Abstract
Pancreatic adenocarcinoma is characterized by poor prognosis, because of late diagnosis and lack of response to chemo- and/or radiation therapies. Resistance to apoptosis mainly causes this insensitivity to conventional therapies. Apoptosis or programmed cell death is a central regulator of tissue homeostasis. Certain genetic disturbances of apoptotic signaling pathways have been found in carcinomas leading to tumor development and progression. In the past few years, the knowledge about the complex pathways of apoptosis has strongly increased and new therapeutic approaches based on this knowledge are being developed. This review will focus on the role of apoptotic proteins contributing to pancreatic cancer development and progression and will demonstrate possible targets to influence this deadly disease.
Similar content being viewed by others
Review
Pancreatic cancer is one of the most malignant tumors with a very poor prognosis. Although pancreatic cancer has an incidence of about 10 cases/100,000 persons it is still the fourth male and fifth female leading cause of cancer-related death in the Western world [1]. Most of the newly diagnosed patients present with an already unresectable tumor stage. The 5-year survival rate of patients with pancreatic cancer receiving surgery and chemotherapy ranges from 1%–2% [2]. One of the reasons for this low survival rate is the insensitivity of pancreatic cancer to most oncologic therapies like chemotherapy, radiotherapy and immunotherapy [3–10]. Tumor development and progression as well as resistance to most oncologic therapies result mainly from lacking response to apoptotic stimuli.
Apoptosis or programmed cell death is a central regulator of tissue homeostasis [reviewed in [11]]. Multicellular organisms eliminate redundant, damaged or infected cells by apoptosis. Because chemotherapy and radiotherapy act primarily by inducing apoptosis, defects in the apoptotic pathway can cause cancer cell resistance [12, 13]. Tumor cells utilize multiple pathways to down-modulate apoptosis [14].
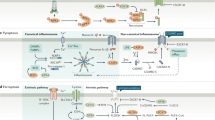
Apoptosis mediated by death receptors belonging to the tumor-necrosis factor (TNF) receptor superfamily is the best-studied pathway in cells (Figure 1) [15, 16]. Members of the TNF receptor family, TNF, Fas (Apo-1, CD95) and TRAIL (TNF-related apoptosis-inducing ligand)-R [16] share a common internal domain, the so-called death domain [17]. These receptors are activated by their natural ligands TNFα, FasL, and TRAIL, respectively. The interaction between receptor and ligand causes trimerization of receptor followed by recruitment of FADD (Fas-associated death domain protein) and procaspase-8 to the death domain forming the DISC (death-inducing signaling complex) [18]. At the DISC, cleavage of procaspase-8 yields the active form of this protease [18]. In type I cells, the amount of activated caspase-8 is sufficient to initiate apoptosis via direct activation of the central effector caspase, caspase-3. In type II cells, the signal enhancing-effect of mitochondria is needed to induce apoptosis [19]. The Bcl-2 family member BID mediates activation of mitochondria in response to death receptor activation. BID is cleaved by active caspase-8 producing tBID, which is translocated to the mitochondria [19]. tBID becomes integrated into the mitochondria membrane and induces release of cytochrome c and other apoptogenic factors from the intermembranous space of mitochondria [20, 21]. In the cytoplasm, cytochrome c forms a complex with Apaf-1 (apoptotic protease activating factor-1), ATP and procaspase-9 termed the apoptosome. Like caspase-8, caspase-9 can be considered an initiator caspase, which is activated by cleavage at the apoptosome and activates in turn executioner caspases, mainly caspase-3, -6 and -7 [22]. Cleavage of death substrates, DNA fragmentation, and cleavage of cytoskeletal proteins finally lead to cell death [22].
Apoptosis mediated by death receptors of the TNF family in type I and type II cells. Apoptosis can be initiated by two alternative pathways: in type I cells the amount of initiator caspases is sufficent to induce executioner caspases directly or in type II cells the enhancing effect of mitochondria is necessary. Active executioner caspases cleave the death substrates, which results in apoptosis.
This complex pathway is controlled and influenced by a variety of different pro- and anti-apoptotic factors. The balance of these effectors is important to ensure tissue homeostasis. Activation or downregulation of pro- and anti-apoptotic genes influence cancer cell viability, cancer cell sensitivity to chemotherapy and radiotherapy, and tumor development and progression (Figure 2).
This review will focus on the knowledge about deregulation of apoptotic proteins and pathways in pancreatic cancer and possible therapeutic approaches based on these findings.
Death receptors
As described above, apoptosis is mediated mainly by members of the TNF death receptor superfamily proteins including Fas (Apo-1, CD95) and TRAIL-R and their natural ligands. Deregulation of these pathways may contribute to abnormal tumor growth [23, 24].
The Fas-FasL system is believed to represent one of the main apoptotic cell death-signaling pathway [25]. Fas or FasL over- and under-expression has been shown in a variety of human carcinomas including lung [26], renal [27] and colon cancer [28]. Findings concerning Fas receptor expression in pancreatic cancer are contradictorily. It has been demonstrated recently that Fas mRNA was increased in pancreatic carcinomas [29]. Contrarily, an in vivo study revealed that both membranous Fas and cytoplasmic Fas receptors could not be detected invasive ductal-type pancreatic adenocarcinomas [30]. Together, these data suggest that tumor cells can evade apoptosis by downregulation of the Fas receptor.
In contrast to FasL, TRAIL is not toxic to normal cells [31]. Unlike other tumor cells, TRAIL receptors are highly expressed at the surface of pancreatic carcinoma cells [32]. Additionally, it has been demonstrated that repeated treatment of different pancreatic cell lines with TRAIL caused sustained and profound cell death [33]. These results show that the TRAIL system in principal is functional in pancreatic cancer but is blocked at some stage downstream in the apoptotic pathway among which is the expression of non-functional receptors, so-called decoy receptors. Approaches to initiate apoptosis by application of TRAIL to cancer cells were successful in mammary carcinomas, intracranial gliomas and colon carcinomas [34]. However, the fact that under certain conditions this therapy induces apoptosis also in normal healthy hepatocytes causing hepatic necrosis [35] dampened the optimistic expectations.
FAP-1
FAP-1 (Fas-associated phosphatase-1) is a non-receptor protein tyrosine phosphatase. It has been demonstrated that FAP-1 can block the function of Fas by interaction with its carboxy-terminal three amino acids [10, 36, 37]. Cell lines resistant to Fas-mediated apoptosis strongly overexpressed FAP-1 and it also was highly expressed in tumor cells in pancreatic carcinoma tissues [37]. In contrast, Capan-1, a pancreatic cancer cell line highly sensitive to Fas-mediated apoptosis, totally lacks FAP-1 expression [37]. Stable transfection of Capan-1 cells with a FAP-1 cDNA strongly decreases the sensitivity to Fas-mediated apoptosis [37]. Inhibition of Golgi anterograde transport by Brefeldin A suggested that FAP-1 could prevent translocation of Fas from intracellular stores to the cell surface [37] resulting in insufficient receptor density at the cell surface. Modulation of FAP-1 expression or suppression of its enzymatic activity may be the basis of a novel therapy for pancreatic cancer.
The role of mitochondria
In type II cells, the apoptosis-enhancing effect of mitochondria is necessary to induce the full apoptotic phenotype. It has been demonstrated that pancreatic cancer cell lines are type II cells [19, 32, 38]. The Bcl-2 family members play the major role in this pathway.
Bcl-2 family
At the center of the cell's decision to live or to die in response to an apoptotic signal is the Bcl-2 family of apoptotic regulators [39]. The Bcl-2 family is the best-characterized group of apoptosis-mediating factors and can be divided into two main groups according to their functional properties; anti-apoptotic proteins like Bcl-xL and Bcl-2 and pro-apoptotic proteins, such as Bax, Bak and Bad. Bcl-2 proteins interact with other molecules through an α-helical domain termed BH-3 domain. This interaction is believed to be important for regulation of apoptosis [40].
Bcl-2 is located at the cytoplasmic face of the mitochondrial outer membrane, at the ER-membrane and the nuclear envelope and may register damage of these compartments and affect their behavior [41]. Bcl-2 directly or indirectly prevents the release of cytochrome c from mitochondria in a variety of tissues [41]. High expression of Bcl-2 is found in various human tumors [42]. Interestingly, it has been shown that Bcl-2 expression is normal or even decreased in pancreatic cancer cells [43, 44]. Preclinic animal and clinical phase II studies using Bcl-2 antisense constructs, such as G3139, in combination with different chemotherapeutics show significant regression of different cancers [45, 46]. However, it is uncertain if this therapeutic approach will be successful in pancreatic cancer showing normal or decreased Bcl-2 expression levels.
Bcl-x exists in two distinct isoforms in humans. Bcl-xL, the longer form, functions in an anti-apoptotic manner. Bcl-xS, the shorter form, in contrast, functions as an apoptosis promoter. Like Bcl-2, Bcl-xL also prevents cytochrome c release from mitochondria [reviewed in [47]]. Different studies showed that most likely every cell type is protected by at least one member of anti-apoptotic Bcl-2-like proteins. In pancreatic carcinoma cells Bcl-xL plays the most important role in protecting from Fas and TRAIL-mediated apoptosis [32]. Furthermore, Bcl-xL is believed to bind to Apaf-1 and may therefore inhibit the association of Apaf-1 with procaspase-9 and thereby prevent caspase-9 activation [48]. Unlike Bcl-2, Bcl-xL is constitutively overexpressed in pancreatic cancer cell lines highly resistant to Fas and TRAIL-mediated apoptosis [32]. Therefore Bcl-xL may be an ideal target for pancreatic cancer therapy. Overexpression of Bcl-xL in cell lines with low Bcl-xL expression like Colo357, showed complete suppression of apoptosis. On the other hand, inhibition of Bcl-xL function by overexpression of Bax or administration of antisense oligonucleotides against Bcl-xL mRNA resulted in sensitization of cells expressing high levels of Bcl-xL like Panc-1 or PancTuI [32]. Additionally, another study revealed that Bcl-xL antisense oligonucleotide inhibited pancreatic cancer cell growth and caused apoptosis by reducing Bcl-xL protein levels in different pancreatic cancer cell lines [49]. Bcl-xL antisense nucleotides also increased the sensitivity to chemotherapeutics like gemcitabine. This was confirmed by another study [50]. Activation of Bcl-2 family member Bcl-xL after repeated exposure to the chemotherapeutic drugs 5-FU and gemcitabine contribute to chemo-resistance of pancreatic cancer cells [50]. Correlation of the molecular data with clinical patient parameters revealed that patients whose tumors exhibited no, faint, or weak Bcl-xL expression lived significantly longer after tumor resection than patients whose tumors exhibited moderate Bcl-xL mRNA expression [51].
Bax is a pro-apoptotic member of the Bcl-2 family that resides in the cytosol and translocates to mitochondria upon induction of apoptosis [52]. It has been demonstrated that ectopically expressed Bax in human embryonic kidney cells (293T cells) induced cytochrome c release and caspase activation [53]. However, overexpression of Bax did not influence the apoptosis rate or expression of Bcl-2 and Bcl-xL in human pancreatic cancer cells transduced with a retroviral expression vector [54]. But Bax significantly increased the sensitivity to chemotherapeutic drugs like gemcitabine and 5-Fu when transduced to pancreatic cancer cell line ASPC-1 cells [54]. Although Bax failed to substantially increase susceptibility of the pancreatic cell line Colo357 towards gemcitabine in vitro, in vivo data from a SCID mouse model suggest that pancreatic tumors overexpressing Bax caused higher sensibility to gemcitabine and therefore stronger tumor regression [B. Schniewind, unpublished data]. That indicates that overexpression of Bax may have therapeutic application in enhancing the efficacy of chemotherapy in pancreatic cancer. A recent study revealed that a binary adenoviral vector system (Ad/GT-Bax + Ad/hTERT-GV16) using the human telomerase reverse transcriptase (hTERT) promoter to induce Bax gene expression was able to induce apoptosis in pancreatic cancer cell lines [55]. Transduced cells not only showed Bax overexpression but also an increased level of caspase-3. Another study revealed that increased levels of Bax and reduced expression of Bcl-2 are involved in the growth-inhibitory effect of cisplatin in pancreatic cancer cells. [56].
Another member of pro-apoptotic proteins of Bcl-2 family is Bak. In pancreatic cancer, Bak expression and apoptosis occur in regions of chronic inflammation surrounding the cancer cells but not in the tumor cells themselves [57]. This may facilitate tumor growth and spread. Recent studies have demonstrated that Bad, a typical pro-apoptotic protein of Bcl-2 family, binds with its BH3 domain to both Bcl-2 and Bcl-xL but mediates its pro-apoptotic functions through inhibition of Bcl-xL, but not Bcl-2 [58].
To restore the function of downregulated pro-apoptotic Bcl-2 family members, there are many efforts to develop peptides and non-peptide agents that mimic the function of these proteins [59].
The key enzymes for arachidonic acid metabolism, lipoxygenases (LOXs) and cyclooxygenases (COXs) influence the development and progression of several human cancers including pancreatic cancer [60]. It has been shown that both COXs and LOXs are upregulated in human pancreatic cancer tissues. Inhibitor studies revealed that blocking these enzymes inhibit pancreatic cancer growth and induce apoptosis. Further investigations pointed out that blockade of LOXs induce apoptosis through cytochrome c release, caspase-9 activation and changes in the levels of Bcl-2 family proteins [61].
In summary, the deregulation of Bcl-2 family both the pro- and the anti-apoptotic proteins play a crucial role in development, growth and expansion of pancreatic cancer. Several approaches based on these findings have been demonstrated to be a tool potentially influencing pancreatic cancer also in a clinical setting.
Caspases and caspase inhibitors
Caspases
The central component of apoptosis is a proteolytic system involving a family of cysteine proteases termed caspases. All caspases are expressed as proenzymes. Activation involves proteolytic processing [62]. Caspases initiate and execute cell death by inactivating anti-apoptotic proteins, shutting down DNA replication and repair [63], reorganization of the cytoskeleton [64] and disruption of the nuclear lamina [65].
Caspases are highly regulated by a variety of different inhibitors and activators. The induced proximity or oligomerization model explains that caspases exist as inactive monomers. The effectors of caspases bring the caspases in close proximity, allowing for their intermolecular autoproteolytic activation [66]. For example, activation of procaspase-8 requires association with its cofactor FADD (Fas-associated protein with death domain) [67] and procaspase-9 must interact with APAF-1 to become functional [68].
Important caspase-8 inhibitors represent the FADD-like ICE inhibitory proteins (FLIPs) [69]. Two forms, FLIPL (long form) and FLIPS (short form) have been characterized [70]. FLIPL is a structural homologue of caspase-8 but it lacks amino acid residues that are critical for caspase activity [70–72]. FLIPL competes with procaspase-8 for binding to FADD at the DISC, thus preventing caspase activation [66]. Very interestingly, it has been shown that FLIPL is overexpressed both in pancreatic cancer cell lines resistant to Fas mediated apoptosis and pancreatic tumors [73]. This may promote progression of malignant pancreatic tumors.
Recent studies suggest that FLIP is not simply an inhibitor of death-receptor-induced apoptosis but it is involved in activation of NF-κB by recruiting adaptor proteins like TRAF [74]. NFκ-B is known to regulate several genes that mediate tumorigenesis and metastasis [reviewed in [75]]. This may also contribute to tumor growth. Downregulation of FLIP in prostate cancer cell line resulted in sensitization to Fas-mediated apoptosis [76]. Recently, it has been demonstrated that natural and synthetic ligands of the transcription factor PPARγ (peroxysome proliferator-activated receptor γ) sensitize tumor cells to apoptosis by decreasing the level of FLIP [77]. Equivalent data concerning pancreatic cancer are eagerly awaited. It has been shown that caspase expression is more or less normal in pancreatic carcinoma. In contrast, effectors blocking caspase activation or function like FLIPs are deregulated in pancreatic cancer leading to resistance to death receptor mediated apoptosis.
A new therapeutic approach uses somatostatin receptor subtype 2 (sst2) to activate caspase-3 and therefore to induce apoptosis in pancreatic cancer [78]. Likewise, sst2 treatment of human cancer cell lines with Diospyrin, a bisnaphthoquinonoid natural product, resulted in induction of apoptosis mediated via activation of caspase-3 and caspase-8 [79].
Irofulven (MGI 114, 6-hydroxymethylacylfulvene) induces apoptosis via caspase activation in pancreatic carcinoma cells [80].
IAP family
The inhibitor of apoptosis (IAP) family including cIAPs, XIAP and survivin can block apoptosis through interaction with members of the caspase family. IAPs are characterized by a domain termed the baculoviral IAP repeat (BIR) necessary for caspase interaction [81, 82]. At least one BIR domain is necessary for suppression of apoptosis [83]. Another structural feature of IAPs is the presence of a carboxy terminal RING zinc finger domain [83]. But it seems that this domain is not always strictly required for inhibition of apoptosis [84–87]. Overexpression of XIAP, cIAP1, cIAP2 and survivin has been demonstrated to suppress apoptosis [reviewed in [83]]. The cellular function of the IAP family members cIAP1, cIAP2 and XIAP is largely unclear. These proteins seem to be involved in pathogenesis of small-cell lung cancer [88], non-small cell lung cancer [89], myeloid leukemia cells [90] and prostrate cancer [91]. Concerning pancreatic cancer, there are only few data on the involvement of cIAP1, cIAP2 and XIAP in the pathogenesis of this type of cancer. Our own results indicate increased levels of cIAP1, cIAP2 and XIAP in pancreatic cancer cell lines highly resistant to Fas- and TRAIL-induced apoptosis (A. Trauzold, unpublished data). Therefore, changes of expression of cIAP1, cIAP2 and XIAP may also have a role in the pathogenesis also in pancreatic cancer.
Survivin
Survivin has been identified as a new member of the inhibitor of apoptosis (IAP) family. Survivin is characterized by a unique structure that discriminates it from all other members of the IAP family. It contains only a single BIR repeat and lacks a carboxy terminal RING finger domain. Survivin is expressed in the G2/M phase of the cell cycle in a cycle-regulated manner [92]. It directly binds to and inhibits both caspase-3 and caspase-7 activity leading to arrest of apoptosis [93].
Survivin expression is not detectable in differentiated normal adult cells of any organ [94] but it is highly expressed in a wide range of cancer tissues [95] including neuroblastoma [96], colorectal [97] and stomach [98] carcinoma. It has been demonstrated recently that survivin is also frequently expressed in malignant pancreatic ductal tumors [99]. Because survivin is a potent caspase-inhibitor, its overexpression in cancer cells is implicated in the resistance to different apoptotic stimuli including chemotherapy. Molecular manipulation of survivin expression may enhance chemotherapy and radiation therapy not only in pancreatic cancer.
SMAC/DIABLO
IAPs are inhibited by a protein named SMAC/DIABLO (Second mitochondria-derived activator of caspase/direct IAP binding protein with low pI) [100, 101]. SMAC/DIABLO is synthesized as a precursor protein and is imported into mitochondria [100]. Upon cellular stress, SMAC/DIABLO is released into the cytosol. In the cytosol it promotes cell death by preventing IAP inhibition of caspases [102]. Like cytochrome c, release of SMAC/DIABLO from the mitochondria is inhibitable by Bcl-2 [100]. SMAC/DIABLO could have a therapeutic application in enhancing the effect of chemotherapeutics by binding IAPs that are overexpressed in a variety of carcinoma cells including pancreatic cancer. Recently it has been shown that SMAC/DIABLO fusion peptide was able to enhance apoptosis induced by diverse anticancer agents including paclitaxel, etoposide and others in MCF-7 breast cancer cells [103].
Growth Factors
Growth factor independence is a hallmark of tumors and is known to give cells a selective growth advantage [104]. Mutations of tumor suppressor genes and oncogenes lead to a loss of regulation in the expression and/or activation of both growth factors and their receptors [105]. Human pancreatic cancer cells are reported to produce multiple growth factors including insulin-like growth factor (IGF) and the receptors IGF-1 receptor (IGF-1R) and IGF-2 receptor, epidermal growth factor (EGF), EGF receptor (EGFR) and ErbB2, transforming growth factor-α (TGF-α), and transforming growth factor-β (TGF-β), fibroblast growth factor (FGF), vascular endothelial growth factors (VEGF) and their receptors [106–115].
Insulin-like growth factor (IGF)
Insulin-like growth factors (IGFs), including IGF-1 and IGF-2, are mitogenic peptides involved in the regulation of cell proliferation, differentiation and apoptosis [116, 117]. Exogenous IGF-1 increased cell proliferation and activated MAPK and AKT signaling pathways in pancreatic cell lines [118, 119]. IGF-2 might function in the switch from the quiescent to the proliferative state [120]. In multiple myeloma cells IGF-1 stimulates sustained activation of NF-κB and AKT and upregulates a series of anti-apoptotic proteins including FLIP, survivin, cIAP-2 and XIAP [121].
IGF-1R shows the highest binding affinity for IGF-1, although IGF-2 can also bind and activate the receptor [117]. IGF-1R as well as IGF-1 and IGF-2 have been found to be upregulated in pancreatic cancer [122]. This leads to down-modulation of apoptosis and survival of cancer cells through the PI3K/AKT pathway.
Epidermal growth factor (EGF)
Epidermal growth factor (EGF) stimulates proliferation and differentiation in a wide range of cell types through its corresponding receptor, EGFR. The EGFR gene is overexpressed in the majority of pancreatic ductal adenocarcinomas and human pancreatic cancer cell lines [123]. EGFR binds EGF and TGF-α with high affinity, and both ligands are overexpressed in pancreatic cancer [123]. Additionally, the overexpression of both the EGF receptor and its ligand in human pancreatic cancer is associated with enhanced tumor aggressiveness and shorter survival following tumor resection [124].
Treatment of pancreatic cancer cell lines with Erbitux (IMC-C225) anti-EGFR antibody in combination with gemcitabine and radiation resulted in complete regression or growth inhibition of cancer cells in a preclinical setting [125]. This combinatory treatment shows potential clinical application in the treatment of pancreatic cancer in humans.
Vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF)
Vascular endothelial growth factor (VEGF) is a potent and specific angiogenic factor. VEGF is known to be a key player for tumor growth [126, 127]. Fibroblast growth factors (FGFs) are mitogenic polypeptides that signal via FGF receptors (FGFRs). Both certain FGFs and VEGF and their receptors are overexpressed in cancer cells including pancreatic cancer cells [111, 112]. A soluble receptor for VEGF (sVEGF) functions as a dominant-negative inhibitor of receptor suppresses tumor angiogenesis and enhances apoptosis of cancer cells [128]. This effect was increased by combined use of sVEGF and a soluble FGF receptor (sFGFR1) in lung and pancreatic cancer cell lines [129].
Transforming growth factor alpha (TGF-α)
TGF-α is structurally and functionally related to members of the EGF family [130]. Numerous human solid tumors express high levels of TGF-α [131]. Recent work has shown that overexpression of TGF-α in liver has a dramatic protective effect on Fas-mediated apoptosis in TGF-α transformed mice. Mice with TGF-α overexpression in the pancreas develop ductal pancreatic cancer [132]. Interestingly, expression of Bcl-xL, an anti-apoptotic protein, was greatly increased in these transgenic mice and may participate in protective role of TGF-α [133]. This may contribute to apoptosis resistance in pancreatic cancer cells.
Transforming growth factor beta (TGF-β)
TGF-β is a multifunctional cytokine regulating a broad spectrum of cellular activity including cell-cycle control, differentiation, and apoptosis [reviewed in [134]]. TGF-β is a factor mediating growth inhibition and induction of apoptosis [134, 135]. TGF-β is involved in many events of apoptotic pathways. It cooperates with Fas [136] and TGF-α [137], down-regulates the anti-apoptotic proteins Bcl-xL and Bcl-2 and activates caspase-1, -3, -8 and -9 [138, 139]. Another mechanism of TGF-β-induced apoptosis has been proposed following the finding that TGF-β down-regulates NF-κB activity by the induction of the expression of IκB α, a specific inhibitor of NF-κB [140]. TGF-β initiates its cellular response by activation of specific downstream intracellular effectors termed SMAD proteins [reviewed in [141]] Escape from TGF-β/SMAD-induced apoptosis is frequently observed in tumors. Mutations and changes of expression levels of TGF-β and SMAD proteins could be observed also in pancreatic cancer tissues [142, 143]. For example, SMAD 4, a tumor suppressor in the TGF-β signaling pathway is genetically inactivated in about 55% of all pancreatic adenocarcinomas. Patients with pancreatic adenocarcinomas with SMAD4 expression live significantly longer [144].
Other factors and signaling pathways
Nuclear factor of κB (NF-κB)
Nuclear factor κB (NFκB) is a sequence-specific transcription factor that is involved in many cellular activities including the inflammatory and immune response. NF-κB also inhibits apoptosis by activation of several anti-apoptotic proteins [[145], reviewed in [146]] like cIAPs, FLIPs and the Bcl-2 family member Bcl-xL [147]. Normally, NF-κB remains as a heterodimeric complex consisting of p50, p65, and is kept by IκBα in an inactive state in the cytoplasm. A variety of stimuli like cytokines, pathogens, carcinogens or stress can lead to degradation of IκBα and p50–p65 heterodimer is translocated to the nucleus, binds the DNA at the promoter region and activates anti-apoptotic genes [148]. It has been demonstrated recently that NF-κB contributes to apoptosis resistance in different cancers including pancreatic cancer [38, 148]. This suggests that NF-κB is an ideal target for chemotherapeutic therapies of cancer. Inhibition of NF-κB by Gliotoxin, MG132 or Sulfasalazine [149] sensitizes pancreatic cancer cells to apoptosis induced by etoposide (VP16) or doxorubicin. Furthermore, inhibition of NF-κB by food-derived polyphenols like quercitin decreased pancreatic cancer growth and prevented metastasis [150]. Quercitin decreased primary tumor growth, increased apoptosis by mitochondrial depolarization, cytochrome c release, caspase 3 activation, and inhibition of NF-κB activation.
PKCμ
Protein kinases control and modulate a variety of cellular activities. Members of the protein kinase C (PKC) family have been shown to be involved in cell proliferation and differentiation [151]. Screening of expression pattern of PKC isoforms pointed out that the expression of PKCμ correlates with the resistance to Fas-mediated apoptosis in different pancreatic cancer cell lines [40]. Cell lines highly resistant to Fas-mediated apoptosis show high PKCμ expression levels [40]. It has been demonstrated that PKCμ regulates apoptosis in several cancer cells including pancreatic carcinoma cells [152, 153]. Overexpression of PKCμ reduces the sensitivity of the cells to apoptosis induced by TNF [153]. This effect correlates with an enhanced expression of anti-apoptotic proteins like cIAP2, TRAF1, cFLIP and survivin as well as increased expression level of NF-κB dependent genes [152–154].
Survival signaling through PI3K/AKT
Survival signals like growth factors, cytokines and hormones activate phosphatidylinositol 3-kinase (PI3K) [155]. Subsequently, PI3K activates AKT/PKB [156] that interferes with the apoptotic machinery. Activated AKT/PKB mediate cell survival via the regulation of numerous apoptotic relevant proteins such as the Bcl-2 family members BAD and Bcl-xL and the transcription factor NF-κB [157, 158]. Survival signaling by AKT is counteracted by PTEN that antagonizes the action of PI3K. PI3K and AKT are overexpressed in a variety of cancers [159, 160]. In addition, PTEN is frequently deleted in advanced tumors [161, 162]. These alterations lead to a 'constitutively active' survival-signaling pathway that enhances the insensitivity of tumor cells to apoptosis induction. Additionally, EGFR directly influences PI3K [163]. Because PI3K mediates survival signals, EGFR overexpression leads to a decrease in the apoptotic response and therefore a stronger survival of cancer cells. Recent work has shown that the IGF-1R suppresses apoptosis by signaling through PI3K and AKT [120]. Activated AKT in turn phosphorylates the intracellular transducer, Bad, which modulates the activity of the apoptosis suppressors Bcl-2 and Bcl-xL [164]. Additionally, AKT can directly phosphorylate caspase-9, which leads to inactivation of caspase-9 [165]. Strategies are pursued that aim to block the enzymatic activity of PI3K and PKB/AKT, in order to prevent inactivation of pro-apoptotic Bad. Wortmannin was shown to be a potent inhibitor of PI3K [160]. In several cancers including non-small-cell lung cancer [160] and pancreatic cancer [166], treatment of cells with wortmannin leads to inhibition of proliferation and increased apoptosis. Additionally, wortmannin enhanced gemcitabine-induced apoptosis in human pancreatic cells in vitro [167] and in vivo [168]. In contrast, studies investigating different pancreatic cancer cell lines pointed out that the PI3K/AKT pathway is not involved in gemcitabine-resistance [A. Arlt, unpublished data]. Neither did the basal AKT-activity correlate with the sensitivity towards gemcitabine treatment, nor did the inhibition of PI3K/AKT alter gemcitabine-induced apoptosis.
p53
The p53 pathway represents the molecular connection between the cell cycle and apoptosis. The p53 gene encodes a 53-kDa nuclear phosphoprotein. p53 inhibits cell growth through activation of cell cycle arrest and apoptosis [169]. The p53 gene is mutated in over 50% of human cancers. Pancreatic cell lines showed mutations in p53 at frequencies of 95% [170]. This enhances tumor development and progression. Currently, many therapeutic approaches were attempted to normalize p53 function in a variety of cancers including pancreatic cancer. Transfer of wild-type p53 induces apoptosis and produces significant growth suppression of pancreatic cancer in vitro and in vivo [170–172].
Conclusion
In the past, many efforts were made to cure patients from pancreatic cancer. Changes of expression and mutations of apoptotic proteins are common in pancreatic cancer cells and contribute to tumor development and progression. Consequently, pancreatic cancer has developed multiple resistance mechanisms to apoptosis. Many efforts to restore apoptosis and thereby reducing tumor growth were made with considerable success at least under preclinical conditions (Figure 3). Future therapies have to translate this knowledge into the clinic. They need to combine various therapeutic strategies and have to modulate selectively the sensitivity of pancreatic cancer cells to apoptosis without affecting normal cells.
Effects causing pancreatic cancer and possible therapeutic approaches. Multiple changes of apoptotic proteins contribute to pancreatic cancer development and progression. But many therapeutic approaches are developed to restore normal sensitivity to apoptotic stimuli and therefore to repress pancreatic cancer.
References
Parker SL, Tong T, Bolden S, Wingo PA: Cancer statistics. CA Cancer J Clin. 1997, 47: 5-27.
Coppola D: Molecular prognostic markers in pancreatic cancer. Cancer Contr. 2000, 7: 421-427.
von Bernstorff W, Spanjaard RA, Chan AK, Lockhart DC, Sadanaga N, Wood I, Peiper M, Goedegebuure PS, Eberlein TJ: Pancreatic cancer cells can evade immune surveillance via nonfunctional Fas (APO-1/CD95) receptors and aberrant expression of functional Fas ligand. Surgery. 1999, 125: 73-84.
Beger HG, Büchler MW, Friess H: Surgical results and indications for adjuvant measures in pancreatic cancer. Chirurg. 1994, 65: 246-252.
Guo X, Friess H, Graber HU, Kashiwagi M, Zimmermann A, Korc M, Büchler MW: KAI1 expression is up-regulated in early pancreatic cancer and decreased in the presence of metastases. Cancer Res. 1996, 56: 4876-4880.
Friess H, Büchler M, Krüger M, Beger HG: Treatment of duct carcinoma of the pancreas with the LH-RH-analogue buserelin. Pancreas. 1992, 7: 516-521.
Andren-Sandberg A, Bäckman PL, Andersson R: Results of adjuvant therapy in resected pancreatic cancer. Int J Pancreatol. 1997, 21: 31-38.
Palmer KR, Kerr M, Knowles G, Cull A, Carter DC, Leonard RC: Chemotherapy prolongs survival in inoperable pancreatic carcinoma. Br J Surg. 1994, 81: 882-885.
von Bernstorff W, Voss M, Freichel S, Schmid A, Vogel I, Johnk C, Henne-Bruns D, Kremer B, Kalthoff H: Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001, 7: 925s-932s.
Ungefroren H, Voss M, Jansen M, Roeder C, Henne-Bruns D, Kremer B, Kalthoff H: Human pancreatic adenocarcinomas express Fas and Fas ligand yet are resistant to Fas-mediated apoptosis. Cancer Res. 1998, 58: 1741-1749.
Igney FH, Krammer PH: Death and anti-death: Tumour resistance to apoptosis. Nature Rev Cancer. 2002, 2: 277-288.
Miyashita T, Reed JC: Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993, 81: 151-157.
Korsmeyer SJ: Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992, 80: 879-886.
Hager JH, Hanahan D: Tumor cells utilize multiple pathways to down-modulate apoptosis. Lessons from a mouse model of islet cell carcinogenesis. Ann N Y Acad Sci. 1999, 887: 150-163.
Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S: The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991, 66: 233-243.
Suda T, Takahashi T, Golstein P, Nagata S: Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993, 75: 1169-1178.
Krammer PH, Galle PR, Moller P, Debatin KM: CD95 (APO-1/Fas)-mediated apoptosis in normal and malignant liver, colon, and hematopoietic cells. Adv Cancer Res. 1998, 75: 251-273.
Sprick MR, Weigand MA, Rieser E, Rauch CT, Juo P, Blenis J, Krammer PH, Walczak H: FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000, 12: 599-609.
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME: Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998, 17: 1675-1687.
Zamzami N, Kroemer G: The mitochondrion in apoptosis: how Pandora's box opens. Nature Rev Mol Cell Biol. 2001, 2: 67-71.
Martinou JC, Green DR: Breaking the mitochondrial barrier. Nature Rev Mol Cell Biol. 2001, 2: 63-67.
Rathmell JC, Thompson CB: The central effectors of cell death in the immune system. Annu Rev Immunol. 1999, 17: 781-828.
Nagata S: Apoptosis by death factor. Cell. 1997, 88: 355-365.
Wyllie AH: Apoptosis and carcinogenesis. Eur J Cell Biol. 1997, 73: 189-197.
Nagata N, Golstein P: The Fas death factor. Science. 1995, 267: 1449-1456.
Niehans GA, Brunner T, Frizelle SP, Liston JC, Salerno CT, Knapp DJ, Green DR, Kratzke RA: Human lung carcinomas express Fas ligand. Cancer Res. 1997, 57: 1007-1012.
Horie S, Kano M, Higashihara E, Moriyama N, Tanaka E, Hirose A, Kakizoe T, Kawabe K: Expression of Fas in renal cell carcinoma. Jpn J Clin Oncol. 1997, 27: 384-388.
von Reyher U, Strater J, Kittstein W, Gschwendt M, Krammer PH, Moller P: Colon carcinoma cells use different mechanisms to escape CD95-mediated apoptosis. Cancer Res. 1998, 58: 526-534.
Kornmann M, Ishiwata T, Kleeff J, Beger HG, Korc M: Fas and Fas-ligand expression in human pancreatic cancer. Ann Surg. 2000, 231: 368-379.
von Bernstorff W, Glickman JN, Odze RD, Farraye FA, Joo HG, Goedegebuure PS, Eberlein TJ: Fas (CD95/APO-1) and Fas ligand expression in normal pancreas and pancreatic tumors. Implications for immune privilege and immune escape. Cancer. 2002, 94: 2552-2560.
Snell V, Clodi K, Zhao S, Goodwin R, Thomas EK, Morris SW, Kadin ME, Cabanillas F, Andreeff M, Younes A: Activity of TNF-related apoptosis-inducing ligand (TRAIL) in haematological malignancies. Br J Haematol. 1997, 99: 618-624.
Hinz S, Trauzold A, Boenicke L, Sandberg C, Beckmann S, Bayer E, Walczak H, Kalthoff H, Ungefroren H: Bcl-xL protects pancreatic adenocarcinoma cells against CD95- and TRAIL-receptor-mediated apoptosis. Oncogene. 2000, 19: 5477-5486.
Matsuzaki H, Schmied BM, Ulrich A, Standop J, Schneider MB, Batra SK, Picha KS, Pour PM: Combination of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and actinomycin D induces apoptosis even in TRAIL-resistant human pancreatic cancer cells. Clin Cancer Res. 2001, 7: 407-414.
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH: Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999, 104: 155-162.
Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Biliar TR, Strom SC: Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000, 6: 564-567.
Sato T, Irie S, Kitada S, Reed JC: FAP-1: a protein tyrosine phosphatase that associates with Fas. Science. 1995, 268: 411-415.
Ungefroren H, Kruse ML, Trauzold A, Roeschmann S, Roeder C, Arlt A, Henne-Bruns D, Kalthoff H: FAP-1 in pancreatic cancer cells: functional and mechanistic studies on its inhibitory role in CD95-mediated apoptosis. J Cell Sci. 2001, 114: 2735-2746.
Trauzold A, Wermann H, Arlt A, Schütze S, Schäfer H, Oestern S, Roeder C, Ungefroren H, Lampe E, Heinrich M, Walczak H, Kalthoff H: CD95 and TRAIL receptor-mediated activation of protein kinase C and NF-κB contributes to apoptosis resistance in ductal pancreatic adenocarcinoma cells. Oncogene. 2001, 20: 4258-4269.
Adams JM, Cory S: The Bcl-2 protein family: Arbiter of cell survival. Science. 1998, 281: 1322-1326.
Kelekar A, Thompson CB: Bcl-2 family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998, 8: 324-330.
Green DR, Reed JC: Mitochondria and apoptosis. Science. 1998, 281: 1309-1312.
Gutierrez-Puente Y, Zapata-Benavides P, Tari AM, Lopez-Berestein G: Bcl-2 related antisense therapy. Semin Oncol. 2002, 29: 71-76.
Evans JD, Cornford PA, Dodson A, Greenhalf W, Foster CS, Neoptolemos JP: Detailed tissue expression of bcl-2, bax, bak and bcl-x in the normal human pancreas and in chronic pancreatitis, ampullary and pancreatic ductal adenocarcinomas. Pancreatology. 2001, 1: 254-262.
Campani D, Esposito I, Boggi U, Cecchetti D, Menicagli M, De Negri F, Colizzi L, Del Chiaro M, Mosca F, Fornaciari G, Bevilacqua G: Bcl-2 expression in pancreas development and pancreatic cancer progression. J Pathol. 2001, 194: 444-450.
Dias N, Stein CA: Potential roles of antisense oligonucleotides in cancer therapy. The example of Bcl-2 antisense oligonucleotides. Eur J Pharm Biopharm. 2002, 54: 263-269.
Ehlert JE, Kubbutat MH: Apoptosis and its relevance in cancer therapy. Onkologie. 2001, 24: 433-440.
Tsujimoto Y: Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria?. Genes Cells. 1998, 3: 697-707.
Hu Y, Benedict MA, Wu D, Inohara N, Nùnez G: Bcl-xL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc Natl Acad Sci U S A. 1998, 95: 4386-4391.
Xu Z, Friess H, Solioz M, Aebi S, Korc M, Kleeff J, Büchler MW: Bcl-x(L) antisense oligonucleotides induce apoptosis and increase sensitivity of pancreatic cancer cells to gemcitabine. Int J Cancer. 2001, 94: 268-274.
Shi X, Liu S, Kleeff J, Friess H, Büchler MW: Acquired resistance of pancreatic cancer cells towards 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology. 2002, 62: 354-362.
Friess H, Lu Z, Andrén-Sandberg A, Berberat P, Zimmermann A, Adler G, Schmid R, Büchler MW: Moderate activation of the apoptosis inhibitor Bcl-xL worsens the prognosis in pancreatic cancer. Ann Surg. 1998, 228: 780-787.
Hsu YT, Wolter KG, Youle RJ: Cytosol-to-membrane redistribution of Bax and Bcl-x(L) during apoptosis. Proc Natl Acad Sci U S A. 1997, 94: 3668-3672.
Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR: Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J Biol Chem. 1999, 274: 2225-2233.
Xu ZW, Friess H, Büchler MW, Solioz M: Overexpression of Bax sensitizes human pancreatic cancer cells to apoptosis induced by chemotherapeutic agents. Cancer Chemother Pharmacol. 2002, 49: 504-510.
Pirocanac EC, Nassirpour R, Yang M, Wang J, Nardin SR, Gu J, Fang B, Moossa AR, Hoffmann RM, Bouvet M: Bax-induction gene therapy of pancreatic cancer. J Surg Res. 2002, 106: 346-351.
Seki T, Ohba N, Makino R, Funatomi H, Mitamura K: Mechanism of growth-inhibitory effect of cisplatin on human pancreatic cancer cells and status of p53 gene. Anticancer Res. 2001, 21: 1919-1924.
Graber HU, Friess H, Zimmermann A, Korc M, Adler G, Schmid R, Büchler MW: Bak expression and cell death occur in peritumorous tissue but not in pancreatic cancer cells. J Gastrointest Surg. 1999, 3: 74-80.
Adachi M, Imai K: The proapoptotic BH3-only protein BAD transduces cell death signals independently of its interaction with Bcl-2. Cell Death and Differ. 2002, 9: 1240-1247.
Baell JB, Huang DC: Prospects for targeting the Bcl-2 family of proteins to develop novel cytotoxic drugs. Biochem Pharmacol. 2002, 64: 851-863.
Ding XZ, Tong WG, Adrian TE: Cyclooxygenases and lipoxygenases as potential targets for treatment of pancreatic cancer. Pancreatology. 2001, 1: 291-299.
Tong WG, Ding XZ, Adrian TE: The mechanisms of lipoxygenase inhibitor-induced apoptosis in human breast cancer cells. Biochem Biophys Res Commun. 2002, 296: 942-948.
Turk B, Stoka V, Rozman-Pungercar J, Cirman T, Droga-Mazovec G, Oreic K, Turk V: Apoptotic pathways: involvement of lysosomal proteases. Biol Chem. 2002, 383: 1035-1044.
Cryns V, Yuan J: Proteases to die for. Genes Dev. 1998, 12: 1551-1570.
Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT: Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997, 278: 294-298.
Takahashi A, Alnemri ES, Lazebnik YA, Fernandes-Alnemri T, Litwack G, RD Moir, Goldman RD, Poirier GG, Kaufmann SH, Earnshaw WC: Cleavage of lamin A by Mch2α but not CPP32: Multiple interleukin 1-β converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis. Proc Natl Acad Sci U S A. 1996, 93: 8395-8400.
Thornberry NA, Lazebnik Y: Caspases: enemies within. Science. 1998, 281: 1312-1316.
Boldin M, Goncharov TM, Goltsev YV, Wallach D: Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/Apo-1-and TNF receptor-induced cell death. Cell. 1996, 85: 803-815.
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X: Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997, 91: 479-489.
Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J: Inhibition of death receptor signals by cellular FLIP. Nature. 1997, 388: 190-195.
Krueger A, Baumann S, Krammer PH, Kirchhoff S: FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001, 21: 8247-8254.
Goltsev YV, Kovalenko AV, Arnold E, Varfolomeev EE, Brodianskii VM, Wallach D: CASH, a novel caspase homologue with death effector domains. J Biol Chem. 1997, 272: 19641-4.
Han DK, Chaudhary PM, Wright ME, Friedmann C, Trask BJ, Riedel RT, Baskin DG, Schwartz SM, Hood L: MRIT, a novel death-effector domain-containing protein, interacts with caspases and Bcl-xL and initiates cell death. Proc Natl Acad Sci U S A. 1997, 94: 11333-8.
Elnemr A, Ohta T, Yachie A, Kayahara M, Kitagawa H, Fujimura T, Ninomiya I, Fushida S, Nishimura GI, Shimizu K, Miwa K: Human pancreatic cancer cells disable function of Fas receptors at several levels in Fas signal transduction pathway. Int J Oncol. 2001, 18: 311-316.
Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovacsovics M, Tschopp J: The caspase-8 inhibitor FLIP promotes activation of NF-κB and Erk signaling pathways. Curr Biol. 2000, 10: 640-648.
Bharti AC, Aggarwal BB: Nuclear factor-κB and cancer: its role in prevention and therapy. Biochem Pharmacol. 2002, 64: 883-888.
Hyer ML, Sudarshan S, Kim Y, Reed JC, Dang JY, Schwartz JY, Norris JS: Downregulation of c-FLIP sensitizes DU145 prostate cancer cells to Fas-mediated apoptosis. Cancer Biol Ther. 2002, 1: 401-406.
Kim Y, Suh N, Sporn M, Reed JC: An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J Biol Chem. 2002, 277: 22320-22329.
Vernejoul F, Faure P, Benali N, Calise D, Tiraby G, Pradayrol L, Susini C, Buscail L: Antitumor effect of in vivo somatostatin receptor subtype 2 gene transfer in primary and metastatic pancreas cancer models. Cancer Res. 2002, 62: 6124-6131.
Chakrabarty S, Roy M, Hazra B, Bhattacharya RK: Induction of apoptosis in human cancer cell lines by diospyrin, a plant-derived bisnaphthoquinoid, and its synthetic derivates. Cancer Lett. 2002, 188: 85-93.
Wang W, Waters SJ, MacDonald JR, Roth C, Shentu S, Freeman J, VonHoff DD, Miller AR: Irofulven (6-hydroxymethylacylfulvene, MGI 114)-induced apoptosis in human pancreatic cancer cells is mediated by ERK and JNK kinases. Anticancer Res. 2002, 22: 559-564.
Birnbaum MJ, Clem RJ, Miller LK: An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifs. J Virol. 1994, 68: 2521-2528.
Crook NE, Clem RJ, Miller LK: An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol. 1993, 67: 2168-2174.
Deveraux QL, Reed JC: IAP family proteins-suppressors of apoptosis. Genes Dev. 1999, 13: 239-252.
Harvey AJ, Soliman H, Kaiser W, Miller LK: Anti- and pro-apoptotic activities of baculovirus and Drosophila IAPs in an insect cell line. Cell Death Differ. 1997, 4: 733-744.
Deveraux QL, Takahashi R, Salvesen GS, Reed JC: X-linked IAP is a direct inhibitor of cell death proteases. Nature. 1997, 388: 300-303.
Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, GS Salvesen, Reed JC: A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem. 1998, 273: 7787-7790.
Roy N, Mahadevan MS, McLean M, Shutler G, Yaraghi Z, Farahani R, Baird S, Besner-Johnson A, Lefebvre C, Kang X, Salih M, Aubry H, Tamai K, Guan X, Ioannou P, Crawford TO, de Jong PJ, Surh L, Ikeda J-E, Korneluk RG, MacKenzie A: The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell. 1995, 80: 167-178.
Ekedahl J, Joseph B, Grigoriev MY, Muller M, Magnusson C, Lewensohn R, Zhivotovsky B: Expression of inhibitor of apoptosis proteins in small- and non-small-cell lung carcinoma cells. Exp Cell Res. 2002, 279: 277-290.
Hofmann HS, Simm A, Hammer A, Silber RE, Bartling B: Expression of inhibitors of apoptosis (IAP) proteins in non-small cell lung cancer. J Cancer Res Clin Oncol. 2002, 128: 554-560.
Zhang J, Li Y, Shen B: Up-regulation of XIAP by M-CSF is associated with resistance of myeloid leukemia cells to apoptosis. Leukemia. 2002, 16: 2163-2165.
McEleny KR, Watson RW, Coffey RN, O'Neill AJ, Fitzpatrick JM: Inhibitors of apoptosis proteins in prostate cancer cell lines. Prostate. 2002, 51: 133-140.
Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC: Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998, 396: 580-583.
Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC: IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998, 58: 5315-5320.
Ambrosini G, Adida C, Altieri DC: A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997, 3: 917-921.
Yamamoto T, Tanigawa N: The role of survivin as a new target of diagnosis and treatment in human cancer. Med Electron Microsc. 2001, 34: 207-212.
Adida C, Berrebi D, Peuchmaur M, Reyes-Mugica M, Altieri DC: Anti-apoptosis gene, survivin, and prognosis of neuroblastoma. Lancet. 1998, 351: 882-883.
Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N: Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998, 58: 5071-5074.
Lu CD, Altieri DC, Tanigawa N: Expression of novel anti-apoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998, 58: 1808-1812.
Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T: Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001, 92: 271-278.
Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL: Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000, 102: 43-53.
Du C, Fang M, Li Y, Li L, Wang X: Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000, 102: 33-42.
Verhagen AM, Vaux DL: Cell death regulation by the mammalian IAP antagonist Diablo/Smac. Apoptosis. 2002, 7: 163-166.
Arnt CR, Chiorean MV, Heldebrant MP, Gores GJ, Kaufmann SH: Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP in situ. J Biol Chem. 2002, 277: 44236-44243.
Nowell PC: The clonal evolution of tumor cell populations. Science. 1976, 194: 23-28.
Porter AC, Vaillancourt RR: Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene. 1998, 17: 1343-1352.
Korc M, Chandrasekar B, Yamanaka Y, Friess H, Buchier M, Beger HG: Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the level of epidermal growth factor and transforming growth factor alpha. J Clin Invest. 1992, 90: 1352-1360.
Chen J, Liu TH: Expression of EGF, TGF-alpha, EGFR and c-erbB2 genes and their gene products in human pancreatic carcinoma cell lines. Zentralbl Pathol. 1994, 140: 265-270.
Freeman JW, Mattingly CA, Strodel WE: Increased tumorigenicity in human pancreatic cell line MIA PaCa-2 is associated with an aberrant regulation of an IGF-1 autocrine loop and lack of expression of the TGF-beta type RII receptor. J Cell Physiol. 1995, 165: 155-163.
Bergmann U, Funatomi H, Yokoyama M, Berger HG, Korc M: Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res. 1995, 55: 2007-2011.
Ishiwata T, Bergmann U, Kornmann M, Lopez M, Beger HG, Korc M: Altered expression of insulin-like growth factor II receptor in human pancreatic cancer. Pancreas. 1997, 15: 367-373.
Korc M: Role of growth factors in pancreatic cancer. Surg Oncol Clin N Am. 1998, 7: 25-41.
Arii S, Mori A, Uchida S, Fujimoto K, Shimada Y, Imamura M: Implication of vascular endothelial growth factor in the development and metastasis of human cancers. Hum Cell. 1999, 12: 25-30.
Kalthoff H, Roeder C, Gieseking J, Humburg I, Schmiegel W: Inverse regulation of human ERBB2 and epidermal growth factor receptors by tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1993, 90: 8972-8976.
Kalthoff H, Roeder C, Brockhaus M, Thiele HG, Schmiegel W: Tumor necrosis factor (TNF) up-regulates the expression of p75 but not p55 TNF receptors, and both receptors mediate, independently of each other, up-regulation of transforming growth factor alpha and epidermal growth factor receptor mRNA. J Biol Chem. 1993, 268: 2762-2766.
Schmiegel W, Roeder C, Schmielau J, Rodeck U, Kalthoff H: Tumor necrosis factor alpha induces the expression of transforming growth factor alpha and the epidermal growth factor receptor in human pancreatic cancer cells. Proc Natl Acad Sci U S A. 1993, 90: 863-867.
Grothey A, Voigt W, Schober C, Muller T, Dempke W, Schmoll HJ: The role of insulin-like growth factor I and its receptor in cell growth, transformation, apoptosis, and chemoresistance in solid tumors. J Cancer Res Clin Oncol. 1999, 125: 166-173.
Prisco M, Romano G, Peruzzi F, Valentinis B, Baserga R: Insulin and IGF-I receptors signaling in protection from apoptosis. Horm Metab Res. 1999, 31: 80-89.
Yao Z, Okabayashi Y, Yutsudo Y, Kitamura T, Ogawa W, Kasuga M: Role of Akt in growth and survival of PANC-1 pancreatic cancer cells. Pancreas. 2002, 24: 42-46.
DeBosch BJ, Deo BK, Kumagai AK: Insulin-like growth factor-1 effects on bovine retinal endothelial cell glucose transport: role of MAP kinase. J Neurochem. 2002, 81: 728-734.
Nair PN, De Armond DT, Adamo ML, Strodel WE, Freeman JW: Aberrant expression and activation of insulin-like growth factor-1 receptor (IGF-1R) are mediated by an induction of IGF-1R promoter activity and stabilization of IGF-1R mRNA and contributes to growth factor independence and increased survival of the pancreatic cancer cell line MIA PaCa-2. Oncogene. 2001, 20: 8203-8214.
Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, Hideshima T, Treon SP, Munshi NC, Richardson PG, Anderson KC: Activation of NF-κB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002, 21: 5673-5683.
Lopez T, Hanahan D: Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002, 1: 339-353.
Ghaneh P, Kawesha A, Evans JD, Neoptolemos JP: Topics: Pancreatic cancer-new horizons in diagnosis and treatment: Molecular prognostic markers in pancreatic cancer. J Hepatobiliary Pancreat Surg. 2002, 9: 1-11.
Shi X, Friess H, Kleeff J, Ozawa F, Büchler MW: Pancreatic cancer: factors regulating tumor development, maintenance and metastasis. Pancreatology. 2001, 1: 517-524.
Buchsbaum DJ, Bonner JA, Grizzle WE, Stackhouse MA, Carpenter M, Hicklin DJ, Bohlen P, Raisch KP: Treatment of pancreatic cancer xenografts with Erbitux (IMC-C225) anti-EGFR antibody, gemcitabine, and radiation. Int J Radiat Oncol Biol Phys. 2002, 54: 1180-1193.
Verheul HM, Pinedo HM: The role of vascular endothelial growth factor (VEGF) in tumor angiogenesis and early clinical development of VEGF-receptor kinase inhibitors. Clin Breast Cancer. 2000, 1 (Suppl 1): S80-84.
Sipos B, Weber D, Ungefroren H, Kalthoff H, Zühlsdorff A, Luther C, Török V, Klöppel G: Vascular endothelial growth factor mediated angiogenic potential of pancreatic ductal carcinomas enhanced by hypoxia: An in vitro and in vivo study. Int J Cancer. 2002, 102: 592-600.
Takayama K, Ueno H, Nakanishi Y, Sakamoto T, Inoue K, Shimizu K, Oohashi H, Hara N: Suppression of tumor angiogenesis and growth by gene transfer of a soluble form of vascular endothelial growth factor receptor into a remote organ. Cancer Res. 2000, 60: 2169-2177.
Ogawa T, Takayama K, Takakura N, Kitano S, Ueno H: Anti-tumor angiogenesis therapy using soluble receptors: enhanced inhibition of tumor growth when soluble fibroblast growth factor receptor-1 is used with soluble vascular endothelial growth factor receptor. Cancer Gene Ther. 2002, 9: 633-640.
Derynck R: Transforming growth factor alpha. Cell. 1988, 54: 593-595.
Webber EM, FitzGerald MJ, Brown PI, Bartlett MH, Fausto N: Transforming growth factor-alpha expression during liver regeneration after partial hepatectomy and toxic injury, and potential interactions between transforming growth factor-alpha and hepatocyte growth factor. Hepatology. 1993, 18: 1422-1431.
Greten FR, Wagner M, Weber CK, Zechner U, Adler G, Schmid RM: TGF alpha transgenic mice. A model of pancreatic cancer development. Pancreatology. 2001, 1: 363-368.
Kanda D, Takagi H, Toyoda M, Horiguchi N, Nakajima H, Otsuka T, Mori M: Transforming growth factor alpha protects against Fas-mediated liver apoptosis in mice. FEBS Lett. 2002, 519: 11-15.
Schuster N, Kreiglstein K: Mechanisms of TGF-β-mediated apoptosis. Cell Tissue Res. 2002, 307: 1-14.
Voss M, Wolff B, Savitskaia N, Ungefroren H, Deppert W, Schmiegel W, Kalthoff H, Naumann M: TGFbeta-induced growth inhibition involves cell cycle inhibitor p21 and pRb independent from p15 expression. Int J Oncol. 1999, 14: 93-101.
Ashley DM, Kong FM, Bigner DD, Hale LP: Endogenous expression of transforming growth factor beta1 inhibits growth and tumorigenicity and enhances Fas-mediated apoptosis in a murine high-grade glioma model. Cancer Res. 1998, 58: 302-309.
Tobin SW, Brown MK, Douville K, Payne DC, Eastman A, Arrick BA: Inhibition of transforming growth factor beta signaling in MCF-7 cells results in resistance to tumor necrosis factor alpha: the role for Bcl-2. Cell Growth Differ. 2001, 12: 109-117.
Shima Y, Nakao K, Nakashima T, Kawakami A, Nakata K, Hamasaki K, Kato Y, Eguchi K, Ishii N: Activation of caspase-8 in transforming growth factor-beta-induced apoptosis of human hepatoma cells. Hepatology. 1999, 30: 1215-1222.
Foghi A, Teerds KJ, van der Donk H, Moore NC, Dorrington J: Induction of apoptosis in thecal/interstitial cells: action of transforming growth factor (TGF) alpha plus TGF beta on bcl-2 and interleukin-1 beta-converting enzyme. J Endocrinol. 1998, 157: 489-494.
Arsura M, Wu M, Sonenshein GE: TGF beta 1 inhibits NF-κB/Rel activity inducing apoptosis of B cells: transcriptional activation of I kappa B alpha. Immunity. 1996, 5: 31-40.
Dijke PT, Goumans M-J, Itoh F, Itoh S: Regulation of cell proliferation by Smad proteins. J Cell Phys. 2002, 191: 1-16.
Antonello D, Moore PS, Zamboni G, Falconi M, Scarpa A: Absence of mutations in the transforming growth factor-beta inducible early gene 1, TIEG1, in pancreatic cancer. Cancer Lett. 2002, 183: 179-183.
Maurice D, Pierreux CE, Howell M, Wilentz RE, Owen MJ, Hill CS: Loss of Smad4 function in pancreatic tumors: C-terminal truncation leads to decreased stability. J Biol Chem. 2001, 276: 43175-43181.
Tascilar M, Skinner HG, Rosty C, Sohn T, Wilentz RE, Offerhaus GJ, Adsay V, Abrams RA, Cameron JL, Kern SE, Yeo CJ, Hruban RH, Goggins M: The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001, 7: 4115-4121.
Beg AA, Baltimore D: An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996, 274: 782-784.
Karin M, Cao Y, Greten FR, Li ZW: NF-κB in cancer: from innocent bystander to major culprit. Nature Rev Cancer. 2002, 2: 301-310.
Karin M, Lin A: NF-κB at the crossroads of life and death. Nature Immunol. 2002, 3: 221-227.
Bharti AC, Aggarwal BB: Nuclear factor-κB and cancer: its role in prevention and therapy. Biochem Pharmacol. 2002, 64: 883-888.
Arlt A, Vorndamm J, Breitenbroich M, Folsch UR, Kalthoff H, Schmidt WE, Schäfer H: Inhibition of NF-κB sensitizes human pancreatic carcinoma cells to apoptosis induced by etoposide (VP16) or doxorubicin. Oncogene. 2001, 20: 859-868.
Mouria M, Gukovskaya AS, Jung Y, Büchler P, OJ Hines, HA Reber, Pandol SJ: Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome c release and apoptosis. Int J Cancer. 2002, 98: 761-769.
Ron D, Kazanietz MG: New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J. 1999, 13: 1658-1676.
Trauzold A, Schmiedel S, Sipos B, Wermann H, Westphal S, Roeder C, Klapper W, Arlt A, Lehnert L, Ungefroren H, Johannes F-J, Kalthoff H: Overexpression of PKCμ regulates apoptosis and proliferation in pancreatic tumour cells. Submitted.
van Lint J, Rykx A, Maeda Y, Vantus T, Sturany S, Malhorta V, Vandenheede JR, Seufferlein T: Protein kinase D: an intracellular traffic regulator on the move. Trends Cell Biol. 2002, 12: 193-200.
Johannes F-J, Horn J, Link G, Haas E, Siemienski K, Wajant H, Pfizenmaier K: Protein kinase Cμ dowregulation of tumor-necrosis-factor-induced apoptosis correlates with enhanced expression of nuclear-factor-κB-dependent protective genes. Eur J Biochem. 1998, 257: 47-54.
Datta SR, Brunet A, Greenberg ME: Cellular survival: a play in three Akts. Genes Dev. 1999, 13: 2905-2927.
Burgering BM, Coffer PJ: Protein kinase B (c-Akt) in phosphoinositol-3-OH kinase signal transduction. Nature. 1995, 376: 599-602.
Graff JR, Konicek BW, McNulty AM, Wang Z, Houck K, Allen S, Paul JD, Hbaiu A, Goode RG, Sandusky GE, Vessella RL, Neubauer BL: Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J Biol Chem. 2000, 275: 24500-24505.
Jones RG, Parsons M, Bonnard M, Chan VS, Yeh WC, Woodgett JR, Ohashi PS: Protein kinase B regulates T lymphocyte survival, nuclear factor κB activation, and Bcl-X(L) levels in vivo. J Exp Med. 2000, 191: 1721-1734.
Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, Tsichlis PN, Testa JR: AKT2, a putative oncogene encoding a number of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci U S A. 1992, 89: 9267-9271.
Boehle AS, Kurdow R, Boenicke L, Schniewind B, Faendrich F, Dohrmann P, Kalthoff H: Wortmannin inhibits growth of human non-small-cell lung cancer in vitro and in vivo. Langenbecks Arch Surg. 2002, 387: 234-239.
Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R: Mutation of Pten/Mmac 1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci U S A. 1999, 96: 1563-1568.
Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A, Khoo W, Fukumoto M, Mak TW: High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998, 8: 1169-1178.
Matsuda K, Idezawa T, You XJ, Kothari NH, Fan H, Korc M: Multiple mitogenic pathways in pancreatic cancer cells are blocked by a truncated epidermal growth factor receptor. Cancer Res. 2002, 62: 5611-5617.
Gilmore AP, Valentijn AJ, Wang P, Ranger AM, Bundred N, O'Hare MJ, Wakeling A, Korsmeyer SJ, Streuli CH: Activation of BAD by therapeutic inhibition of epidermal growth factor receptor and transactivation by insulin-like growth factor receptor. J Biol Chem. 2002, 277: 27643-27650.
Kermer P, Ankerhold R, Klocker N, Krajewski S, Reed JC, Bahr M: Caspase-9: involvement in secondary death of axotomized rat retinal ganglion cells in vivo. Brain Res Mol Brain Res. 2000, 85: 144-150.
Ng SWS, Tsao M-S, Nicklee T, Hedley DW: Wortmannin inhibits PKB/Akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human cancer xenografts in immunodeficient mice. Clin Cancer Res. 2001, 7: 3269-3275.
Ng SSW, Tsao MS, Chow S, Hedley DW: Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000, 60: 5451-5455.
Ng SS, Tsao MS, Nicklee T, Hedley DW: Wortmannin inhibits pkb/akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human pancreatic cancer xenografts in immunodeficient mice. Clin Cancer Res. 2001, 7: 3269-3275.
Deppert W, Gohler T, Koga H, Kim E: Mutant p53: "gain of function" through perturbation of nuclear structure and function?. J Cell Biochem Suppl. 2000, 35: 115-122.
Moore PS, Sipos B, Orlandini S, Sorio C, Real FX, Lemoine NR, Gress T, Bassi C, Klöppel G, Kalthoff H, Ungefroren H, Lohr M, Scarpa A: Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001, 439: 798-802.
Ghaneh P, Greenhalf W, Humphreys M, Wilson D, Zumstein L, Lemoine NR, Neoptolemos JP: Adenovirus-mediated transfer of p53 and p16 (INK4a) results in pancreatic cancer regression in vitro and in vivo. Gene Ther. 2001, 8: 199-208.
Cascallo M, Calbo J, Gelpi JL, Mazo A: Modulation of drug cytotoxicity by reintroduction of wild-type p53 gene (Ad5CMV-p53) in human pancreatic cancer. Cancer Gene Ther. 2000, 7: 545-556.
Acknowledgments
This work was supported by grants of DFG (SFB 415/A 3) and IZKF/C 6. We thank A. Trauzold and H. Ungefroren for carefully reading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contribution
SW conducted literature review and wrote this article. HK supported this work by critical reading the manuscript and supervised the final editing. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Westphal, S., Kalthoff, H. Apoptosis: Targets in Pancreatic Cancer. Mol Cancer 2, 6 (2003). https://doi.org/10.1186/1476-4598-2-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-4598-2-6