Abstract
Background
Transforming growth factor β1 (TGFβ1) is a potent inhibitor of epithelial cell growth, thus playing an important role in tissue homeostasis. Most carcinoma cells exhibit a reduced sensitivity for TGFβ1 mediated growth inhibition, suggesting TGFβ1 participation in the development of these cancers. The tumor suppresor gene DPC4/SMAD4, which is frequently inactivated in carcinoma cells, has been described as a key player in TGFβ1 mediated growth inhibition. However, some carcinoma cells lacking functional SMAD4 are sensitive to TGFβ1 induced growth inhibition, thus requiring a SMAD4 independent TGFβ1 pathway.
Results
Here we report that mature TGFβ1 is a ligand for the integrin αVβ6, independent of the common integrin binding sequence motif RGD. After TGFβ1 binds to αVβ6 integrin, different signaling proteins are activated in TGFβ1-sensitive carcinoma cells, but not in cells that are insensitive to TGFβ1. Among others, interaction of TGFβ1 with the αVβ6 integrin resulted in an upregulation of the cell cycle inhibitors p21/WAF1 and p27 leading to growth inhibition in SMAD4 deleted as well as in SMAD4 wildtype carcinoma cells.
Conclusions
Our data provide support for the existence of an alternate TGFβ1 signaling pathway that is independent of the known SMAD pathway. This alternate pathway involves αVβ6 integrin and the Ras/MAP kinase pathway and does not employ an RGD motif in TGFβ1-sensitive tumor cells. The combined action of these two pathways seems to be necessary to elicit a complete TGFβ1 signal.
Similar content being viewed by others
Background
The normal function of transforming growth factor β1 (TGFβ1) is essential for the entire organism, representing a multifunctional regulator of cell growth and differentiation [1–5]. TGFβ1 is a potent inhibitor of epithelial cell proliferation. Upon binding of TGFβ1, TGFβ1-receptors phosphorylate SMAD2 or SMAD3 [6–12]. Phosphorylated SMAD2/3 associates with SMAD4 and, as a complex, moves into the nucleus, where it regulates gene expression [13–15].
SMAD4 (DPC4) is essential for this TGFβ1 signaling and transcriptional activation process [16]. In epithelial cells, TGFβ1 decreases c-myc, cdc2 and cyclin D1 expression, and it increases the expression of c-jun and c-fos [17–23]. Activation of the TGFβ1 signal pathway in epithelial cells leads to an increased expression of the cell cycle inhibitors p21WAF1 and p15Ink4b and to a release of formerly sequestered p27KIP [24–26]. It is assumed that the cooperative action of these cell cycle inhibitors results in the growth arrest mentioned above, although p15Ink4b does not seem to be necessary in this regard. In addition to mutations in the TGFβ1-receptors, in a large number of carcinomas disruptions of this signaling pathway by the alteration of a single protein such as p15Ink4b, p16, and p21Waf1 are found [2, 27–39]. This may result in resistance to the growth-inhibiting action of TGFβ1.
In several cell lines, particularly in pancreatic carcinoma cells, resistance to TGFβ1 could be attributed to a loss of function of the SMAD4 (DPC4) protein [40–43]. However, the pancreatic carcinoma cell line BxPC-3, although homozygously deleted for SMAD4, is growth inhibited by TGFβ1 [30, 44]. It is thus speculated that alternative signaling pathways in addition to the SMAD pathway may exist.
After binding to αVβ6 integrin, latent TGFβ1 is activated by processing of latent TGFβ1 by cleavage of the latency-associated Peptide (LAP) [45–57]. Recently, the interaction of latent TGFβ1 with αVβ6 integrin has been shown [45]. After binding of latent TGFβ1 to αVβ6 integrin, latent TGFβ1 is activated by cleavage of the latency-associated peptide (LAP) [45]. This αVβ6 integrin is also expressed by pancreatic carcinoma cells [58–63]. We hypothesized that there is a SMAD-independent TGFβ1 signaling pathway in TGFβ1-sensitive carcinoma cells. To address this question, several carcinoma cell lines with different degrees of TGFβ1 sensitivity were chosen as a model system. We investigated the interaction of TGFβ1 with the αVβ6 integrin and its influence on selected target genes known to be involved in cell cycle-regulated growth inhibition. Here, we demonstrate an alternate TGFβ1 signaling pathway via αVβ6 integrin contributing to TGFβ1 growth inhibiton in TGFβ1 sensitive carcinoma cells.
Results
Mature TGFβ1 induces cytoskeletal immobilization of proteins and tyrosine phosphorylation via integrin αVβ6 only in TGFβ1 sensitive cells
Only integrins that have bound their ligands are anchored to the cytoskeleton [64, 65]. In our experiments, mature TGFβ1, αVβ6 integrin, and F-actin colocalize (Figure 1), suggesting association with and activation of this integrin. To further support this finding, we stimulated cells and performed co-immunoprecipitated various integrin subunits of cytoskeletal anchored proteins [66, 67] (additional file 1, 2, 3 and 4). Our data strongly suggest that mature TGFβ1 associates with αVβ6 integrin (additional file 1, 2, 3 and 4).
Colocalization of TGFβ 1 , α V β 6 integrin and the cytoskeleton. Panc-1 cells were stimulated with mature TGFβ1 and stained using anti TGFβ1 (labeled with goat anti-rabbit IgG conjugate, A-11046), αV/β6 (labeled with goat anti-rabbit IgG conjugate, A-11046) and Actin antibodies. Magnification 1000×.
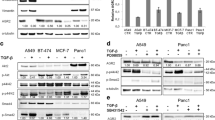
To determine whether binding of mature TGFβ1 leads to integrin-mediated signaling, we looked at the status of integrin-cytoskeleton-associated proteins [66, 67] after incubation with mature TGFβ1 in selected carcinoma cell lines with different degrees of sensitivity to TGFβ1 (Table 1). Cytoskeletal anchored proteins were precipitated with anti αV and β6-antibodies. Immobilization of proteins to the cytoskeleton (Triton-X insoluble fraction, Figure 2B) as well as tyrosine phosphorylation of these proteins (Figure 2A) induced through mature TGFβ1 was only seen in the TGFβ1-sensitive carcinoma cell lines (Figure 2 and additional file 5). Notably, tyrosine phosphorylation of cytoskeletally anchored proteins is further enhanced after combined treatment with mature TGFβ1 and fibronectin in TGFβ1 sensitive cells (Figure 3). In contrast, in the TGFβ1-resistant AsPC-1 and Capan-1 cells, the interaction of mature TGFβ1 with αVβ6 integrin resulted in an immobilization of high molecular weight proteins to the cytoskeleton without tyrosine phosphorylation (Figure 2). Again, stimulation of TGFβ1 sensitive cells BxPC-3, LoVo [68], SW48 [68], Keratinocytes, HeLa and DLD1 [69], results in an enhanced cytoskeletal immobilization and tyrosine phosphorylation of cellular proteins in response to stimulation with mature TGFβ1 (additional file 5). Remarkably, preincubation with the MEK1 inhibitor PD98059 resulted in a reduced cytoskeletal immobilization and tyrosine phosphorylation of cellular proteins in response to stimulation with mature TGFβ1. This finding is in agreement with other observations that MEK1-mediated signal transduction is involved in cytoskeletal remodeling and integrin engagement [70, 71].
Phosphorylation and immobilization of proteins associated with the integrin-cytoskeleton-complex. Cytoskeletally anchored αVβ6 was immunoprecipitated after TGFβ1 stimulation (10 nM for 10 minutes) followed by Western analysis with antibodies against tyrosine-phosphorylated proteins (A) or Western blotting after biotinylation of all proteins and streptavidin detection (B). Presence of TGFβ1 (C), αV and β6 integrin (D) in the co-precipitates is also demonstrated. TGFβ-receptor-I and II (TGFβRI and TGFβRII) are expressed at nearly equal levels in all cell lines as demonstrated by western blotting from whole cell extracts (E). In part the cells were preincubated with αV- and β6-antibodies (1:100 each for 30 min) or with a TGFβ antibody (15 μg/ml for 30 min).
Enhanced Tyrosine Phosphorylation of proteins associated with the integrin-cytoskeleton-complex. Cytoskeletally anchored αVβ6 was immunoprecipitated after TGFβ1 and/or fibronectin stimulation (10 nM for 10 minutes) followed by Western analysis with antibodies against tyrosine-phosphorylated proteins (A). Reprobing with αV and β6 antibodies show equal anounts of precipitates used (B).
Activation of p125FAK, a central step in integrin-associated signaling [72, 73], was determined to assess integrin-mediated signaling. BcPC-3 cells are sensitive to TGFβ1 but are SMAD4 deleted. We incubated BxPC-3 cells with mature TGFβ1 and observed an association on the cytoskeleton connected with integrin αVβ6 and activation of p125FAK (Figure 4). Indeed, TGFβ1 antibodies, cytochalasin D and BAPTA-AM [66] abolished the association on the cytoskeleton connected with integrin αVβ6 and activation of p125FAK. These data further suggest that TGFβ1 mediated activation of p125FAK depends on free intracellular calcium and an intact actin cytoskeleton.
In order to test whether TGFβ1 signaling via αVβ6 is specific for SMAD4 deleted BxPC-3 cells or if this is a general phenomenon, we investigated signaling in TGFβ1-sensitive carcinoma cell lines HeLa, MCF-7 and MDA-MB-231. TGFβ1 induced recruitment of p125Fak, p130Cas and Sos1/2 to the cytoskeleton. Enhanced expression of c-jun, c-fos, p21WAF1 and p27KIP, while downregulating PCNA, is dependent on ERK1/2 signaling, an intact cytoskeleton and intracellular calcium (Figures 5, 6A, 7, 8 and additional files 6, 7 and 8). We also confirmed the purity of the commercially available mature TGFβ1 used in these experiments by silver stained non-reducing SDS-PAGE, with latent TGFβ1 as control (Figure 6B). We also demonstrated the SMAD4 deficiency of the BxPC-3 cells used (Figure 6C).
p125FAK activation by mature TGFβ 1 via integrin α V β 6 . Stimulation of BxPC-3 with mature TGFβ1 (10 nM for 10 minutes), immunoprecipitation with αV- and β6 integrin antibodies after preparation of the cytoskeleton, followed by probing with pp125Fak and p125FAK antibodies. In part the cells were preincubated with αV- and β6-antibodies (1:100 each for 30 min), with a TGFβ antibody (15 μg/ml for 30 min), cytochalasin D and BAPTA AM, respectively.
Cell cycle genes in response to TGFβ 1 . Western Blot analysis of HeLa cells stimulated with 10 nM of mature TGFβ1 for the time indicated. Cytoskeletally anchored proteins are differentially marked. In part the cells were preincubated with αV- and β6-antibodies (1:100 each for 30 min), with a TGFβ-RII antibody (15 μg/ml for 30 min), cytochalasin D, BAPTA AM and MEK1 inhibitor PD98059, respectively.
Enhanced level of cytoskeletal anchored proteins in response to TGFβ 1 (A). Western Blot analysis of BxPC-3 and HeLa cells as indicated after stimulation with TGFβ1 for the time indicated. Cytoskeletally anchored proteins are differentially marked. In part the cells were preincubated with αV- and β6-antibodies (1:100 each for 30 min), with a TGFβ-RII antibody (15 μg/ml for 30 min), cytochalasin D, BAPTA AM and MEK1 inhibitor PD98059, respectively. Purity of the TGFβ 1 used (B). Ten nanogram of mature TGFβ1 and latent TGFβ1 were subjected to non-reducing SDS-PAGE dollowed by silver staining. No latant TGFβ1 could be detected in the mature TGFβ1 used for stimulation. BxPC-3 cells are SMAD4-/-(C). One hundred microgram of whole cell extract from BxPC-3 and HeLa cells were probed with p125FAK and SMAD4 antibodies on the same membrane. As reported, BxPC-3 cells are found to be SMAD4-/-.
Cell cycle genes in response to TGFβ 1 . Western Blot analysis of MCF-7 and MDA-MB 231 cells as indicated after stimulation with TGFβ1 for the time indicated. Cytoskeletally anchored proteins are differentially marked. In part the cells were preincubated with αV- and β6-antibodies (1:100 each for 30 min), with a TGFβ-RII antibody (15 μg/ml for 30 min), cytochalasin D, BAPTA AM and MEK1 inhibitor PD98059, respectively.
Cell cycle genes in response to TGFβ 1 . Western Blot analysis of BxPC-3, LOVO, SW48 and DLD1 cells as indicated after stimulation with TGFβ1 for the time indicated. Cytoskeletally anchored proteins are differentially marked. In part the cells were preincubated with αV- and β6-antibodies (1:100 each for 30 min), with a TGFβ-RII antibody (15 μg/ml for 30 min), cytochalasin D, BAPTA AM and MEK1 inhibitor PD98059, respectively.
TGFβ1/αVβ6 integrin signaling is independent of the known TGFβ1 signaling pathway
To explain the TGFβ1 sensitivity of SMAD4-deleted cells, it is speculated that after binding of TGFβ1 to its receptor, activated SMAD2/3 may translocate to the nucleus and activate gene expression even in the absence of SMAD4. To exclude this possibility, cellular proteins were divided into cytoplasmatic and nuclear fractions after TGFβ1 stimulation, and localization and phosphorylation of SMAD2/3 were investigated. In the SMAD4 deleted BxPC-3 cells, TGFβ1 resulted in phosphorylation of SMAD2/3, but the activated SMAD proteins were retained in the cytoplasmatic fraction (Figure 9). Remarkably, in NP-9 cells [74], SMAD2/3 are translocated into the nucleus upon TGFβ1 stimulation (additional file 9(A)) but we could not observe an enhanced tyrosine phosphorylation of cytoskeletal anchored proteins (additional file 9(B)).
Activation and nuclear translocation of SMAD2/3 in response to TGFβ 1 (A). Nuclear and cytoplasmatic fraction of cellular proteins (BxPC-3) after stimulation with 10 nM of TGFβ1 for 10 minutes and Western blot analysis for SMAD2/3 and phosphorylated SMAD2/3. Purity of cytoplasmic and nuclear fraction (B). Cytoplasmic and nuclear extracts from K562 cells were probed with p125FAK, PCNA and Iκ Bα antibodies at the same time. As predicted, p125FAK cold exclusively be detected in the cytoplasmic extract, whereas PCNA is found in the nucleus. Iκ Bα served as loading control.
TGFβ1 mediated growth inhibition is dependent on αVβ6 integrin
Influence of TGFβ1 on cell growth is well established, but the mechanisms are not fully understood [75–79]. Here, we assayed for the possible synergistic function of αVβ6 integrin on mature TGFβ1 mediated growth inhibition in Panc-1 cells. As shown in the additional file 10, combined treatment with αV and β6 blocking antibodies almost completely abolished the effect of mature TGFβ1 on the growth of Panc-1 cells. We therefore postulate that the growth inhibition of TGFβ1 is synergistically influenced by αVβ6 integrin.
Discussion
A recent study demonstrated an interaction of latent TGFβ1 with αVβ6 integrin, which led to an activation of latent TGFβ1 [45]. Incubation of different tumor cells with mature TGFβ1 resulted in a direct binding of TGFβ1 to αVβ6 integrin. Certain integrins appear to be preferentially associated with specific growth factor receptors [80]. The interaction of these two receptor classes seems to take place via the actin cytoskeleton. We were able to exclude such signal pathway association, since in our cytoskeletal preparations, no TGFβ1-receptors were detectable, indicating that mature TGFβ1 is a ligand for αVβ6.
It has been reported that activated integrins are associated with the cytoskeleton. Here, we show that binding of mature TGFβ1 to αVβ6 integrin resulted in an association of the cytoskeleton (Figure 10). In a variety of integrin-mediated signaling pathways, tyrosinephosphorylation of proteins immobilized to the cytoskeleton is enhanced [66, 67]. The same was true in our experimental settings only for the TGFβ1-sensitive cells. This upregulation of tyrosine phosphorylation was inhibited by preincubation with a TGFβ1 neutralizing antibody or by blocking of αVβ6 integrin, thus again proving mature TGFβ1 as an initial signaling ligand for αVβ6.
Binding of mature TGFβ1 to αVβ6 integrin exerts several downstream effects in TGFβ1-sensitive cells (Figure 9). One is a marked phosphorylation of p125FAK. This phosphorylation is dependent on the integrity of the cytoskeleton, as disruption of actin filaments by cytochalasin D completely eliminated this effect, findings which have also been reported for several integrin signaling pathways [66, 67]. Moreover, incubation of the TGFβ1 sensitive carcinoma cells with TGFβ1 caused immobilization of the docking protein p130cas and of the guanine nucleotide exchange factor SOS to the cytoskeleton. Beyond this, a marked induction of the cell cycle inhibitors p21WAF1 and p27KIP and a decrease in PCNA expression was detectable.
Finally, TGFβ1 caused an activation of p21Ras and the MAP kinases ERK1 and ERK2. This TGFβ1-induced expression profile was not affected by preincubation of SMAD4 deleted BxPC-3 cells with a TGFβ1-RII blocking antibody, which was able to completely block TGFβ1-induced SMAD2/3 phosphorylation, thus demonstrating the independence of the TGFβ1-signaling from the known SMAD pathway in BxPC-3 cells. In contrast, preincubation with αV- and β6-blocking antibodies curbed the TGFβ1-induced regulation of p21/WAF1, p27, c-fos, and the p21Ras and ERK1/2 activation, verifying that the binding of TGFβ1 to the αVβ6 integrin is a prerequisite for the activation of the signal pathway via the αVβ6 integrin. Preincubation of the cells with the MEK1 inhibitor PD98059 curbed the TGFβ1-induced regulation of these genes as well, indicating the involvement of the MAP kinase pathway in TGFβ1 signaling in BxPC-3 cells. As shown recently, the growth-stimulatory effect of the TGFβ superfamily member BMP-2on CAPAN-1 cells was blocked by this inhibitor as well [81–83], supporting our findings.
Indeed, cytoskeletal immobilization of p130cas and SOS was not prevented by the MEK1 inhibitor PD 98059. Thus, these proteins are good candidates to link the integrin-mediated TGFβ1 signaling to the MAP kinase pathway, as was shown previously for signaling events induced by fluid stress or integrin mediated cell-adhesion in other cell types [71, 84–91].
In order to generalize the integrin mediated TGFβ1-pathway identified in the SMAD4 deleted pancreatic tumor cell line BxPC-3, we investigated TGFβ1 signaling in the cervical carcinoma cell line HeLa and the mammary carcinoma cell lines MCF-7 and MDA-MB-231, harboring a wildtype SMAD4-gene. TGFβ1 bound to αVβ6-integrin in these cells as well, and this interaction resulted both in an immobilization of p130Cas and SOS1/2 and in tyrosine phosphorylation of cytoskeleton-associated proteins such as p125FAK. TGFβ1 stimulation of these cells acvtivated p21Ras and MAPK ERK1/2, upregulated c-fos, c-jun/AP1, p21/WAF1 and p27 expression, and resulted a decrease of PCNA, similar to its actions in BxPC-3 cells. Preincubation with a TGFβ-RII blocking antibody attenuated the TGFβ1 induced pattern, contrary to SMAD 4 deleted BxPC-3 cells. This preincubation also decreased activation of p21Ras and of MAPK ERK1/2, indicating the participation of the Ras/MAPK-pathway in TGFβ1 induced transcriptional activation.
The same attenuation of TGFβ1 induced gene expression and the decrease in p21Ras and MAPK ERK1/2 activation was observable after preincubation of SMAD4 wildtype cells with αVβ6-blocking antibodies, demonstrating that TGFβ1 signaling via αVβ6-integrin also is linked to the Ras/MAPK-pathway, and that both pathways have synergistic effects in TGFβ1-signaling. Full TGFβ1 induced transcriptional activation is only reached if both pathways are completed. This finding is supported by the observation that activation of p21/Ras and MAPK ERK1/2 induced by TGFβ1 is only reverted to the control level by the combination of the TGFβ-RII blocking antibody and the αVβ6-blocking antibodies, or by inhibition of MEK1.
Linking of the TGFβ-R pathway to the Ras/MAPK pathway is dependent on a functional SMAD4 gene product, because TGFβ1 induced gene expression and activation of Ras and ERK1/2 is attenuated by the TGFβ-RII blocking antibody only in SMAD4 wild type cells, whereas in the SMAD4 deleted BxPC-3 cells, no such influence was observable.
Based on our results, we suggest the following model of TGFβ1-signaling, which offers an explanation for the different growth responses to TGFβ1 (Fig. 10). In the TGFβ1-sensitive cell lines with intact SMAD pathway, the TGFβ1 response can be attributed to both the common SMAD signaling pathway and the integrin pathway described above. In the cell line BxPC-3, lacking the SMAD4 gene product, the SMAD4-independent pathway can explain the TGFβ1 sensitivity via αVβ6 integrin, the cytoskeleton and the Ras/MAP kinase pathway, resulting in an upregulation of the cell cycle inhibitiors p21/WAF1 and p27, which in turn results in the TGFβ1-induced growth inhibition (additional file 10).
The cell lines Capan-1 and AsPC-1 are not only resistant to TGFβ1 because of their alterations in the SMAD pathway, but also because they cannot complete the alternate pathway, as demonstrated above. Furthermore, this alternate pathway may explain the TGFβ1 resistance of cells with no detectable defect in the SMAD pathway [92–101], as one can imagine that the cooperative action of the both pathways is necessary to exert the complete growth inhibitory effect of TGFβ1. Comparable effects have been described for the synergistic operation of growth factor receptor and anchorage dependent integrin signaling [102–119].
Recombinant mature TGFβ1 does not contain a RGD motif, and thus binding of TGFβ1 to the αVβ6 integrin and the subsequent activation of this integrin must rely on a novel motif distinct from RGD. For αVβ6 integrin, a novel non-RGD ligand recognition motif was recently described with the consensus motif DLXXL [120].
This motif has been detected on several proteins, including laminin, collagen and fibrinogen [120]. A BLAST search for this sequence in TGFβ1 revealed a 70% similar motif in two parts of the molecule; one in the LAP (data not shown) and one in the mature TGFβ1. In mature TGFβ1, the DLXXL motif is freely accessible for interactions on the outside of the molecule. Therefore, it may be speculated that TGFβ1 binding to αVβ6 via this novel ligand recognition motif is facilitating the signaling. Moreover, a non-RGD ligand binding pocket in addition to the usual RGD binding site has been demonstrated for fibrinogen and the αIIbβ3 integrin [121], supporting our findings.
Conclusions
We demonstrate an alternate TGFβ1 signaling pathway via αVβ6 integrin, independent of SMAD4. This pathway seems to be required for full TGFβ1 induced transcriptional activation, which explains the TGFβ1 sensitivity of those cells lacking DPC4/SMAD4 function that still react with growth inhibition.
Methods
Cell Culture and TGFβ1 stimulation
All cells were obtained from from ATCC and maintained in DMEM supplemented with 17% fetal calf serum. Recombinant human proteins (mature TGFβ1, TNF-α, Fibronectin and Laminin 1) were purchased from R&D Systems. 106 cells were grown overnight in 6 cm diameter dishes with DMEM/10 % FCS. After washing twice with PBS (pH 7.4), fresh DMEM without FCS was added to the monolayer. Cells were stimulated with 10 nM of mature TGFβ1 or with fibronectin as described below. In blocking experiments, cells were preincubated with either a TGFβ1-RII-blocking antibody (R&D Systems # AF-241-NA, 15 μg/ml for 30 min), αV and β6-blocking antibodies (Santa Cruz, sc-6617 and sc-6632 respectively, 1:100 each for 30 min), or the MEK1 inhibitor PD98059 (New England Biolabs # 9900S, 7.5 ng/ml for 10 min) before stimulation with mature TGFβ1.
Indirect immunofluorescence
For indirect immunofluorescence, 104 cells were cultured on glass coverslips, stimulated with 10 nM mature TGFβ1 for 10 minutes, stained as described [66, 67] and viewed using a Zeiss LSM-510 confocal microscope. Antibodies used were: actin (sc-8432), TGFβ1 (sc-146), αV (sc-6617) and β6 (sc-6632). The following fluorochrome-labeled antibodies were used (AlexaFluor, Molecular Probes): goat anti-mouse IgG (H+L) conjugate (#A-11032; red), goat anti-rabbit IgG (H+L) conjugate (#A-11046; blue), and donkey anti-goat IgG (H+L) conjugate (#A-11055; green).
Preparation of cytoplasmatic proteins and of nuclei
Cellular fractionation was performed as described in earlier reports [122–125]. Cells were scraped into 100 μl of ice-cold buffer A [10 mM Hepes (pH 7.9); 1.5 mM MgCl2; 10 mM KCl; 0.5 mM DTT; 0.05% NP-40]. Nuclei were pelleted in a microcentrifuge for 10 sec. at 4°C and 15,000 G. The supernatant was used to analyze cytoplasmatic proteins, nuclei were resuspended in 60 μl of ice cold buffer B [20 mM Hepes (pH 7.9); 25% (v/v) glycerol; 420 mM KCl; 1.5 mM MgCl2; 0.2 mM EDTA; 0.5 mM DTT; 0.5 mM PMSF] and incubated on ice for 15 min.
Preparation of actin filaments of the cytoskeleton and immunoprecipitation
The cell monolayer was incubated with cell extraction buffer [0.1 % Triton X-100, 80 mM KCl, 20 mM imidazole, 2 mM MgCl2, 2 mM EGTA, pH 7.8] for 5 min at 4°C. The Triton-insoluble fractions were then scraped into cold Triton X-100 lysis buffer [50 mM Tris/HCl (pH 7.4); 100 mM NaCl; 50 mM NaF; 5 mM EDTA; 40 mM glycerophosphate; 1 mM sodium orthovanadate; 100 μM PMSF; 1 μM leupeptin; 1 μM pepstatin A; 1% (v/v) Triton X-100] and incubated for 20 min on ice, and clarified by centrifugation at 13000 g for 5 min at 4°C. For immunoprecipitation, the lysates were incubated for 4 h at 4°C with 1 μg of antibody (all from Santa Cruz) pre-adsorbed on Protein A-Sepharose beads (Pharmacia). Immune complexes were washed five times with cold Triton X-100 lysis buffer. For re-precipitation, the pellet was boiled in 10 μl 0.1% SDS for 5 min and diluted 1:20 in the Triton X lysis buffer followed by the precipitation procedure. All samples were boiled in Laemmli denaturing buffer and analyzed by Western blotting. Whole cell lysates serving as positive controls were prepared by incubating monolayers with denaturing Laemmli buffer.
Treatment with Cytochalasin and Calcium Chelator
To disrupt the actin filaments of the cytoskeleton, the cell monolayer was treated with 25 nM cytochalasin D for 20 min at 37°C; TGFβ1 was then applied in the presence of 25 nM cytochalasin D. For chelating intracellular calcium, the cells were preincubated with 5 μM of 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid, acetoxymethyl ester (BAPTA-AM) for 15 min. TGFβ1 was then applied in the presence of 5 μM of BAPTA.
[3H]-thymidine incorporation assay
For the TGFβeta1 growth inhibition assay, cells were seeded in 96-well microtitier plates at 104 cells/well in 100 μl of culture medium containing 10% FCS. After 24 h, medium was replaced by culture medium supplemented with 0.5% FCS. After an additional 24 h, cells were treated with 10 nM of mature TGFβ1. After incubation with TGFβ1 for 21 h, cells were pulsed with 200 nCi of [3H]-thymidine (1.74 TBq/mmol; Amersham, UK) for 3 h without changing the medium. Cells were washed once with PBS, incubated with trypsin for 10 min and collected by using a Skatron cell harvester. Radioactivity incorporated was determined by liquid scintillation counting.
Western Blot
Proteins were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Roche) as described previously [66]. Blot membranes were blocked for 3 h at 37°C in PBS containing 5 % skim milk and probed with the respective antibodies (16 h at 4°C). The following antibodies were used in a dilution of 1:1,000: TGFβ1 (Santa Cruz [sc], sc-146), p-Tyr (sc-7020), β6-integrin (sc-6632), αV-integrin (sc-6617), p125FAK (sc-557), TGFβ1-RI (sc-402), TGFβ1-RII (sc-400-G), ERK1/2-P (sc-7383), SMAD2/3 (sc-6033), SOS1/2 (sc-259), p130cas (UBI-06-500), PCNA (sc-56), p21WAF1 (sc-6246), p27KIP (sc-1641), c-fos (sc-7202), c-jun (sc-44), raf1 (sc-133), p21Ras (sc-35) and phospho-threonine antibody (New England Biolabs, # 9381). Detection antibodies (all from Dako; 1:5,000 for 1 h at room temperature) were mouse-anti-goat Ig, mouse-anti-rat Ig, rabbit-anti-mouse Ig, and porcine-anti-rabbit Ig-HRP [66]. To visualize all transferred proteins, we used the ECL protein biotinylation labeling modules (RPN 2202, Amersham) and streptavidin alkaline phosphatase (V020402, Amersham) in accordance with the manufacturer's instructions.
Ras activation assay
Only activated p21Ras is able to bind Raf1, leading to a Raf1-translocation to the cell membrane. After stimulation with 10 nM mature TGFβ1 for 10 minutes, cells were incubated in sterile water until they lysed. The membrane fraction was lysed in Triton X-100 lysis buffer. Precipitation against Raf1 and analysis for p21Ras followed.
References
Shi Y, Massague J: Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003, 113: 685-700.
Massague J, Blain SW, Lo RS: TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000, 103: 295-309.
Massague J: TGFbeta signaling: receptors, transducers, and Mad proteins. Cell. 1996, 85: 947-950.
Massague J: Receptors for the TGF-beta family. Cell. 1992, 69: 1067-1070.
Cheifetz S, Weatherbee JA, Tsang ML, Anderson JK, Mole JE, Lucas R, Massague J: The transforming growth factor-beta system, a complex pattern of cross-reactive ligands and receptors. Cell. 1987, 48: 409-415.
Fink SP, Mikkola D, Willson JK, Markowitz S: TGF-beta-induced nuclear localization of Smad2 and Smad3 in Smad4 null cancer cell lines. Oncogene. 2003, 22: 1317-1323. 10.1038/sj.onc.1206128
Feng XH, Liang YY, Liang M, Zhai W, Lin X: Direct interaction of c-Myc with Smad2 and Smad3 to inhibit TGF-beta-mediated induction of the CDK inhibitor p15(Ink4B). Mol Cell. 2002, 9: 133-143.
Feng XH, Lin X, Derynck R: Smad2, Smad3 and Smad4 cooperate with Sp1 to induce p15(Ink4B) transcription in response to TGF-beta. Embo J. 2000, 19: 5178-5193. 10.1093/emboj/19.19.5178
Stopa M, Anhuf D, Terstegen L, Gatsios P, Gressner AM, Dooley S: Participation of Smad2, Smad3, and Smad4 in transforming growth factor beta (TGF-beta)-induced activation of Smad7. THE TGF-beta response element of the promoter requires functional Smad binding element and E-box sequences for transcriptional regulation. J Biol Chem. 2000, 275: 29308-29317. 10.1074/jbc.M003282200
Labbe E, Silvestri C, Hoodless PA, Wrana JL, Attisano L: Smad2 and Smad3 positively and negatively regulate TGF beta-dependent transcription through the forkhead DNA-binding protein FAST2. Mol Cell. 1998, 2: 109-120.
Goto D, Yagi K, Inoue H, Iwamoto I, Kawabata M, Miyazono K, Kato M: A single missense mutant of Smad3 inhibits activation of both Smad2 and Smad3, and has a dominant negative effect on TGF-beta signals. FEBS Lett. 1998, 430: 201-204. 10.1016/S0014-5793(98)00658-9
Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH, Miyazono K, ten Dijke P: TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. Embo J. 1997, 16: 5353-5362. 10.1093/emboj/16.17.5353
Song CZ, Siok TE, Gelehrter TD: Smad4/DPC4 and Smad3 mediate transforming growth factor-beta (TGF-beta) signaling through direct binding to a novel TGF-beta-responsive element in the human plasminogen activator inhibitor-1 promoter. J Biol Chem. 1998, 273: 29287-29290. 10.1074/jbc.273.45.29287
Feng XH, Zhang Y, Wu RY, Derynck R: The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for smad3 in TGF-beta-induced transcriptional activation. Genes Dev. 1998, 12: 2153-2163.
Lagna G, Hata A, Hemmati-Brivanlou A, Massague J: Partnership between DPC4 and SMAD proteins in TGF-beta signalling pathways. Nature. 1996, 383: 832-836. 10.1038/383832a0
Yang X, Li C, Xu X, Deng C: The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc Natl Acad Sci U S A. 1998, 95: 3667-3672. 10.1073/pnas.95.7.3667
Cutry AF, Kinniburgh AJ, Twardzik DR, Wenner CE: Transforming growth factor alpha (TGF alpha) induction of C-FOS and C-MYC expression in C3H 10T1/2 cells. Biochem Biophys Res Commun. 1988, 152: 216-222.
Mercier T, Gaillard-Sanchez I, Martel P, Seillan-Heberden C: Constitutive overexpression of c-fos protein in rat liver epithelial cells decreases TGF-beta synthesis and increases TGF-beta 1 receptors. Biochim Biophys Acta. 1995, 1266: 64-72. 10.1016/0167-4889(94)00240-F
Zhang Y, Feng XH, Derynck R: Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature. 1998, 394: 909-913. 10.1038/29814
Kutz SM, Providence KM, Higgins PJ: Antisense targeting of c-fos transcripts inhibits serum- and TGF-beta 1-stimulated PAI-1 gene expression and directed motility in renal epithelial cells. Cell Motil Cytoskeleton. 2001, 48: 163-174. 10.1002/1097-0169(200103)48:3<163::AID-CM1006>3.0.CO;2-C
Chen CR, Kang Y, Siegel PM, Massague J: E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to c-myc repression. Cell. 2002, 110: 19-32.
Kowalik TF: Smad about E2F. TGFbeta repressionof c-Myc via a Smad3/E2F/p107 complex. Mol Cell. 2002, 10: 7-8.
Seoane J, Pouponnot C, Staller P, Schader M, Eilers M, Massague J: TGFbeta influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat Cell Biol. 2001, 3: 400-408. 10.1038/35070086
Yang L, Yang J, Venkateswarlu S, Ko T, Brattain MG: Autocrine TGFbeta signaling mediates vitamin D3 analog-induced growth inhibition in breast cells. J Cell Physiol. 2001, 188: 383-393. 10.1002/jcp.1125
Badiavas EV, Zhou L, Falanga V: Growth inhibition of primary keratinocytes following transduction with a novel TGFbeta-1 containing retrovirus. J Dermatol Sci. 2001, 27: 1-6. 10.1016/S0923-1811(01)00091-3
Voss M, Wolff B, Savitskaia N, Ungefroren H, Deppert W, Schmiegel W, Kalthoff H, Naumann M: TGFbeta-induced growth inhibition involves cell cycle inhibitor p21 and pRb independent from p15 expression. Int J Oncol. 1999, 14: 93-101.
Moskaluk CA, Kern SE: Cancer gets Mad: DPC4 and other TGFbeta pathway genes in human cancer. Biochim Biophys Acta. 1996, 1288: M31-3. 10.1016/S0304-419X(96)00033-9
Chen C, Wang XF, Sun L: Expression of transforming growth factor beta (TGFbeta) type III receptor restores autocrine TGFbeta1 activity in human breast cancer MCF-7 cells. J Biol Chem. 1997, 272: 12862-12867. 10.1074/jbc.272.19.12862
Frey RS, Mulder KM: TGFbeta regulation of mitogen-activated protein kinases in human breast cancer cells. Cancer Lett. 1997, 117: 41-50. 10.1016/S0304-3835(97)00211-5
Simeone DM, Pham T, Logsdon CD: Disruption of TGFbeta signaling pathways in human pancreatic cancer cells. Ann Surg. 2000, 232: 73-80. 10.1097/00000658-200007000-00011
Adnane J, Bizouarn FA, Chen Z, Ohkanda J, Hamilton AD, Munoz-Antonia T, Sebti SM: Inhibition of farnesyltransferase increases TGFbeta type II receptor expression and enhances the responsiveness of human cancer cells to TGFbeta. Oncogene. 2000, 19: 5525-5533. 10.1038/sj.onc.1203920
Hata A: TGFbeta signaling and cancer. Exp Cell Res. 2001, 264: 111-116. 10.1006/excr.2000.5140
Rooke HM, Crosier KE: The smad proteins and TGFbeta signalling: uncovering a pathway critical in cancer. Pathology. 2001, 33: 73-84. 10.1080/00313020120034948
Bandyopadhyay A, Zhu Y, Malik SN, Kreisberg J, Brattain MG, Sprague EA, Luo J, Lopez-Casillas F, Sun LZ: Extracellular domain of TGFbeta type III receptor inhibits angiogenesis and tumor growth in human cancer cells. Oncogene. 2002, 21: 3541-3551. 10.1038/sj.onc.1205439
Lei X, Bandyopadhyay A, Le T, Sun L: Autocrine TGFbeta supports growth and survival of human breast cancer MDA-MB-231 cells. Oncogene. 2002, 21: 7514-7523. 10.1038/sj.onc.1205966
Dumont N, Arteaga CL: A kinase-inactive type II TGFbeta receptor impairs BMP signaling in human breast cancer cells. Biochem Biophys Res Commun. 2003, 301: 108-112. 10.1016/S0006-291X(02)02977-7
Bruning A, Runnebaum IB: CAR is a cell-cell adhesion protein in human cancer cells and is expressionally modulated by dexamethasone, TNFalpha, and TGFbeta. Gene Ther. 2003, 10: 198-205. 10.1038/sj.gt.3301887
Ellenrieder V, Buck A, Gress TM: TGFbeta-regulated transcriptional mechanisms in cancer. Int J Gastrointest Cancer. 2002, 31: 61-69. 10.1385/IJGC:31:1-3:61
Ryu B, Kern SE: The Essential Similarity of TGFbeta and Activin Receptor Transcriptional Responses in Cancer Cells. Cancer Biol Ther. 2003, 2: 164-170.
Amendt C, Mann A, Schirmacher P, Blessing M: Resistance of keratinocytes to TGFbeta-mediated growth restriction and apoptosis induction accelerates re-epithelialization in skin wounds. J Cell Sci. 2002, 115: 2189-2198.
Khoo NK, Bechberger JF, Shepherd T, Bond SL, McCrae KR, Hamilton GS, Lala PK: SV40 Tag transformation of the normal invasive trophoblast results in a premalignant phenotype. I. Mechanisms responsible for hyperinvasiveness and resistance to anti-invasive action of TGFbeta. Int J Cancer. 1998, 77: 429-439. 10.1002/(SICI)1097-0215(19980729)77:3<429::AID-IJC20>3.3.CO;2-Q
Peng B, Fleming JB, Breslin T, Grau AM, Fojioka S, Abbruzzese JL, Evans DB, Ayers D, Wathen K, Wu T, Robertson KD, Chiao PJ: Suppression of tumorigenesis and induction of p15(ink4b) by Smad4/DPC4 in human pancreatic cancer cells. Clin Cancer Res. 2002, 8: 3628-3638.
Bartsch D, Barth P, Bastian D, Ramaswamy A, Gerdes B, Chaloupka B, Deiss Y, Simon B, Schudy A: Higher frequency of DPC4/Smad4 alterations in pancreatic cancer cell lines than in primary pancreatic adenocarcinomas. Cancer Lett. 1999, 139: 43-49. 10.1016/S0304-3835(98)00380-2
Giehl K, Seidel B, Gierschik P, Adler G, Menke A: TGFbeta1 represses proliferation of pancreatic carcinoma cells which correlates with Smad4-independent inhibition of ERK activation. Oncogene. 2000, 19: 4531-4541. 10.1038/sj.onc.1203806
Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D: The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999, 96: 319-328.
Lu M, Munger JS, Steadele M, Busald C, Tellier M, Schnapp LM: Integrin alpha8beta1 mediates adhesion to LAP-TGFbeta1. J Cell Sci. 2002, 115: 4641-4648. 10.1242/jcs.00145
Ludbrook SB, Barry ST, Delves CJ, Horgan CM: The integrin alphavbeta3 is a receptor for the latency-associated peptides of transforming growth factors beta1 and beta3. Biochem J. 2003, 369: 311-318. 10.1042/BJ20020809
Saharinen J, Hyytiainen M, Taipale J, Keski-Oja J: Latent transforming growth factor-beta binding proteins (LTBPs)--structural extracellular matrix proteins for targeting TGF-beta action. Cytokine Growth Factor Rev. 1999, 10: 99-117. 10.1016/S1359-6101(99)00010-6
Munger JS, Harpel JG, Giancotti FG, Rifkin DB: Interactions between growth factors and integrins: latent forms of transforming growth factor-beta are ligands for the integrin alphavbeta1. Mol Biol Cell. 1998, 9: 2627-2638.
Saharinen J, Taipale J, Monni O, Keski-Oja J: Identification and characterization of a new latent transforming growth factor-beta-binding protein, LTBP-4. J Biol Chem. 1998, 273: 18459-18469. 10.1074/jbc.273.29.18459
Yuan X, Downing AK, Knott V, Handford PA: Solution structure of the transforming growth factor beta-binding protein-like module, a domain associated with matrix fibrils. Embo J. 1997, 16: 6659-6666. 10.1093/emboj/16.22.6659
Yang Y, Dignam JD, Gentry LE: Role of carbohydrate structures in the binding of beta1-latency-associated peptide to ligands. Biochemistry. 1997, 36: 11923-11932. 10.1021/bi9710479
Grainger DJ, Wakefield L, Bethell HW, Farndale RW, Metcalfe JC: Release and activation of platelet latent TGF-beta in blood clots during dissolution with plasmin. Nat Med. 1995, 1: 932-937.
Annes JP, Munger JS, Rifkin DB: Making sense of latent TGFbeta activation. J Cell Sci. 2003, 116: 217-224. 10.1242/jcs.00229
Altmann CR, Chang C, Munoz-Sanjuan I, Bell E, Heke M, Rifkin DB, Brivanlou AH: The latent-TGFbeta-binding-protein-1 (LTBP-1) is expressed in the organizer and regulates nodal and activin signaling. Dev Biol. 2002, 248: 118-127. 10.1006/dbio.2002.0716
Le M, Gohr CM, Rosenthal AK: Transglutaminase participates in the incorporation of latent TGFbeta into the extracellular matrix of aging articular chondrocytes. Connect Tissue Res. 2001, 42: 245-253.
Saika S, Miyamoto T, Tanaka T, Ishida I, Ohnishi Y, Ooshima A: Latent TGFbeta binding protein-1 and fibrillin-1 in human capsular opacification and in cultured lens epithelial cells. Br J Ophthalmol. 2001, 85: 1362-1366. 10.1136/bjo.85.11.1362
Linder S, Castanos-Velez E, von Rosen A, Biberfeld P: Immunohistochemical expression of extracellular matrix proteins and adhesion molecules in pancreatic carcinoma. Hepatogastroenterology. 2001, 48: 1321-1327.
Streit M, Schmidt R, Hilgenfeld RU, Thiel E, Kreuser ED: Adhesion receptors in malignant transformation and dissemination of gastrointestinal tumors. J Mol Med. 1996, 74: 253-268. 10.1007/s001090050027
Lohr M, Trautmann B, Gottler M, Peters S, Zauner I, Maier A, Kloppel G, Liebe S, Kreuser ED: Expression and function of receptors for extracellular matrix proteins in human ductal adenocarcinomas of the pancreas. Pancreas. 1996, 12: 248-259.
Weinel RJ, Rosendahl A, Pinschmidt E, Kisker O, Simon B, Santoso S: The alpha 6-integrin receptor in pancreatic carcinoma. Gastroenterology. 1995, 108: 523-532.
Weinel RJ, Rosendahl A, Neumann K, Chaloupka B, Erb D, Rothmund M, Santoso S: Expression and function of VLA-alpha 2, -alpha 3, -alpha 5 and -alpha 6-integrin receptors in pancreatic carcinoma. Int J Cancer. 1992, 52: 827-833.
Timar J, Chopra H, Rong X, Hatfield JS, Fligiel SE, Onoda JM, Taylor JD, Honn KV: Calcium channel blocker treatment of tumor cells induces alterations in the cytoskeleton, mobility of the integrin alpha IIb beta 3 and tumor-cell-induced platelet aggregation. J Cancer Res Clin Oncol. 1992, 118: 425-434.
Beck R, Nebe B, Guthoff R, Rychly J: Inhibition of lens epithelial cell adhesion by the calcium antagonist Mibefradil correlates with impaired integrin distribution and organization of the cytoskeleton. Graefes Arch Clin Exp Ophthalmol. 2001, 239: 452-458.
Schmidt C, Pommerenke H, Durr F, Nebe B, Rychly J: Mechanical stressing of integrin receptors induces enhanced tyrosine phosphorylation of cytoskeletally anchored proteins. J Biol Chem. 1998, 273: 5081-5085. 10.1074/jbc.273.9.5081
Pommerenke H, Schmidt C, Durr F, Nebe B, Luthen F, Muller P, Rychly J: The mode of mechanical integrin stressing controls intracellular signaling in osteoblasts. J Bone Miner Res. 2002, 17: 603-611.
Carethers JM, Pham TT: Mutations of transforming growth factor beta 1 type II receptor, BAX, and insulin-like growth factor II receptor genes in microsatellite unstable cell lines. In Vivo. 2000, 14: 13-20.
Therrien JP, Loignon M, Drouin R, Drobetsky EA: Ablation of p21waf1cip1 expression enhances the capacity of p53-deficient human tumor cells to repair UVB-induced DNA damage. Cancer Res. 2001, 61: 3781-3786.
Houle F, Rousseau S, Morrice N, Luc M, Mongrain S, Turner CE, Tanaka S, Moreau P, Huot J: Extracellular signal-regulated kinase mediates phosphorylation of tropomyosin-1 to promote cytoskeleton remodeling in response to oxidative stress: impact on membrane blebbing. Mol Biol Cell. 2003, 14: 1418-1432. 10.1091/mbc.E02-04-0235
Fincham VJ, James M, Frame MC, Winder SJ: Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. Embo J. 2000, 19: 2911-2923. 10.1093/emboj/19.12.2911
Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD: FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000, 2: 249-256. 10.1038/35010517
Achison M, Elton CM, Hargreaves PG, Knight CG, Barnes MJ, Farndale RW: Integrin-independent tyrosine phosphorylation of p125(fak) in human platelets stimulated by collagen. J Biol Chem. 2001, 276: 3167-3174. 10.1074/jbc.M007186200
Farre L, Casanova I, Guerrero S, Trias M, Capella G, Mangues R: Heterotopic implantation alters the regulation of apoptosis and the cell cycle and generates a new metastatic site in a human pancreatic tumor xenograft model. Faseb J. 2002, 16: 975-982. 10.1096/fj.01-0973com
Liboi E, Di Francesco P, Gallinari P, Testa U, Rossi GB, Peschle C: TGF beta induces a sustained c-fos expression associated with stimulation or inhibition of cell growth in EL2 or NIH 3T3 fibroblasts. Biochem Biophys Res Commun. 1988, 151: 298-305.
Laiho M, DeCaprio JA, Ludlow JW, Livingston DM, Massague J: Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990, 62: 175-185.
Inagaki M, Moustakas A, Lin HY, Lodish HF, Carr BI: Growth inhibition by transforming growth factor beta (TGF-beta) type I is restored in TGF-beta-resistant hepatoma cells after expression of TGF-beta receptor type II cDNA. Proc Natl Acad Sci U S A. 1993, 90: 5359-5363.
Herrera RE, Makela TP, Weinberg RA: TGF beta-induced growth inhibition in primary fibroblasts requires the retinoblastoma protein. Mol Biol Cell. 1996, 7: 1335-1342.
Edens M, Leof EB: In vitro assays for measuring TGF-beta growth stimulation and inhibition. Methods Mol Biol. 2000, 142: 1-11. 10.1385/1-59259-053-5:1
Giancotti FG, Ruoslahti E: Integrin signaling. Science. 1999, 285: 1028-1032. 10.1126/science.285.5430.1028
Huang W, Rudkin GH, Carlsen B, Ishida K, Ghasri P, Anvar B, Yamaguchi DT, Miller TA: Overexpression of BMP-2 modulates morphology, growth, and gene expression in osteoblastic cells. Exp Cell Res. 2002, 274: 226-234. 10.1006/excr.2002.5483
Wyatt LE, Chung CY, Carlsen B, Iida-Klein A, Rudkin GH, Ishida K, Yamaguchi DT, Miller TA: Bone morphogenetic protein-2 (BMP-2) and transforming growth factor-beta1 (TGF-beta1) alter connexin 43 phosphorylation in MC3T3-E1 Cells. BMC Cell Biol. 2001, 2: 14- 10.1186/1471-2121-2-14
Zerath E, Holy X, Noel B, Malouvier A, Hott M, Marie PJ: Effects of BMP-2 on osteoblastic cells and on skeletal growth and bone formation in unloaded rats. Growth Horm IGF Res. 1998, 8: 141-149.
Mainiero F, Gismondi A, Soriani A, Cippitelli M, Palmieri G, Jacobelli J, Piccoli M, Frati L, Santoni A: Integrin-mediated ras-extracellular regulated kinase (ERK) signaling regulates interferon gamma production in human natural killer cells. J Exp Med. 1998, 188: 1267-1275. 10.1084/jem.188.7.1267
Barberis L, Wary KK, Fiucci G, Liu F, Hirsch E, Brancaccio M, Altruda F, Tarone G, Giancotti FG: Distinct roles of the adaptor protein Shc and focal adhesion kinase in integrin signaling to ERK. J Biol Chem. 2000, 275: 36532-36540. 10.1074/jbc.M002487200
Whitlock BB, Gardai S, Fadok V, Bratton D, Henson PM: Differential roles for alpha(M)beta(2) integrin clustering or activation in the control of apoptosis via regulation of akt and ERK survival mechanisms. J Cell Biol. 2000, 151: 1305-1320. 10.1083/jcb.151.6.1305
Aplin AE, Stewart SA, Assoian RK, Juliano RL: Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J Cell Biol. 2001, 153: 273-282. 10.1083/jcb.153.2.273
Sablina AA, Chumakov PM, Levine AJ, Kopnin BP: p53 activation in response to microtubule disruption is mediated by integrin-Erk signaling. Oncogene. 2001, 20: 899-909. 10.1038/sj.onc.1204156
Brunton VG, Fincham VJ, McLean GW, Winder SJ, Paraskeva C, Marshall JF, Frame MC: The protrusive phase and full development of integrin-dependent adhesions in colon epithelial cells require FAK- and ERK-mediated actin spike formation: deregulation in cancer cells. Neoplasia. 2001, 3: 215-226. 10.1038/sj.neo.7900149
Ahmed N, Niu J, Dorahy DJ, Gu X, Andrews S, Meldrum CJ, Scott RJ, Baker MS, Macreadie IG, Agrez MV: Direct integrin alphavbeta6-ERK binding: implications for tumour growth. Oncogene. 2002, 21: 1370-1380. 10.1038/sj.onc.1205286
Weyts FA, Li YS, van Leeuwen J, Weinans H, Chien S: ERK activation and alpha v beta 3 integrin signaling through Shc recruitment in response to mechanical stimulation in human osteoblasts. J Cell Biochem. 2002, 87: 85-92. 10.1002/jcb.10278
Kleeff J, Maruyama H, Friess H, Buchler MW, Falb D, Korc M: Smad6 suppresses TGF-beta-induced growth inhibition in COLO-357 pancreatic cancer cells and is overexpressed in pancreatic cancer. Biochem Biophys Res Commun. 1999, 255: 268-273. 10.1006/bbrc.1999.0171
Calonge MJ, Massague J: Smad4/DPC4 silencing and hyperactive Ras jointly disrupt transforming growth factor-beta antiproliferative responses in colon cancer cells. J Biol Chem. 1999, 274: 33637-33643. 10.1074/jbc.274.47.33637
Inman GJ, Allday MJ: Resistance to TGF-beta1 correlates with a reduction of TGF-beta type II receptor expression in Burkitt's lymphoma and Epstein-Barr virus-transformed B lymphoblastoid cell lines. J Gen Virol. 2000, 81: 1567-1578.
Lee S, Cho YS, Shim C, Kim J, Choi J, Oh S, Zhang W, Lee J: Aberrant expression of Smad4 results in resistance against the growth-inhibitory effect of transforming growth factor-beta in the SiHa human cervical carcinoma cell line. Int J Cancer. 2001, 94: 500-507. 10.1002/ijc.1494
Paterson IC, Davies M, Stone A, Huntley S, Smith E, Pring M, Eveson JW, Robinson CM, Parkinson EK, Prime SS: TGF-beta1 acts as a tumor suppressor of human malignant keratinocytes independently of Smad 4 expression and ligand-induced G(1) arrest. Oncogene. 2002, 21: 1616-1624. 10.1038/sj.onc.1205217
Macias-Silva M, Li W, Leu JI, Crissey MA, Taub R: Up-regulated transcriptional repressors SnoN and Ski bind Smad proteins to antagonize transforming growth factor-beta signals during liver regeneration. J Biol Chem. 2002, 277: 28483-28490. 10.1074/jbc.M202403200
Berger DH, Feng XH, Yao J, Saha D, Beauchamp RD, Lin X: Resistance to transforming growth factor-beta occurs in the presence of normal Smad activation. Surgery. 2002, 132: 310-316. 10.1067/msy.2002.126097
Schwarte-Waldhoff I, Schmiegel W: Smad4 transcriptional pathways and angiogenesis. Int J Gastrointest Cancer. 2002, 31: 47-59. 10.1385/IJGC:31:1-3:47
Nicolas FJ, Hill CS: Attenuation of the TGF-beta-Smad signaling pathway in pancreatic tumor cells confers resistance to TGF-beta-induced growth arrest. Oncogene. 2003, 22: 3698-3711. 10.1038/sj.onc.1206420
Stoika R, Yakymovych M, Souchelnytskyi S, Yakymovych I: Potential role of transforming growth factor beta1 in drug resistance of tumor cells. Acta Biochim Pol. 2003, 50: 497-508.
Yamanaka I, Koizumi M, Baba T, Yamashita S, Suzuki T, Kudo R: Epidermal growth factor increased the expression of alpha2beta1-integrin and modulated integrin-mediated signaling in human cervical adenocarcinoma cells. Exp Cell Res. 2003, 286: 165-174. 10.1016/S0014-4827(03)00065-X
Kabir-Salmani M, Shiokawa S, Akimoto Y, Sakai K, Nagamatsu S, Nakamura Y, Lotfi A, Kawakami H, Iwashita M: Alphavbeta3 integrin signaling pathway is involved in insulin-like growth factor I-stimulated human extravillous trophoblast cell migration. Endocrinology. 2003, 144: 1620-1630. 10.1210/en.2002-220886
Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE: Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003, 278: 12384-12389. 10.1074/jbc.M208544200
Smyth SS, Patterson C: Tiny dancers: the integrin-growth factor nexus in angiogenic signaling. J Cell Biol. 2002, 158: 17-21. 10.1083/jcb.200202100
Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA: Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol. 2002, 157: 149-160. 10.1083/jcb.200109079
Hermanto U, Zong CS, Li W, Wang LH: RACK1, an insulin-like growth factor I (IGF-I) receptor-interacting protein, modulates IGF-I-dependent integrin signaling and promotes cell spreading and contact with extracellular matrix. Mol Cell Biol. 2002, 22: 2345-2365. 10.1128/MCB.22.7.2345-2365.2002
Lee JW, Juliano RL: The alpha5beta1 integrin selectively enhances epidermal growth factor signaling to the phosphatidylinositol-3-kinase/Akt pathway in intestinal epithelial cells. Biochim Biophys Acta. 2002, 1542: 23-31. 10.1016/S0167-4889(01)00161-6
Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL: Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J Biol Chem. 2001, 276: 46707-46713. 10.1074/jbc.M106176200
Gleeson LM, Chakraborty C, McKinnon T, Lala PK: Insulin-like growth factor-binding protein 1 stimulates human trophoblast migration by signaling through alpha 5 beta 1 integrin via mitogen-activated protein Kinase pathway. J Clin Endocrinol Metab. 2001, 86: 2484-2493. 10.1210/jc.86.6.2484
Lai CF, Feng X, Nishimura R, Teitelbaum SL, Avioli LV, Ross FP, Cheng SL: Transforming growth factor-beta up-regulates the beta 5 integrin subunit expression via Sp1 and Smad signaling. J Biol Chem. 2000, 275: 36400-36406. 10.1074/jbc.M002131200
Renshaw MW, Price LS, Schwartz MA: Focal adhesion kinase mediates the integrin signaling requirement for growth factor activation of MAP kinase. J Cell Biol. 1999, 147: 611-618. 10.1083/jcb.147.3.611
Skinner MA, Wildeman AG: beta(1) integrin binds the 16-kDa subunit of vacuolar H(+)-ATPase at a site important for human papillomavirus E5 and platelet-derived growth factor signaling. J Biol Chem. 1999, 274: 23119-23127. 10.1074/jbc.274.33.23119
Aplin AE, Juliano RL: Integrin and cytoskeletal regulation of growth factor signaling to the MAP kinase pathway. J Cell Sci. 1999, 112 ( Pt 5): 695-706.
Gu J, Tamura M, Yamada KM: Tumor suppressor PTEN inhibits integrin- and growth factor-mediated mitogen-activated protein (MAP) kinase signaling pathways. J Cell Biol. 1998, 143: 1375-1383. 10.1083/jcb.143.5.1375
Zheng B, Clemmons DR: Blocking ligand occupancy of the alphaVbeta3 integrin inhibits insulin-like growth factor I signaling in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1998, 95: 11217-11222. 10.1073/pnas.95.19.11217
Gotoh A, Ritchie A, Takahira H, Broxmeyer HE: Thrombopoietin and erythropoietin activate inside-out signaling of integrin and enhance adhesion to immobilized fibronectin in human growth-factor-dependent hematopoietic cells. Ann Hematol. 1997, 75: 207-213. 10.1007/s002770050344
Genersch E, Schuppan D, Lichtner RB: Signaling by epidermal growth factor differentially affects integrin-mediated adhesion of tumor cells to extracellular matrix proteins. J Mol Med. 1996, 74: 609-616. 10.1007/s001090050064
Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE: Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. 1995, 6: 1349-1365.
Kraft S, Diefenbach B, Mehta R, Jonczyk A, Luckenbach GA, Goodman SL: Definition of an unexpected ligand recognition motif for alphav beta6 integrin. J Biol Chem. 1999, 274: 1979-1985. 10.1074/jbc.274.4.1979
Hu DD, White CA, Panzer-Knodle S, Page JD, Nicholson N, Smith JW: A new model of dual interacting ligand binding sites on integrin alphaIIbbeta3. J Biol Chem. 1999, 274: 4633-4639. 10.1074/jbc.274.8.4633
Fujioka S, Sclabas GM, Schmidt C, Niu J, Frederick WA, Dong QG, Abbruzzese JL, Evans DB, Baker C, Chiao PJ: Inhibition of constitutive NF-kappa B activity by I kappa B alpha M suppresses tumorigenesis. Oncogene. 2003, 22: 1365-1370. 10.1038/sj.onc.1206323
Sclabas GM, Fujioka S, Schmidt C, Fan Z, Evans DB, Chiao PJ: Restoring apoptosis in pancreatic cancer cells by targeting the nuclear factor-kappaB signaling pathway with the anti-epidermal growth factor antibody IMC-C225. J Gastrointest Surg. 2003, 7: 37-43; discussion 43. 10.1016/S1091-255X(02)00088-4
Fujioka S, Sclabas GM, Schmidt C, Frederick WA, Dong QG, Abbruzzese JL, Evans DB, Baker C, Chiao PJ: Function of nuclear factor kappaB in pancreatic cancer metastasis. Clin Cancer Res. 2003, 9: 346-354.
Dong QG, Sclabas GM, Fujioka S, Schmidt C, Peng B, Wu T, Tsao MS, Evans DB, Abbruzzese JL, McDonnell TJ, Chiao PJ: The function of multiple IkappaB : NF-kappaB complexes in the resistance of cancer cells to Taxol-induced apoptosis. Oncogene. 2002, 21: 6510-6519. 10.1038/sj.onc.1205848
Mineo C, Anderson RG, White MA: Physical association with ras enhances activation of membrane-bound raf (RafCAAX). J Biol Chem. 1997, 272: 10345-10348. 10.1074/jbc.272.16.10345
Acknowledgements
CS acknowledges support from the German Research Foundation. GMS is a recipient of a Fellowship of the Cancer League of Bern, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
CS performed all assays and drafted the manuscript. MPK and GMS provided suggestions and comments for its finalization. All authors read and approved the final manuscript.
A retraction note to this article can be found online at http://dx.doi.org/10.1186/1476-4598-3-2.
An erratum to this article is available at http://dx.doi.org/10.1186/1476-4598-3-2.
Electronic supplementary material
12943_2003_37_MOESM1_ESM.png
Additional File 1: Portable Network Graphic (PNG) File showing that mature TGFβ1 binds to αVβ6 integrin. The cells indicated were stimulated for ten minutes with 10 nM of either mature TGFβ1, tumor necrosis factor α (TNFα) or fibronectin (FN). Cytoskeletal anchored proteins were extracted, co-immunoprecipitated (IP) and analyzed (Blot) with the antibodies indicated. (PNG 83 KB)
12943_2003_37_MOESM2_ESM.png
Additional File 2: Portable Network Graphic (PNG) File showing that mature TGFβ1 binds to αVβ6 integrin. The cells indicated were stimulated for ten minutes with 10 nM of either mature TGFβ1, tumor necrosis factor α (TNFα), laminin-1 (Lam1) or fibronectin (FN). Cytoskeletal anchored proteins were extracted, co-immunoprecipitated (IP) and analyzed (Blot) with the antibodies indicated. (PNG 87 KB)
12943_2003_37_MOESM3_ESM.png
Additional File 3: Portable Network Graphic (PNG) File showing that mature TGFβ1 binds to αVβ6 integrin and the speciticity of the signals detected as well. The cells indicated were stimulated for ten minutes with 10 nM of either mature TGFβ1, tumor necrosis factor α (TNFα), or fibronectin (FN). Cytoskeletal anchored proteins were extracted, co-immunoprecipitated (IP) and analyzed (Blot) with the antibodies indicated. (PNG 79 KB)
12943_2003_37_MOESM4_ESM.png
Additional File 4: Portable Network Graphic (PNG) File showing the speciticity of the signals detected. The cells indicated were stimulated for ten minutes with 10 nM of either mature TGFβ1, tumor necrosis factor α (TNFα), or fibronectin (FN). Cytoskeletal anchored proteins were extracted, and analyzed (Blot) with secondary antibodies (α-mouse HRP plus α-rabbit HRP plus α-goat HRP conjugated antibodies.) (PNG 27 KB)
12943_2003_37_MOESM5_ESM.png
Additional File 5: Portable Network Graphic (PNG) File showing enhanced cytoskeletal immobilization and tyrosine phosphorylation of cellular proteins in response to stimulation with mature TGFβ1. Cytoskeletally anchored αVβ6 was immunoprecipitated after TGFβ1 stimulation (10 nM for 10 minutes) followed by Western analysis with antibodies against tyrosine-phosphorylated proteins (A) or Western blotting after biotinylation of all proteins and streptavidin detection (B). In part the cells were preincubated with αV- and β6-antibodies (1:100 each for 30 min) or with a TGFβ-RII antibody (15 μg/ml for 30 min). (PNG 64 KB)
12943_2003_37_MOESM6_ESM.png
Additional File 6: Portable Network Graphic (PNG) File showing cell cycle genes in response to TGFβ1. Western Blot analysis of HeLa, MCF-7 and Keratinocytes (Keratino) cells as indicated after stimulation with TGFβ1 for the time indicated. Cytoskeletally anchored proteins are differentially marked. In part the cells were preincubated with αV- and β6-antibodies (1:100 each for 30 min), with a TGFβ-RII antibody (15 μg/ml for 30 min), cytochalasin D, BAPTA AM and MEK1 inhibitor PD98059, respectively. (PNG 38 KB)
12943_2003_37_MOESM7_ESM.png
Additional File 7: Portable Network Graphic (PNG) File showing that PCNA regulation is dependent on αVβ6 integrins, intact cytoskeleton and free intracellular calcium. BxPC-3 cells were stimulated with 10 nM of mature TGFβ1 for 6 hours. In part the cells were preincubated with αV- and β6-antibodies (1:100 each for 30 min), with a TGFβ antibody (15 μg/ml for 30 min), cytochalasin D and BAPTA AM, respectively. Whole cell extract was probed with PCNA antibodies. Actin served as loading control. (PNG 49 KB)
12943_2003_37_MOESM8_ESM.png
Additional File 8: Portable Network Graphic (PNG) File showing tha regulation of p27, p21, c-fos and c-jun are dependent on αVβ6 integrins, intact cytoskeleton and free intracellular calcium. BxPC-3 cells were stimulated with 10 nM of mature TGFβ1 for 6 hours. In part the cells were preincubated with αV- and β6-antibodies (1:100 each for 30 min), with a TGFβ-RII antibody (15 μg/ml for 30 min), cytochalasin D and BAPTA AM, respectively. Whole cell extract was probed with PCNA antibodies. Actin served as loading control. (PNG 77 KB)
12943_2003_37_MOESM9_ESM.png
Additional File 9: Portable Network Graphic (PNG) File showing activation and nuclear translocation of SMAD2/3 in response to TGFβ1 (A). Nuclear and cytoplasmatic fraction of cellular proteins (NP9) after stimulation with 10 nM of TGFβ1 for 10 minutes and Western blot analysis for SMAD2/3 and phosphorylated SMAD2/3. Cytoskeletally anchored αVβ6 was immunoprecipitated after TGFβ1 stimulation (10 nM for 10 minutes) followed by Western analysis with antibodies against tyrosine-phosphorylated proteins (C) or Western blotting after biotinylation of all proteins and streptavidin detection (D). In part the cells were preincubated with αV- and β6-antibodies (1:100 each for 30 min) or with a TGFβ antibody (15 μg/ml for 30 min). (PNG 43 KB)
12943_2003_37_MOESM10_ESM.xls
Additional File 10: Microsoft Excel spreadsheet showing TGFβ1 elicited growth inhibition of Panc-1 cells is dependent on αVβ6 integrin function. The assay was performed as described in the "Methods" section. (XLS 16 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kracklauer, M.P., Schmidt, C. & Sclabas, G.M. TGFβ1 signaling via αVβ6 integrin. Mol Cancer 2, 28 (2003). https://doi.org/10.1186/1476-4598-2-28
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-4598-2-28