Abstract
Background
The hypoxia-inducible factor (HIF) transcription complex, which is activated by low oxygen tension, controls a diverse range of cellular processes including angiogenesis and erythropoiesis. Under normoxic conditions, the α subunit of HIF is rapidly degraded in a manner dependent on hydroxylation of two conserved proline residues at positions 402 and 564 in HIF-1α in the oxygen-dependent degradation (ODD) domain. This allows subsequent recognition by the von Hippel-Lindau (VHL) tumor suppressor protein, which targets HIF for degradation by the ubiquitin-proteasome pathway. Under hypoxic conditions, prolyl hydroxylation of HIF is inhibited, allowing it to escape VHL-mediated degradation. The transcriptional regulation of the erythropoietin gene by HIF raises the possibility that HIF may play a role in disorders of erythropoiesis, such as idiopathic erythrocytosis (IE).
Results
Patients with IE were screened for changes in the HIF-1 α coding sequence, and a change in the ODD domain that converts Pro-582 to Ser was identified in several patients. This same change, however, was also detected at a significant frequency, 0.073, in unaffected controls compared to 0.109 in the IE patient group. In vitro hydroxylation assays examining this amino acid change failed to reveal a discernible effect on HIF hydroxylation at Pro-564.
Conclusion
The Pro582Ser change represents a common polymorphism of HIF-1α that does not impair HIF-1α prolyl hydroxylation. Although the Pro582Ser polymorphism is located in the ODD domain of HIF-1α it does not diminish the association of HIF-1α with VHL. Thus, it is unlikely that this polymorphism accounts for the erythrocytosis in the group of IE patients studied.
Similar content being viewed by others
Background
Hypoxia-inducible factor (HIF) is the master regulator of a wide range of cellular functions including glycolysis, erythropoiesis and angiogenesis [1, 2]. HIF is a dimeric transcription complex composed of an α subunit (either HIF-1α, HIF-2α, or HIF-3α) and a β subunit (also known as ARNT), and it binds to target genes containing hypoxia response elements (HRE) in mammalian cells [3, 4]. There is an expanding list of HIF target genes that are involved in a diverse range of cellular processes that include angiogenesis, cell survival and proliferation, energy and glucose metabolism, apoptosis, and erythropoiesis [3].
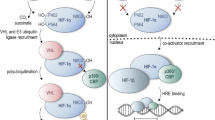
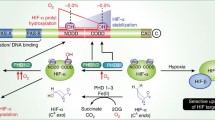
Both subunits of HIF are constitutively expressed, but under normoxic conditions, the α subunit of the complex is also constitutively hydroxylated on two conserved proline residues (Pro-402 and Pro-564 in the case of human HIF-1α) [5–8]. This prolyl hydroxylation, catalyzed by a family of intracellular prolyl hydroxylases designated PHD/HPH [9, 10], allows recognition of the α subunits by the von Hippel-Lindau tumor suppressor protein (VHL) [11], a component of an E3 ubiquitin ligase complex that targets these subunits for degradation by the ubiquitin-proteasome pathway [4, 12]. This complex also includes Elongin C, Elongin B, Cullin2, and Rbx1 [13]. Thus, under normoxic conditions, HIF-1α (and the other α subunits) is essentially undetectable. Under hypoxic conditions, HIF-1α prolyl hydroxylation, which is dependent on molecular oxygen, is inhibited, thereby allowing HIF-1α to escape VHL-mediated degradation. This therefore provides a framework for understanding how oxygen tension can be directly coupled to HIF activation.
Knowledge of the regulation of HIF has important implications for understanding, and potentially treating, ischemic cardiac and cerebrovascular disease. Moreover, HIF plays a critical role in cancer [14–16], as HIF-induced angiogensis is instrumental in promoting cell growth in solid tumors and metastasis [17]. Elevated HIF expression occurs under pathophysiologic conditions such as in the hypoxic cores of enlarging solid tumors. HIF levels can also be augmented by cancer-inducing mutations in oncogenes such as p53, Src, and most notably, VHL [12]. VHL mutations occur in the context of the von Hippel-Lindau syndrome, a tumor predisposition syndrome in which a germline mutation is followed by somatic mutation of the remaining allele, thereby allowing the development of the classic triad of renal cell carcinoma, pheochomocytoma, and hemangioblastoma, in conformance with Knudsen's two hit hypothesis. Importantly, bi-allelic VHL mutations also occur in the majority of sporadic clear cell carcinomas of the kidney. Most, but not all, of these mutations impair or abolish the capacity of VHL to serve as an E3 ubiquitin ligase for HIF [18, 19], consistent with the central importance of this pathway in the pathogenesis of these tumors.
The critical importance of HIF in the transcriptional regulation of the erythropoietin (Epo) also raises the possibility of involvement of the HIF-VHL pathway in disorders of erythropoiesis, such as primary erythrocytosis. This disorder is characterized by over production of red cells and inappropriately normal or elevated Epo levels. Indeed, in patients with autosomal recessive Chuvash polycythemia [20–22], an Arg200Trp mutation of VHL impairs its ability to polyubiquitinate HIF [23], thereby providing an explanation for elevated Epo levels. In the present studies, we screened the HIF-1α gene for base changes in patients with idiopathic erythrocytosis. Sequencing the VHL binding region of HIF-1 α detected one patient who was homozygous for a Pro582Ser polymorphism and an additional eight patients heterozygous for the same polymorphism. However, screening of a group of normal controls detected the same Pro582Ser polymorphism, and functional studies failed to reveal a defect in HIF-1α hydroxylation. Collectively, these studies identify Pro582Ser as a common polymorphism of HIF-1α that does not appear to affect either HIF-1α hydroxylation or subsequent recognition by VHL.
Results and Discussion
We sequenced exon 12 of HIF-1 α from a group of 55 patients, all of whom had raised or inappropriately normal Epo levels. Sequencing of the Epo receptor (EpoR) gene from these patients as previously described [24] failed to reveal any mutations. With the exception noted below, none of the patients displayed mutations in VHL [22]. From this group, we detected a heterozygous C to T transition at nucleotide 1744 of HIF-1α in 8 individuals (compare Figure 1A with 1B). An additional individual was homozygous for the same base change (Figure 1C), and both his parents were heterozygous. This individual was heterozygous for the Arg200Trp VHL mutation (C598T) seen in Chuvash polycythemia, and clinical features of this patient, D1, have been described in a previous report [22]. The C1744T polymorphism in the HIF-1 α gene abolishes the restriction site for Tsp 45I enzyme (Figure 1D), and a group of 48 caucasian normal anonymised controls showing an equal male/female distribution were screened employing this diagnostic site. Seven were also found to have lost this site (in 6 of these, the C1744T polymorphism was directly confirmed by sequencing), thus giving a predicted frequency for this polymorphism of 0.073 in the normal group compared to 0.109 for the erythrocytosis patients. Sequencing of exons 9 and 15 of HIF-1 α, which includes Pro402 and Asn803, failed to detect any further base changes in this group of patients. It might be noted that of nine patients that we previously reported as having Chuvash polycythemia [22], three of these were found to be heterozygous for the C1744T polymorphism in HIF-1 α.
Detection of C1744T polymorphism. Sequencing of exon 12 of the HIF-1α detected a heterozygous base change of C to T at nucleotide 1744 in 8 patients (Figure 1B) when compared to the normal sequence (Figure 1A). One patient, D1, was homozygous for this base change (Figure 1C). The position of the mutation is indicated by an arrow. Bases are as follows: G = black; A = green; T = red; C = blue. The C1744T mutation destroys the restriction site for Tsp 45I enzyme and the normal exon 12 PCR product is restricted in the presence of this enzyme into 4 fragments of 213, 156, 139 and 92 bp; in the presence of the C1744T mutation the 156 and 139 bp fragments are replaced with one of 295 bp. (Figure 1D). M1: 100 bp DNA ladder; M2: 25 bp ladder; 1: C1744 control individual; 2: heterozygous individual for C1744T polymorphism; 3: individual D1 homozygous for C1744T polymorphism.
The C1744T polymorphism predicts an amino acid change of proline to serine at codon 582 (Pro582Ser). This residue is of potential interest because it is embedded in a Phe-Asp-Gln-Leu-Ser-Pro motif (where Pro is Pro-582), which bears homology to the Leu-Xaa-Xaa-Leu-Ala-Pro motif (where Xaa is any amino acid and Pro is the hydroxylacceptor proline) present at the previously identified sites of hydroxylation at Pro-402 and Pro-564. Of relevance here is the fact that mutations can be tolerated at all positions of this motif with the exception of the hydroxylacceptor proline [25]; hence, it is conceivable that the proline (582) itself may be a hydroxylation site. It may also be noted that this proline is conserved in all mammalian HIF-1α proteins for which sequence information is available, but is not present in either HIF-2α or HIF-3α, or in HIF-1α from Xenopus laevis [26].
To test the possibility that Pro-582 itself might be a site of hydroxylation by PHD, we first prepared proteins corresponding to HIF-1α (531–575) or HIF-1α (569–589) fused to GST, and then immobilized these on GSH-agarose. The bound protein was then treated with PHD2, washed, and incubated with 35S-labelled VHL to assess the extent of prolyl hydroxylation. As shown in Fig. 2A, under conditions where PHD2 hydroxylates the HIF-1α (531–575) peptide (lane 3), which contains the primary site of hydroxylation (Pro-564), we found no evidence that it hydroxylates the HIF-1α (569–589) peptide (lane 4), at least in a manner which permits subsequent recognition by VHL.
Effects of the Pro582Ser polymorphism on HIF-1α hydroxylation. (A) GST pulldown assay. 5 μg of recombinant (His)6FlagPHD2 were incubated with 3 μg of either GST, GST-HIF-1α (531–575), or GST-HIF-1α (569–589) prebound to GSH agarose under hydroxylation conditions, as indicated. The resins were washed, and were subsequently incubated with 35S-labeled, in vitro translated HA-VHL. Bound VHL was eluted and then subjected to SDS-PAGE and autoradiography. The position of HA-VHL is indicated. "In" designates 10% of the input HA-VHL. One of two representative results is shown. (B) VBC pulldown assay. 35S labeled, in vitro translated wild type or Pro582Ser GAL4-HIF-1α (531–652) was incubated with 1 μg of (His)6-PHD2 for the indicated times. Hydroxylated reaction products were then isolated by first incubating with (His)6-Flag-VBC, and then by immunoprecipitation with anti-Flag antibodies coupled to agarose. The immunoprecipitated reaction products were subjected to SDS-PAGE and autoradiography. "In" designates 10% of the substrate. One of three representative results is shown. (C) GST pulldown assay. 1.5 μg of recombinant (His)6PHD2 was incubated with 5 μg of either GST, GST-HIF-1α (531–652), or GST-HIF-1α (531–652) Pro582Ser prebound to GSH agarose under hydroxylation conditions, as indicated. The resins were washed, and were subsequently incubated with 35S-labeled, in vitro translated FlagVHL Arg200Trp. Bound VHL was eluted and then subjected to SDS-PAGE and autoradiography. The position of VHL Arg200Trp is indicated. "In" designates 10% of the input VHL Arg200Trp. One of two representative results is shown.
While Pro-582 may not itself be a site of prolyl hydroxylation, it is in the vicinity of Pro-564, which is the primary site of prolyl hydroxylation in HIF-1α. Hence, the Pro582Ser substitution might affect HIF-1α hydroxylation at Pro-564. To examine this, we incubated 35S-labelled wild type or Pro582Ser GAL4-HIF-1α (531–652) with PHD2. Hydroxylated substrate was then isolated by incubation with VHL-elonginB-elongin C (VBC) followed by immunoprecipitation of the latter [10, 25]. As shown in Fig. 2B, both wild type and Pro582Ser GAL4-HIF-1α (531–652) are hydroxylated in a similar time-dependent fashion by PHD2 (compare lanes 2–5 with lanes 7–10). Thus, the Pro582Ser polymorphism does not adversely affect the in vitro hydroxylation at Pro-564 by PHD. This conclusion is consistent with binding studies that have shown that the tight interaction of HIF-1α ODD peptides containing Hyp-564 to VHL does not require this residue [26, 27]. It is also consistent with the structure of HIF-1α (549–582) Pro564Hyp cocrystallized with VHL, in which Pro-582, unlike Hyp-564, was not visualized [26].
Finally, it is possible that the Pro582Ser polymorphism in HIF-1α might only manifest a functional change in the context of other mutations, such as the Arg200Trp mutation in the VHL protein. To test this, we hydroxylated WT or Pro582Ser GST-HIF-1α (531–652) with PHD2 and tested the hydroxylated protein for interaction with VHL Arg200Trp. As shown in Fig. 2C, this mutant VHL binds to hydroxylated forms of both WT and Pro582Ser GST-HIF-1α (531–652) (lanes 3 and 4, respectively). Collectively, these results provide evidence that the Pro582Ser polymorphism does not interfere with recognition by Hyp-564 HIF-1α by either WT (Fig. 2B) or Arg200Trp (Fig. 2C) VHL. The latter observation indicates that this polymorphism is unlikely to account for the erythrocytosis seen in the patient mentioned above who was heterozygous for the Arg200Trp mutation in VHL.
It has been recently reported that human hormone-refractory prostate cancers possess mutations in the ODD of HIF-1α [28]. One mutation that was identified turned out, in fact, to be the Pro582Ser mutation examined here. A second mutation was an Glu591Gly mutation. Neighboring normal tissue possessed wild type HIF-1α sequence [28]. A previous report also documented the occurrence of the Pro582Ser polymorphism in both renal cell carcinomas and normal tissue [29]. While we cannot rule out the formal possibility that the Pro582Ser polymorphism observed in the current studies or the previous report might affect HIF-1α transcriptional activity, or HIF-1α stability in the context of the full length protein, the results presented here indicate that it does not affect either hydroxylation at Pro-564 or its subsequent recognition by VHL.
Conclusions
The C1744T transition is present in patients with IE but is also a common polymorphism which is predicted to result in Pro582Ser change in the HIF-1α amino acid sequence. While this residue resides in the ODD domain of HIF-1α, in vitro studies fail to reveal any impairment in hydroxylation or subsequent recognition by VHL. Hence, we conclude that the erythrocytosis observed in the patients of the present study is likely due to other mechanisms.
Methods
Patients
Idiopathic erythrocytosis patients from the UK and Ireland with elevated hemoglobin (Hb) (greater than 18 g/dL in males and 16.5 g/dL in females), raised packed cell volume (PCV) (above 0.51 in males and 0.48 in females), with no splenomegaly and the absence of known secondary causes were recruited to our registry. Approval was obtained from Queen's University, Belfast, Research Ethics Committee for these studies and the use of the anonymised controls and informed consent was provided according to the Declaration of Helsinki.
Sequencing exons 9, 12 and 15 of the HIF-1α gene
Genomic DNA was isolated from peripheral blood using a Nucleon BACC 1 DNA extraction kit (Nucleon Biosciences, Manchester, UK). PCR was performed in a 100 μL reaction containing 10 % DMSO, 1.5 mmol/L MgCl2, 200 μmol/L dNTP, 0.5 μmol/L each of forward and reverse primer, 2.5 units of Thermo-Start DNA Polymerase (ABgene, Epsom, Surrey, UK) and 100 ng genomic DNA, in 1× Thermo-Start Standard Buffer provided by the manufacturer. The following primer sets were used: HIF EX9F 5'-AAATCTTGAAATGTTCCTGTCCA-3' and HIF EX9R 5'-GGGGCAAAAATATTTAAGCAAA-3' for exon 9; HIF EX12F 5'-TTGCTGAAGACACAGAAGCAA-3' and HIF EX12R 5'-TTGACTCAAAGCGACAGATAACA-3' for exon 12; HIF EX15F 5'-CCTAACACATTGTGGGTGTTT-3' and HIF EX15R 5'-AGAAAATGAGCTGTCTGTGATCC-3' for exon 15. Amplification was performed with an initial heat activation step of 15 min at 95°C followed by 35 cycles of 95°C for 1 min, 55°C for 1 min and 72°C for 1 min in the GeneAmp PCR system 2700 (Applied Biosystems, Warrington, UK). PCR products were purified using Concert Rapid PCR Purification System (Life Technologies, Paisley, UK) and were sequenced using ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction Kit Version 3 on ABI 3100 DNA Genetic Analyzer (Applied Biosystems).
Mutation screen for C1744T base change
The C1744T polymorphism abolishes the restriction site for Tsp 45I enzyme; thus, 16 μL of PCR-amplified exon 12 were digested with 10 U of Tsp 45I (New England Biolabs, Hitchin, UK) for 2 hours at 37°C and examined by agarose electrophoresis.
Plasmids
pcDNA3-GAL4-HIF-1α (531–652) Pro582Ser was prepared by QuikChange mutagenesis using pcDNA3-GAL4-HIF-1α (531–652) [25] as a template and the following two oligonucleotides: 5'-CCTTCGATCAGTTGTCAAGCTTAGAAAGCAGTTCCGC-3' and 5'-GCGGAACTGCTTTCTAAGCTTGACAACTGATCGAAGG-3'. pGEX-HIF-1α (569–589) was constructed by subcloning into the BamH I/Xho I site of pGEX-5X-1 a duplex comprised of the following two oligonucleotides:
5'-GATCCATGATGATGACTTCCAGTTAAGATCTTTCGATCAGTTGTCACCATTAGAGAGCTCTTCCGCAAGCTAATCTAGAC-3' and
5'-TCGAGTCTAGATTAGCTTGCGGAAGAGCTCTCTAATGGTGACAACTGATCGAAAGATCTTAACTGGAAGTCATCATCATG-3'. pGEX-HIF-1α (531–652) was constructed by standard recombinant DNA methods. pGEX-HIF-1α (531–652) Pro582Ser was constructed by subcloning the 0.4 kb Eco RI/Spe I fragment of pcDNA3-GAL4-HIF-1α (531–652) Pro582Ser into the Eco RI/Spe I site of pGEX-HIF-1α (531–652). pcDNA3-FlagVHL Arg200Trp was prepared by QuikChange mutagenesis using pcDNA3-FlagVHL [30] as a template and the following two oligonucleotides:
5'-CAAATGTGCAGAAAGACCTCGAGTGGCTGACACAGGAGCGC-3' and 5'-GCGCTCCTGTGTCAGCCACTCGAGGTCTTTCTGCACATTTG-3'.
Where appropriate, sequences of recombinant plasmids were verified using a Big Dye Terminator kit and an ABI automated sequencer. The sources of pGEX-HIF-1α (531–575) and pcDNA3-HA-VHL have been described [25, 30].
Proteins
(His)6PHD2, (His)6Flag-PHD2, and (His)6Flag-VBC were purified from baculovirus-infected Sf9 cells as described [25]. GST, GST-HIF-1α (531–575), GST-HIF-1α (569–589), GST-HIF-1α (531–652), and GST-HIF-1α (531–652) Pro582Ser were purified from E. coli transformed with the appropriate plasmids by affinity chromatography on GSH-agarose [31].
In vitro prolyl hydroxylation assays
GST pulldown assays
The assay was performed in two steps. First, GST, GST-HIF-1α (531–575), GST-HIF-1α (569–589), GST-HIF-1α (531–652), or GST-HIF-1α (531–652) Pro582Ser immobilized on 10 μl of GSH-agarose was incubated with recombinant PHD2 in 20 μl of buffer A (20 mM Hepes, pH 7.9, 100 mM KCl, 1 mM DTT) supplemented with 100 μM FeCl2, 1 mM 2-oxoglutarate, 5 mM ascorbate, and 0.2 μg/μl bovine liver catalase (Sigma) for 1 hr at 30°C. The resins were washed once with buffer A, and then incubated with 5 μl of 35S-labelled, in vitro translated WT or Arg200Trp VHL (prepared using pcDNA3-HA-VHL or pcDNA3-FlagVHL Arg200Trp as templates, respectively, and a TnTQuick T7 reticulocyte lysate kit) in 500 μl of buffer A supplemented with 0.2% NP-40 and 0.2% BSA with rocking for 1 hr at 4°C. The resins were washed three times with buffer A supplemented with 0.2% NP-40, eluted with 2× SDS-PAGE loading buffer followed by heating at 100°C for 3 min, and the eluates then subjected to SDS-PAGE and autoradiography.
VBC pulldown assays
VBC pulldown assays using (His)6PHD2 were performed essentially as described [25], except that 1 mM DTT was substituted for 1.4 mM β-mercaptoethanol and bovine liver catalase was included in the hydroxylation reactions at a final concentration of 0.3 μg/μl.
References
Semenza GL: HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001, 13: 167-171. 10.1016/S0955-0674(00)00194-0
Maxwell P: Oxygen homeostasis and cancer: insights from a rare disease. Clinical Medicine. 2002, 2: 356-362.
Semenza GL: Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends in Molecular Medicine. 2001, 7: 345-350. 10.1016/S1471-4914(01)02090-1
Ratcliffe PJ: From erythropoietin to oxygen: hypoxia-inducible factor hydroxylases and the hypoxia signal pathway. Blood Purification. 2002, 20: 445-450. 10.1159/000065201
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG: HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001, 292: 464-8.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim Av, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ: Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001, 292: 468-72.
Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ: Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001, 20: 5197-206. 10.1093/emboj/20.18.5197
Yu F, White SB, Zhao Q, Lee FS: HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci U S A. 2001, 98: 9630-5. 10.1073/pnas.181341498
Bruick RK, McKnight SL: A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001, 294: 1337-40. 10.1126/science.1066373
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ: C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001, 107: 43-54.
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ: The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999, 399: 271-5. 10.1038/20459
Huang LE, Bunn HF: Hypoxia-inducible factor and its biomedical relevance. J Biol Chem. 2003, 278: 19575-8. 10.1074/jbc.R200030200
Kim W, Kaelin WG: The von Hippel-Lindau tumor suppressor protein: new insights into oxygen sensing and cancer. Curr Opin Genet Dev. 2003, 13: 55-60. 10.1016/S0959-437X(02)00010-2
Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin G: Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002, 1: 237-246. 10.1016/S1535-6108(02)00043-0
Mandriota SJ, Turner JJ, Davies DR, Murray PG, Morgan NV, Swoter HM, Wykoff CC, Maher ER, Harris AL, Ratcliffe PJ, Maxwell PH: HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppression function in the nephron. Cancer Cell. 2002, 1: 459-468. 10.1016/S1535-6108(02)00071-5
Maranchie JK, Vasselli JR, Riss J, Bonifaciono JS, Linehan WM, Klausner RD: The contribution of VHL substrate binding and HIF-1 alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell. 2002, 1: 247-255. 10.1016/S1535-6108(02)00044-2
Melnyk O, Zimmerman M, Kim KJ, Shuman M: Neutralizing anti-vascular endothelial growth factor antibody inhibits further growth of established prostate cancer and metastases in a pre-clinical model. J Urol. 1999, 161: 960-963. 10.1097/00005392-199903000-00071
Clifford SC, Cockman ME, Smallwood AC, Mole DR, Woodward ER, Maxwell PH, Ratcliffe PJ, Maher ER: Contrasting effects on HIF-1alpha regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel-Lindau disease. Hum Mol Genet. 2001, 10: 1029-38. 10.1093/hmg/10.10.1029
Hoffman MA, Ohh M, Yang H, Klco JM, Ivan M, Kaelin WG: von Hippel-Lindau protein mutants linked to type 2C VHL disease preserve the ability to downregulate HIF. Hum Mol Genet. 2001, 10: 1019-27. 10.1093/hmg/10.10.1019
Ang SO, Chen H, Gordeuk VR, Sergueeva AI, Polyakova LA, Miasnikova GY, Kralovics R, Stockton DW, Prchal JT: Endemic polycythemia in Russia: Mutation in the VHL gene. Blood Cells Molecules and Diseases. 2002, 28: 57-62. 10.1006/bcmd.2002.0488.
Pastore YD, Jelinek J, Ang SO, Guan Y, Lui E, Jedlickova K, Krishnamurti L, Prchal JT: Mutations in the VHL gene in sporadic apparently congenital polycythemia. Blood. 2003, 101: 1591-1595. 10.1182/blood-2002-06-1843
Percy MJ, McMullin MF, Jowitt SN, Potter M, Treacy M, Watson WH, Lappin TRJ: Chuvash-type congenital polycythemia in 4 families of Asian and Western European ancestry. Blood. 2003, 102: 1097-1099. 10.1182/blood-2002-10-3246
Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Lui E, Sergueeva AI, Miasnikova GY, Mole D, Maxwell P, Stockton DW, Semena GL, Prchal JT: Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nature Genetics. 2002, 32: 614-21. 10.1038/ng1019
Percy MJ, McMullin MF, Roques A, Westwood N, Acharya J, Hughes AE, Lappin TRJ, Pearson TM: Erythrocytosis due to a mutation in erythropoietin receptor. British Journal of Haematology. 1998, 100: 407-410.
Huang J, Zhao Q, Mooney SM, Lee FS: Sequence determinants in hypoxia-inducible factor-1alpha for hydroxylation by the prolyl hydroxylases PHD1, PHD2, and PHD3. J Biol Chem. 2002, 277: 39792-800. 10.1074/jbc.M206955200
Hon W-C, Wilson MJ, Harlos K, Claridge TDW, Schofield CJ, Pugh CW, Maxwell PH, Ratcliffe PJ, Stuart DI, Jones EY: Structural basis for the recognition of hydroxyproline in HIF-1α by pVHL. Nature. 2002, 417: 975-978. 10.1038/nature00767
Min JH, Yang H, Ivan M, Gertler F, Kaelin WG, Pavletich NP: Structure of an HIF-1alpha-pVHL complex: hydroxyproline recognition in signaling. Science. 2002, 296: 1886-9. 10.1126/science.1073440
Anastasiadis AG, Ghafer MA, Salamon L, Vacherot F, Benedit P, Chen M-W, Shabsigh A, Burchardt M, Chopin DK, Shabsigh R, Buttyan R: Human hormone-refractory prostate cancers can harbor mutations in the O2-dependent degradation domain of hypoxia inducible factor-1α (HIF-1α). J Cancer Res Clin Oncol. 2002, 128: 358-362. 10.1007/s00432-002-0346-1
Clifford SC, Astuti D, Hooper L, Maxwell PH, Ratcliffe PJ, Maher ER: The pVHL-associated SCF ubiquitin ligase complex: molecular genetic analysis of elongin B and C, Rbx1 and HIF-1 alpha in renal cell carcinoma. Oncogene. 2001, 20: 5067-5074. 10.1038/sj.onc.1204602
Yu F, White SB, Zhao Q, Lee FS: Dynamic, Site-Specific Interaction of Hypoxia Inducible Factor-1α with the von Hippel-Lindau Tumor Suppressor Protein. Cancer Res. 2001, 61: 4136-4142.
Smith DB, Johnson KS: Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S transferase. Gene. 1988, 67: 31-40. 10.1016/0378-1119(88)90005-4
Acknowledgements
We wish to thank all the clinicians that have referred erythrocytosis patients from UK and Ireland to our registry and provided patient samples. We thank Dr. Quan Zhao for preparing the pGEX-HIF-1α (531–652) Pro582Ser construct.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
MJP conceived and participated in the design of the study, performed analysis and drafted the manuscript. SMM and AF participated in the design, execution, and interpretation of the in vitro prolyl hydroxylation assays. MFM participated in the design of the study and collected patient data and samples. TRJL participated in the conception and design of the experiments. FSL coordinated the in vitro prolyl hydroxylation assays and participated in the interpretation of the data. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Percy, M.J., Mooney, S.M., McMullin, M.F. et al. A common polymorphism in the oxygen-dependent degradation (ODD) domain of hypoxia inducible factor-1α (HIF-1α) does not impair Pro-564 hydroxylation. Mol Cancer 2, 31 (2003). https://doi.org/10.1186/1476-4598-2-31
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-4598-2-31