Heart Program. Circulation. 1996, 94: 952-956. 10.1161/01.CIR.94.5.952
Filion KB, El Khoury F, Bielinski M, Schiller I, Dendukuri N, Brophy JM: Omega-3 fatty acids in high-risk cardiovascular patients: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2010, 10: 24- 10.1186/1471-2261-10-24
Saravanan P, Davidson NC: Fish oil and arrhythmias. Pro-arrhythmic effects of fish oils. BMJ. 2009, 338: b393- 10.1136/bmj.b393
Saravanan P, Davidson NC, Schmidt EB, Calder PC: Cardiovascular effects of marine omega-3 fatty acids. Lancet. 2010, 376: 540-550. 10.1016/S0140-6736(10)60445-X
Ribas V, Sánchez-Quesada JL, Antón R, Camacho M, Julve J, Escolà-Gil JC: Human apolipoprotein A-II enrichment displaces paraoxonase from HDL and impairs its antioxidant properties: a new mechanism linking HDL protein composition and antiatherogenic potential. Circ Res. 2004, 95: 789-797. 10.1161/01.RES.0000146031.94850.5f
Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Wagner AC, Hama S: An oral apoJ peptide renders HDL antiinflammatory in mice and monkeys and dramatically reduces atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2005, 25: 1932-1937. 10.1161/01.ATV.0000174589.70190.e2
Tall A: Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008, 263: 256-3-
Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, Reddy ST, Sevanian A, Fonarow GC, Fogelman AM: Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000 Sep, 41 (9): 1495-1508.
Garner B, Waldeck AR, Witting PK, Rye KA, Stocker R: Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J Biol Chem. 1998, 273 (11): 6088- 10.1074/jbc.273.11.6088
Lin CY, Duan H, Mazzone T: Apolipoprotein E-dependent cholesterol efflux from macrophages: kinetic study and divergent mechanisms for endogenous versus exogenous apolipoprotein E. J Lipid Res. 1999, 40: 1618-1627.
Zheng C, Khoo C, Furtado J, Sacks FM: Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation. 2010, 121: 1722-1734. 10.1161/CIRCULATIONAHA.109.875807
Holzer M, Birner-Gruenberger R, Stojakovic T, El-Gamal D, Binder V, Wadsack C, Heinemann A, Marsche G: Uremia Alters HDL Composition and Function. J Am Soc Nephrol. 2011, 22 (9): 1631-1641. Epub 2011 Jul 29, 10.1681/ASN.2010111144
Robbins RA, Nelson KJ, Gossman GL, Koyama S, Rennard SI: Complement activation by cigarette smoke. Am J Physiol. 1991, 260: L254-L259.
Hamilton KK, Zhao J, Sims PJ: Interaction between apolipoproteins A-I and A-II and the membrane attack complex of complement. Affinity of the apoproteins for polymeric C9. J Biol Chem. 1993, 268: 3632-3638.
Roman J, Ritzenthaler JD, Gil-Acosta A, Rivera HN, Roser-Page S: Nicotine and fibronectin expression in lung fibroblasts: implications for tobacco-related lung tissue remodeling. FASEB J. 2004, 18: 1436-1438.
Dhami R, Gilks B, Xie C, Zay K, Wright JL, Churg A: Acute cigarette smoke-induced connective tissue breakdown is mediated by neutrophils and prevented by alpha1-antitrypsin. Am J Respir Cell Mol Biol. 2000, 22: 244-252.
de Roos B, Geelen A, Ross K, Rucklidge G, Reid M, Duncan G: Identification of potential serum biomarkers of inflammation and lipid modulation that are altered by fish oil supplementation in healthy volunteers. Proteomics. 2008, 8: 1965-1974. 10.1002/pmic.200700457
de Roos B: Proteomic analysis of human plasma and blood cells in nutritional studies: development of biomarkers to aid disease prevention. Expert Rev Proteomics. 2008, 5: 819-826. 10.1586/14789450.5.6.819
de Roos B: Nutrition proteomics and biomarker discovery. Expert Rev Proteomics. 2009, 6: 349-351. 10.1586/epr.09.52
Friedewald W, Levy R, Fredrickson D: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972, 18: 499-502.
de la Fuente-Arrillaga C, Ruiz ZV, Bes-Rastrollo M, Sampson L, Martinez-González MA: Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010, 13: 1364-1372. 10.1017/S1368980009993065
Elosua R, Marrugat J, Molina L, Pons S, Pujol E: Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish men. The MARATHOM Investigators. Am J Epidemiol. 1994, 139: 1197-1209.
Acknowledgments
We thank the Proteomic Platform of the Park Scientific of Barcelona, Spain, and Silvia Barceló-Batllori and Irene Orera from the Proteomics Unit of the Instituto de Investigaciones Sanitarias Aragón (IIS), Spain, for their effective work and advices in the development of this study.
Sources of support
Grants from Ferrer International, Spanish Ministry of Health FIS PS09/0673 and PI10/00387 and RTIC C06/01 (RECAVA) supported this work. RMG was partially supported by a fellowship from Instituto Danone. The sponsors were not influential in the study design, data collection, analysis, interpretation of results, or writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None of the authors declared a conflict of interest.
Authors’ contributions
EB carried out the proteomic studies, the immunoassays and drafted the manuscript. RMG carried out the subject’s selection and participated in the collection of data. AC participated in the design of the study, performed the statistical analysis and corrected the draft. SF participated in the inmunoassays analysis and corrected the English. AB collected the data and helped into the subject’s selection and administrated the omega-3 supplementation. IJ and JV carried out the mass spectrometry analysis. JV helped in the coordination of the proteomic analysis and in the draft corrections. FC conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
12944_2012_749_MOESM1_ESM.doc
Additional file 1:Normalized volumes of differentially expressed protein spot-features. The file is a table that shows the normalized volumes of the spots that resulted statistically different in their expression. (DOC 72 kb) (DOC 72 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Burillo, E., Mateo-Gallego, R., Cenarro, A. et al. Beneficial effects of omega-3 fatty acids in the proteome of high-density lipoprotein proteome. Lipids Health Dis 11, 116 (2012). https://doi.org/10.1186/1476-511X-11-116
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-511X-11-116