Abstract
Background
Peroxisome proliferator-activated receptors gamma (PPARγ) ligands have been shown to inhibit the growth of non-small cell lung cancer (NSCLC) cells. However, the mechanisms underlying this effect remain incompletely elucidated.
Methods
Cell proliferation and apoptosis were measured by cell viability, MTT and caspase3/7 activity assays. Phosphorylation/protein expression and gene silence/overexpression of AMPKα, phosphoinositide-dependent protein kinase 1 (PDK1), Egr-1 and PPARγ were performed by Western blot and siRNA/transfection assays. Dual-Luciferase Reporter Kit was used to measure the PPAR response elements (PPRE) reporter and PDK1 promoter activities, and ChIP assay was used to detect the Egr-1 protein binding to the DNA site in the PDK1 gene promoter.
Results
We found that ciglitazone, one synthetic PPARγ ligand, inhibited growth and induced apoptosis of NSCLC cells through decreased expression of PDK1, which was not blocked by GW9662 (a specific PPARγ antagonist). Overexpression of PDK1 overcame the effect of ciglitazone on cell growth and caspase 3/7 activity. Ciglitazone increased the phosphorylation of AMPKα and c-Jun N-terminal kinase (JNK), and the inhibitor of AMPK (compound C), but not JNK (SP600125), reversed the effect of ciglitazone on PDK1 protein expression. Ciglitazone reduced PDK1 gene promoter activity, which was not observed in cells exposed to compound C, but not silenced of PPARγ siRNA. Combination of ciglitazone and metformin further reduced PDK1 expression and promoter activity. Furthermore, we showed that ciglitazone induced the protein expression of Egr-1, which was not observed in cells silencing of AMPKα. Moreover, silencing of Egr-1 abrogated the effect of ciglitazone on PDK1 promoter activity and cell growth. On the contrary, overexpression of Egr-1 enhanced the effect of ciglitazone on PDK1 gene promoter activity. ChIP assays demonstrated that ciglitazone induced Egr-1 protein bind to the specific DNA site in the PDK1 gene promoter.
Conclusion
Collectively, our results demonstrate that ciglitazone inhibits PDK1 expression through AMPKα-mediated induction of Egr-1 and Egr-1 binding to the specific DNA site in the PDK1 gene promoter, which is independent of PPARγ. Activation of AMPKα by metformin enhances the effect of ciglitazone. In turn, this leads to inhibition of NSCLC cell proliferation.
Similar content being viewed by others
Background
Lung cancer remains the leading cause of cancer-related mortality in the United States, and 30% to 40% of newly diagnosed patients with non-small cell lung cancer (NSCLC) present with regionally advanced and unresectable stage III disease [1]. Despite recent advances in understanding the molecular biology of lung cancer and the introduction of multiple new chemotherapeutic agents for its treatment, the poor outcomes related to lung cancer have not changed substantially [2, 3]. This justifies the continuing search for agents with therapeutic potential against NSCLC.
Peroxisome proliferator-activated receptors (PPARs isotypes α, β/δ,γ) are ligand-inducible nuclear transcription factors that heterodimerize with retinoid X receptors and bind to PPAR response elements (PPRE) located in the promoter region of PPAR target genes [4]. The role of PPARγ, one PPAR isotype, has been extensively studied thanks to the availability of synthetic PPARγ agonists including antidiabetic drugs, such as rosiglitazone, ciglitazone, and pioglitazone [5]. These drugs are also effective in regulating cell activation, differentiation, proliferation, and apoptosis through both PPARγ-dependent and -independent signaling [6, 7]. However, the detailed mechanisms responsible for these effects remain incompletely elucidated.
Stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) is a mitogen-activated protein kinase family member that is activated by diverse stimuli and plays a critical role in regulating cell fate, being implicated in a multitude of diseases ranging from cancer to neurological, immunological and inflammatory conditions. JNK signaling is required for normal mammary gland development and has a suppressive role in mammary tumorigenesis [8]. AMP-activated protein kinase (AMPK), a heterotrimeric protein complex with serine/threonine kinase activity, has been involved in the regulation of a number of physiological processes including β-oxidation of fatty acids, lipogenesis, protein and cholesterol synthesis, as well as cell cycle inhibition and apoptosis. AMPK has been shown to act upstream and downstream of known tumor suppressors. However, whether AMPK acts as a bona fide tumor suppressor or a oncogene and, of particular importance, if AMPK should be targeted for activation or inhibition during cancer therapy, is controversial [9]. Early growth response-1 (Egr-1) is a Cys2-His2-type zinc-finger transcription factor. A broad range of extracellular stimuli is capable of activating Egr-1, thus mediating growth, proliferation, differentiation or apoptosis. Egr-1 is, therefore, participating in the progression of a variety of diseases such as atherosclerosis or cancer. A growing body of evidence suggests that Egr-1 functions as a tumor suppressor [10–12].
In an effort to explore the anti-tumor effects of ciglitazone on potential targets, we turned our attention to 3-phosphoinositide-dependent protein kinase 1 (PDK1), a master regulator of signal cascades that is involved in suppression of apoptosis and promotion of tumor growth including lung cancer [13]. Reduction of PDK1 by small interfering RNA (siRNA) in several cancer cells results in significant growth inhibition [14–17]. These observations suggest that PDK1 can be used as a target for cancer therapies.
Here, we report that ciglitazone inhibits NSCLC proliferation by inhibiting PDK1 expression through activation of AMPKα and induction of Egr-1 that is independent of PPARγ.
Results
Ciglitazone decreased growth and induced apoptosis in lung cancer cells, and inhibited PDK1 protein expression independent of PPARγ
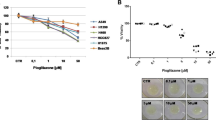
We first examined the effect of ciglitazone on growth and apoptosis of lung cancer cells. We found that ciglitazone inhibited growth of lung cancer cell H1650 in the time- and dose-dependent manner, with significant inhibition observed at 20 μM at 48 h (Figure 1A, upper panel). Similar results were also observed in other NSCLC cell lines (Figure 1A, lower panel). We also showed that ciglitazone induced caspase 3/7 activity in H1650 cells indicating increase in apoptosis (Figure 1B). We then examined whether ciglitazone affected the expression of PDK1. We found that ciglitazone inhibited PDK1 protein expression in a time- and dose-dependent manner, with an effective response of 20 μM at 24 h in H1650 cells (Figure 1C). Reduction of PDK1 protein expression by ciglitazone was also found in other NSCLC cell lines (Figure 1D).
We then tested whether the effects of ciglitazone on PDK1 were mediated through the activation of PPARγ. We showed that, while ciglitazone increased the PPRE luciferase activity (activation of PPAR) (Figure 2A), the effects of ciglitazone on PDK1 expression were not eliminated in the presence of GW9662, a specific PPARγ antagonist (Figure 2B) and in cells (H1299 and H1650) silencing of PPARγ (not shown). The result suggests that PPARγ-independent signals mediate the effect of ciglitazone on PDK1 protein expression.
Ciglitazone decreased growth and induced apoptosis in lung cancer cells. A, H1299 cells were treated with increased concentrations of ciglitazone for up to 72 h (upper panel). NSCLC cells indicated were treated with ciglitazone (20 μM) or up to 48 h (lower panel). The cell viability was determined using the MTT assay as described in the Materials and Methods section in three separate experiments. B, Caspase 3/7 activity assay was performed on H1299 cells treated with or without ciglitazone for 48 h. Relative caspase 3/7 activity is indicated as percentage of untreated control cells. Results represent those obtained in three experiments. *indicates significant difference as compared to the untreated control group (P < 0.05). C, Cellular protein was isolated from H1299 cells that were cultured with increased concentrations of ciglitazone for up to 24 h (upper), or with ciglitazone (20 μM) for indicated period of time (lower), followed by Western blot. D, Cellular protein were isolated from NSCLC cells (H1299, PC9, A549, H1957, H358 and H1650) that were cultured with ciglitazone (20 μM) for up to 24, followed by Western Blot.
Ciglitazone inhibited PDK1 protein expression independent of PPARγ. A, H1299 and H1650 cells were transfected with control or PPRE X3-TK-luc reporter (from Addgene) for 24 h, followed by treating with ciglitazone for an additional 24 h. Afterwards, the Luciferase reporter activity was measured using Luciferase Assay System (Promega) according to manufacturer's instructions. The bars represent the mean ± SD of at least three independent experiments for each condition. *indicates significant difference as compared to the untreated control group (P < 0.05). B, Cellular protein was isolated from H1299 and H1650 cells cultured for 1 h in the presence or absence of GW9662 (20 μM) before exposing the cells to ciglitazone (20 μM) for an additional 24 h, then subjected to Western blot analysis. C, H1299 cells were transfected with control or PDK1 siRNA (80 nM) for 40 h, followed by exposing the cells to ciglitazone (20 μM) for an additional 24 h. Afterwards, the luminescence of viable cells was detected using Cell Titer-Glo Luminescent Cell Viability Assay kit. D-E, H1299 cells were transfected with the control and PDK1 expression vectors using the oligofectamine reagent according to the manufacturer’s instructions. After 24 h of incubation, cells were treated with or without ciglitazone for an additional 24 h. Afterwards, the luminescence of viable cells was detected using Cell Titer-Glo Luminescent Cell Viability Assay kit (D). In separate experiment, the relative caspase 3/7 activity (E) is indicated as percentage of untreated control cells. The bars represent the mean ± SD of at least four independent experiments for each condition. Insert on the top panel shows a Western blot for PDK1 protein. *indicates significant difference as compared to the untreated control group (P < 0.05). **Indicates significance of combination treatment as compared with ciglitazone alone (P < 0.05).
Next, to test whether ciglitazone affects cell growth through PDK1-mediated signals, we blocked the PDK1 gene using PDK1 siRNA. We showed that knockdown of PDK1 significantly reduced PDK1 production, while the control siRNA had no effect (Figure 2C, upper panel). Cells exposed to PDK1 siRNA showed a slight reduction in cell proliferation at baseline; however, they showed significant reduction in growth in the presence of ciglitazone as determined by cell viability assay (Figure 2C, lower panel). Overexpression of PDK1 has been reported to correlate with tumor progression [15]. We found that overexpression of PDK1 abrogated the effect of ciglitazone on cell growth (Figure 2D, lower panel) and caspase 3/7 activity (Figure 2E). Transfection with PDK1 expression vector was confirmed by Western blot (Figure 2D-E, upper panel). Together, this suggested that ciglitazone not only inhibited growth but also increased apoptosis of lung cancer cells through, at least in part, the inhibition of PDK1.
The role of AMPKα and SAPK/JNK in mediating the effect of ciglitazone on PDK1 protein expression
Studies by this group and others also demonstrated a role for AMPK in mediating the effect of PPARγ ligands, such as thiazolinediones (TZDs) compounds, in different cell systems [18, 19]. We showed that ciglitazone increased phosphorylation of AMPKα and SAPK/JNK with maximal effect observed at 2–4 h in H1650 cells (Figure 3A-B). Interestingly, the inhibitors of AMPK, compound C, but not of SAPK/JNK, SP600125, blocked the inhibitory effect of ciglitazone on PDK1 protein expression in both H1650 and H1299 cells (Figure 3C). Similarly, silencing of AMPKα abrogated the effect of ciglitazone on PDK1 protein (Figure 3D). This indicates the specificity of AMPKα activation in this process. Interestingly, combination treatment of ciglitazone and metformin, an activator of AMPK, further reduced the PDK1 protein expression (Figure 3E).
The role of AMPK and SAPK/JNK in mediating the effect of ciglitazone on PDK1 protein expression. A-B, Cellular protein were isolated from H1299 cells that were cultured with ciglitazone for up to 24, followed by Western Blot for phosphor-AMPKα, SAPK/JNK and total AMPKα, SAPK/JNK. C, Cellular protein was isolated from H1299 and H1650 cells treated with SP600125 (10 μM) or compound C (20 μM) for 1 h before exposure of the cells to ciglitazone for an additional 24 h. Afterwards, Western blot was performed. D, Cellular protein was isolated from H1299 cells transfected with control or AMPKα siRNA (80 nM) for 40 h, followed by exposing the cells to ciglitazone for an additional 24 h. Afterwards, Western blot was performed. E, Cellular protein was isolated from H1299 cells treated with ciglitazone and metformin (5 mM) for 24 h, followed by Western Blot. GAPDH served as internal controls for normalization purposes. The bar graph represents the mean ± SD of PDK1/GAPDH of at least three independent experiments. *Indicates significant difference from untreated control. **Indicates significance of combination treatment as compared with ciglitazone alone (P < 0.05).
Ciglitazone decreases PDK1 promoter activity independent of PPARγ activation
We also examined if the effects of ciglitazone on PDK1 expression occurred at the transcriptional level. As shown in Figure 4A, the PDK1 gene promoter contains multiple transcription factor binding sites including PPRE, Egr-1, nuclear factor-κB (NF-κB) and p53, among others. We found that NSCLC cells transfected with wild-type PDK1 promoter-luciferase reporter construct showed decreased activity when exposed to ciglitazone (Figure 4B). As expected, metformin enhanced the inhibitory effect of ciglitazone (Figure 4B).
Next, we assessed whether PPARγ activation played a role in mediating the effect of ciglitazone on PDK1 promoter activity. The effect of ciglitazone on inhibition of PDK1 promoter activity was not abrogated by PPARγ siRNA (Figure 4C, lower panel). Note that PPARγ siRNA blocked PPARγ protein expression (Figure 4C, upper panel). As expected, we found that compound C reduced the effect of ciglitazone on PDK1 promoter activity (Figure 4D).
Ciglitazone decreased PDK1 promoter activity. A, The human PDK1 wild type reporter construct schematics are presented. These regions contain several transcription factor binding sites including PPRE, Egr-1, p53 and NF-κB. B, H1299 and H1650 lung cancer cells (1 × 105 cells) were transfected with a wild type human PDK1 promoter reporter construct ligated to luciferase reporter gene and an internal control Renilla Luciferase Reporter Vector for 24 h. Afterward, cells were treated with ciglitazone for an additional 24 h. C, H1299 cells were transfected with control or PPARγ siRNAs (80 nM) together with a wild type ILK promoter construct for 30 h, then cells were exposed to ciglitazone for an additional 24 h. Insert on the top shows Western blot result for PPARγ protein. GAPDH served as internal control for normalization purposes. D, H1299 cells (1 × 105 cells) were transfected with a wild type human PDK1 promoter reporter construct ligated to luciferase reporter gene and an internal control Renilla Luciferase Reporter Vector as described in Materials and Methods for 24 h. Afterwards, cells were treated with SP600125 (10 μM) for 1 h before exposure of the cells to ciglitazone for an additional 24 h. The ratio of firefly luciferase to renilla luciferase activity was quantified as described in Material and Methods. The bars represent the mean ± SD of at least four independent experiments for each condition. *Indicates significant increase of activity as compared to controls. **Indicates significance of combination treatment as compared with ciglitazone alone (P < 0.05).
The role of transcription factor Egr-1 in mediating the effect of ciglitazone on expression of PDK1 and cell growth
We further tested the role of the transcription factors in mediating the effect of ciglitazone on PDK1 expression in human lung carcinoma cells. We showed that ciglitazone significantly induced the expression of Egr-1 protein in a time–dependent manner, while it had little effect on p65 and p53 (Figure 5A, upper panel). Note that a synergy was observed in the combination of ciglitazone and metformin treatment (Figure 5A lower panel). Interestingly, we also found that silencing of AMPKα abolished the effect of ciglitazone on Egr-1 protein expression, further suggesting the critical role of AMPKα activation in this process (Figure 5B). Next, we found that while cells transfected with Egr-1 siRNA slightly increased PDK1 promoter activity at baseline, it greatly antagonized the inhibitory effect of ciglitazone on PDK1 promoter activity. Note that the control siRNA had no effect (Figure 5C, lower panel). Egr-1 siRNA reduced the production of Egr-1 protein (Figure 5C, upper panel). Furthermore, it eliminated the ciglitazone-reduced PDK1 protein expression, whereas the control siRNA had no effect (Figure 5D). Consistent with these findings, we found that cells transfected with Egr-1 siRNA blocked the inhibitory effects of ciglitazone on cell growth (Figure 5E). The control siRNA had no effect.
Ciglitazone induces Egr-1 protein expression; silencing of Egr-1 abrogates the effect of ciglitazone on PDK1 promoter activity, protein expression and cell proliferation. A, Cellular proteins were isolated from H1299 cells treated with ciglitazone (20 μM) for the indicated time period (upper panel) or with metformin (5 mM) for 24 h (lower panel). Afterwards, Western blot analyses were performed for detecting Egr-1, p65 and p53 proteins. B, H1299 cells were transfected with control or AMPKα siRNA (80 nM) for 30 h before exposing the cells to ciglitazone (20 μM) for an additional 24 h followed by Western blot. C, H1299 cells were transfected with control or Egr-1 siRNA (80 nM) together with a wild type PDK1 promoter construct for 30 h, then cells were exposed to ciglitazone (20 μM) for an additional 24 h. Insert shows the Western blot result for Egr-1 protein. D, H1299 cells were transfected with control or Egr-1 siRNA (80 nM) for 30 h before exposing the cells to ciglitazone (20 μM) for an additional 24 h, followed by Western blot. E, H1299 cells were transfected with control or Egr-1 siRNA (80 nM) for 30 h before exposure of the cells to ciglitazone (20 μM) for an additional 24 h. Afterwards, the luminescence of viable cells was detected using Cell Titer-Glo Luminescent Cell Viability Assay kit. The bars represent the mean ± SD of at least four independent experiments for each condition. *Indicates significant difference as compared to the control. **Indicates significance of combination treatment as compared with ciglitazone alone (P < 0.05).
However, cells co-transfected with an Egr-1 expression vector showed little or no synergistic effect on PDK1 promoter activity (Figure 6A), suggesting the specificity of Egr-1. Next, by ChIP assays, we showed that ciglitazone induced Egr-1 protein binding to the Egr-1 DNA site in the PDK1 gene promoter (Figure 6B).
Overexpression of Egr-1 reduces PDK1 promoter activity. A, H1299 cells (1x105 cells) were transfected with control and Egr-1 expression reporter constructs, and together with a wild type PDK1 promoter construct and an internal control Renilla Luciferase Reporter Vector as described in Material and Methods section for 24 h, then treated with ciglitazone (20 μM) for an additional 24 h. The insert in upper panel represents Western blot results for Egr-1 protein. B, H1299 cells were lysed after exposure of ciglitazone (20 μM) for 24 h, and nuclei were isolated and then sonicated. Chromatin from H1650 cells was immunoprecipitated using antibodies against Egr-1 protein or preimmune serum (pre-immune). PCR analysis using primers surrounding the Egr-1 site shows that this DNA sequence (−4392 to −4402 bp) is specifically immunoprecipitated indicating that Egr-1 binds to endogenous DNA sites in the PDK1 promoter. A non- Egr-1 sequence was used as control. Aliquots of the chromatin were also analyzed before immunoprecipitation (input). C, Diagram demonstrates that ciglitazone inhibits PDK1 expression through AMPKα-mediated induction of Egr-1 protein expression and Egr-1 protein binding to the DNA sequence in the PDK1 gene promoter independent of PPARγ. Activation of AMPKα enhances the effect of ciglitazone on Egr-1 and PDK1 protein expression. In turn, this results in inhibition of NSCLC cell proliferation.
Discussion
The expression of PPARγ and the effects of PPARγ ligands on cell growth have been extensively studied in many carcinoma cell types including lung [6, 20]. However, the exact mechanisms mediating the effects of PPARγ ligands on cell growth inhibition are not fully understood. We have found that ciglitazone, a TZD and one of the synthetic PPARγ ligands, inhibited growth and induced apoptosis of NSCLC cells through reduction of PDK1, a kinase and master regulator of a number of downstream signal cascades that are involved in suppression of apoptosis and promotion of tumor growth including lung cancer [13, 21]. Inhibition of PDK1 in several cancer cells results in significant cell growth inhibition [14–17]. These observations suggest that PDK1 can be considered as a key mediator of neoplasia and a promising anticancer target. This result, together with the finding that exogenous PDK1 diminishes the effect of ciglitazone on cancer cell growth, suggests a critical role of PDK1 in this process.
The concentrations of ciglitazone used here, found significantly inhibition of PDK1 gene expression and cell growth , are consistent or even lower with those reported by others which showed a significant effect on cell growth and apoptosis at clinically achievable concentrations [22–25]. For example, ciglitazone inhibited the growth of androgen-dependent and -independent human prostate cancer cells starting at 10 and reached maximal at even 45 μM concentrations [23]. In another study, ciglitazone showed to significantly inhibit cell viability and proliferation of brain tumor stem cells starting at 5 and continued to 25 μM concentration [25].
We demonstrated that ciglitazone inhibited the expression of PDK1 protein independent of PPARγ signals. Consistent with this, the PPARγ-independent signals mediating the effects of PPARγ ligands on gene expression and cell proliferation including lung cancer have been shown in other studies [24, 26, 27] although PPARγ-dependent signals were observed [28, 29]. We reasoned that targeting PDK1 may also involve such mechanisms by which ciglitazone inhibits NSCLC cell growth. Given the fact that silencing of the PPARγ gene by siRNA had no effect on blockage of the effect of ciglitazone on PDK1 promoter activity, additional experiments are required to explore the contributions of PPARγ-independent mechanisms in these processes.
Interestingly, PDK1 knockdown alone did not affect cell proliferation significantly. However, inhibition of PDK1 in the setting of ciglitazone treatment resulted in largely growth inhibition. This suggests that other factors are important for control of NSCLC cell proliferation. It also suggests that the growth inhibitory effects of ciglitazone may occur by concomitant actions on pathways other then PDK1. Report shown that ciglitazone exerts effects on several other targets that were implicated in control of lung cancer growth [30, 31].
In this study, we showed that activation of AMPKα played a vital role in mediating the effect of ciglitazone on PDK1 expression. In addition, activation of AMPK enhanced the effect of ciglitazone on PDK1 expression and promoter activity. Data demonstrated that synthetic PPARγ ligands regulated several kinase signaling pathways including AMPK in different cells [32, 33]. Activation or inactivation of AMPK has been shown to link synthetic PPARγ agonists-mediated signaling to the transcriptional regulation of genes that are crucial for cell growth inhibition [32, 34]. Considering the recent data for the dual role of AMPK [9], we believed that more dedicated studies are required to further elucidate the biological function and relevant signaling of this kinase.
Having demonstrated the important role of PDK1, we further investigated whether the ciglitazone–mediated downregulation of PDK1 reflected inhibition of transactivation of the PDK1 gene. Our results suggested that increased Egr-1 protein expression and binding to the upstream areas of PDK1 gene promoter played an important role in mediating the effect of ciglitazone. Knockdown of Egr-1 abrogated the effect of ciglitazone on PDK1 expression and on cell proliferation, whereas overexpression of Egr-1 had no further effect of ciglitazone on PDK1 promoter activity confirming the inhibitory property of this transcription factor. It also suggested the specificity of Egr-1 played in this process. To our knowledge, the role of Egr-1 in regulation of PDK1 expression has never been reported. Egr-1 functions as a tumor suppressor in many cancers [10–12]. Loss of Egr-1 expression has been associated with invasion and anti-apoptotic events, whereas overexpression of Egr-1 suppressed the tumorigenicity and metastatic potential in several cancer cells including lung [34, 35]. However, opposite role of Egr-1 were also found in several studies [36, 37]. Thus Egr-1 is considered to play dual roles depending on the cell types and environment. One study showed that several PPARγ ligands including TZD induced the expression of Egr-1 through PPARγ-independent pathway in breast cancer cells [38]. Thus, other factors responsible for this effect (in addition to Egr-1) need further exploration. ChIP assays showed that Egr-1 protein occupancy of the Egr-1 sites in the upstream areas of PDK1 gene promoter was enhanced by exposure of cells to ciglitazone. Further studies are required by site-directed mutagenesis experiments to confirm this. Moreover, the detail mechanisms responsible for the effect of metformin in this process needs to be determined.
Conclusion
Our results demonstrate that ciglitazone inhibits PDK1 expression through AMPKα-mediated induction of Egr-1 protein expression and Egr-1 binding to specific DNA sequences in the PDK1 gene promoter, which is independent of PPARγ activation. Activation of AMPKα by metformin enhances the effect of ciglitazone on Egr-1 and PDK1 protein expression. In turn, this leads to inhibition of NSCLC cell proliferation (Figure 6C). This study provides a novel mechanism by which the antidiabetic drug inhibits human lung cancer cell growth, and targeting the PDK1 may be a potential therapeutic strategy for inhibition of lung cancer growth.
Materials and methods
Culture and chemicals
The human NSCLC cell lines A549, H1650, PC9, H1975, H1299 and H358 were obtained from the Cell Line Bank at the Laboratory Animal Center of Sun Yat-sen University starting March 2012 (Guangzhou, China) and grown in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, HEPES buffer, 50 IU/mL penicillin/streptomycin, and 1 μg amphotericin (complete medium). All cell lines have been tested and authenticated for absence of Mycoplasma, genotypes, drug response, and morphology using a commercially available kit (Invitrogen, Shanghai, China) in the Laboratory and Animal Center at Sun Yat-sen University in April 2010 and August 2012. Polyclonal antibodies specific for PDK1, phosphor-AMPKα (thr172) phosphor-p-SAPK/JNK (Thr183/Tyr185) and total AMPKα and SAPK/JNK were purchased from Cell Signaling (Beverly, MA). Polyclonal antibodies against PPARγ, AMPKα, p53, p65 and Egr-1 were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA). Ciglitazone, SP600125, GW9662, compound C, metformin and other chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA) unless otherwise indicated.
Western blot analysis
Protein concentrations were determined by the Bio-Rad protein assay. Equal amounts of protein from whole cell lysates were solubilized in 2x SDS-sample buffer and separated on 10% SDS polyacrylamide gels. Membranes were incubated with antibodies against PDK1, PPARg phosphor-AMPKα (thr172) phosphor-p-SAPK/JNK (Thr183/Tyr185) and total AMPKα and SAPK/JNK, p53, p65 and Egr-1. The membranes were washed and incubated with incubation with a secondary goat antibody raised against rabbit IgG conjugated to horseradish peroxidase (Cell Signaling, Beverly, MA, USA). The membranes were washed again and transferred to freshly made ECL solution (Pierce, Rockford, IL, USA) for 1 min, and exposed to X-ray film.
MTT cell viability assay
Cell viability was measured using the 3-(4, 5-dimethylthiazol-2- yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Briefly, NSCLC cells (5 × 103 cells/well) were counted and seeded into a 96-well microtiterplate. The cells were treated with increasing concentrations of ciglitazone for up to 72 h. After incubation, 10 μL MTT solution (5 g/L) was added to each well and incubated at 37°C for an additional 4 h. Supernatant was removed, then 150 μL DMSO was added to each well and oscillated for 10 min. Absorbance at 490 nm was determined through the use of ELISA reader (Perkin Elmer, Victor X5, USA). Each experiment was repeated at least three times. Cell viability (%) was calculated as (absorbance of test sample/absorbance of control) × 100%.
CellTiter-Glo luminescent cell viability assay
Human lung carcinoma cells were treated with compound C for 2 h or were transfected with control or Egr-1 siRNA or PDK1 expression vectors for 24 h before exposure of the cells to ciglitazone for an additional 24 h in 96-well plates in DMEM media with 0.5% FBS. Afterwards, cell viability was measured using the CellTiter-Glo Luminescent Cell Viability Assay kit (Promega, Shanghai, China) according to the instructions of the manufacturer.
Detection of caspase-3/7 activity
Enzymatic activity of caspase-3/7 was measured using the Caspase-Glo 3/7 Assay kit (Promega, Shanghai, China) according to the manufacturer’s instruction. Briefly, NSCLC cells were seeded in 96-well plates and treated with or without 20 μM of ciglitazone for 48 h. Afterwards, the cells were lysed and incubated with 100 μL of Apo-ONE Caspase-3/7 reagent (substrate and buffer in the ratio of 1:100). After 1 h incubation in the dark at RT, the fluorescence of each well was measured at 485–520 nm by reading in an Epoch microplate reader (Biotek Instruments; Winooski, VT, USA).
Treatment with AMPKα, PDK1, Egr-1 and PPARγ small interfering RNA (siRNA)
The siRNA human PDPK1 (EHU071261) was ordered from Sigma (Shanghai, China). The AMPKα (Cat No.sc-45312), Egr-1 siRNA (Cat No. sc-105070), PPARγ siRNA (Cat No. sc-29455), and control nonspecific siRNA oligonucleotides (Cat No. sc-37007) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). For the transfection procedure, cells were grown to 60% confluence, and PDK1, Egr-1, and PPARγ and control siRNAs were transfected using the oligofectamine reagent (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. Briefly, Lipofectamine was incubated with serum–free medium for 10 min., mixed with siRNA (80 nM), incubated for 20 min at room temperature before the mixture was diluted with medium and added to cells. After culturing for 30 h, cells were washed, resuspended in new culture media in the presence or absence of ciglitazone for an additional 24 h for Western Blot, cell growth, luciferase report assays and other experiments.
Transient transfection assays
The original human PDK1 promoter construct was a gift from Dr. Michalik at the University of Lausanne and have been reported previously [39]. The PDK1 promoter construct contains approximately 1500 base pairs of the 5’ flanking region of the human PDK1 gene connected to the pGL3 basic luciferase reporter vector [39]. Briefly, NSCLC cells were seeded at a density of 5 × 105 cells/well in 6-well dishes and grown to 50 –60% confluence. For each well, 2 μg of the control or PPRE X3-TK-luc reporter (Addgene, plasmid 1015) [40], above PDK1 plasmid DNA constructs, or overexpression of PDK1 (pDONR223-PDPK1) (Addgene plasmid 23801) [41] or Egr-1 expression vectors [obtained from Dr. Thomas Eling (NIEHS, USA) and have been reported previously] [42], with or without 0.2 μg of the internal control phRL-TK Renilla Luciferase Reporter Vector were co-transfected into the cells with the oligofectamine reagent (Invitrogen, Shanghai, China). In parallel experiments, NSCLC cells transfected with Egr-1, PPARγ, or control siRNAs (80 nM each) for 30 h followed by exposed the cells to ciglitazone for an additional 24 h. The preparation of cell extracts and measurement of luciferase activities were determined using the Dual-Luciferase Reporter Kit (Promega, Shanghai, China). Firefly luciferase activity was normalized with Renilla luciferase activity within each sample.
Chromatin immunoprecipitation assay (ChIP)
ChIP assays were performed as described by other study [43]. Briefly, cells were incubated in 1% formaldehyde for 10 min at 37°C, quenched with 125 mmol/L glycine, lysed in SDS buffer with protease inhibitors (Roche), 0.5 mmol/L phenylmethyl-sulfonyl fluoride and sonicated. Fragmented chromatin was pre-cleared by adding salmon sperm-DNA/protein A-agarose beads. A portion of the supernatant was kept as “input” material. The remaining cleared chromatin was incubated overnight with or without 5 μg of anti-Egr-1 antibody or normal human IgG (Upstate Biotechnology, Shanghai, China). DNA (10 μg) from each immunoprecipitation was reserved for input controls. DNA was purified with QIAquick PCR purification column (QIAGEN Sciences, MA) and genomic sequences of interest were amplified by PCR using primers Egr-1 forward (−4392/-4402) 5′- GAGGGTGGACACAGTTGAGTCAG-3′ and reverse ′5-TGGACAACATTAGCAAGACCCTG-3′. A total of 2% of each IP was assayed by PCR using primers specific for the region of interest (218 bp).
Statistical analysis
All experiments were repeated a minimum of three times. All data were expressed as means ± SD. and then processed using SPSS10.0 software. Statistical significance was determined with Student’s t test (two-tailed) comparison between two groups of data set. Asterisks shown in the figures indicate significant differences of experimental groups in comparison with the corresponding control condition (P < 0.05, see figure legends).
Abbreviations
- PPARγ:
-
Peroxisome proliferator-activated receptors gamma
- PDK1:
-
Phosphoinositide-dependent protein kinase 1
- Egr-1:
-
Early growth response-1
- siRNA:
-
Small interfering RNA
- ChIP:
-
Chromatin immunoprecipitation assay
- AMPKα:
-
AMP-activated protein kinase alpha
- SAPK/JNK:
-
Stress-activated protein kinase/c-Jun N-terminal kinase
- NSCLC:
-
Non-small cell lung cancer
- PPRE:
-
PPAR response elements
- NF-κB:
-
Nuclear factor-κB
- TZDs:
-
Thiazolinediones.
References
Lee CB, Stinchcombe TE, Rosenman JG, Socinski MA: Therapeutic advances in local-regional therapy for stage III non-small-cell lung cancer: evolving role of dose-escalated conformal (3-dimensional) radiation therapy. Clin Lung Cancer. 2006, 8: 195-202. 10.3816/CLC.2006.n.047
Dempke WC, Suto T, Reck M: Targeted therapies for non-small cell lung cancer. Lung Cancer. 2010, 67: 257-274. 10.1016/j.lungcan.2009.10.012
Siegel R, Naishadham D, Jemal A: Cancer statistics, 2013. CA Cancer J Clin. 2013, 63: 11-30. 10.3322/caac.21166
Tan NS, Michalik L, Desvergne B, Wahli W: Multiple expression control mechanisms of peroxisome proliferator-activated receptors and their target genes. J Steroid Biochem Mol Biol. 2005, 93: 99-105. 10.1016/j.jsbmb.2004.12.025
Choi JH, Banks AS, Kamenecka TM, Busby SA, Chalmers MJ, Kumar N, Kuruvilla DS, Shin Y, He Y, Bruning JB, Marciano DP, Cameron MD, Laznik D, Jurczak MJ, Schürer SC, Vidović D, Shulman GI, Spiegelman BM, Griffin PR: Antidiabetic actions of a non-agonist PPARgamma ligand blocking Cdk5-mediated phosphorylation. Nature. 2011, 477: 477-481. 10.1038/nature10383
Terrasi M, Bazan V, Caruso S, Insalaco L, Amodeo V, Fanale D, Corsini LR, Contaldo C, Mercanti A, Fiorio E, Lo Re G, Cicero G, Surmacz E, Russo A: Effects of PPARgamma agonists on the expression of leptin and vascular endothelial growth factor in breast cancer cells. J Cell Physiol. 2013, 228: 1368-1374. 10.1002/jcp.24295
Li S, Zhou Q, He H, Zhao Y, Liu Z: Peroxisome proliferator-activated receptor gamma agonists induce cell cycle arrest through transcriptional regulation of Kruppel-like factor 4 (KLF4). J Biol Chem. 2013, 288: 4076-4084. 10.1074/jbc.M111.317487
Cellurale C, Girnius N, Jiang F, Cavanagh-Kyros J, Lu S, Garlick DS, Mercurio AM, Davis RJ: Role of JNK in mammary gland development and breast cancer. Cancer Res. 2012, 72: 472-481. 10.1158/0008-5472.CAN-11-1628
Liang J, Mills GB: AMPK: a contextual oncogene or tumor suppressor?. Cancer Res. 2013, 73: 2929-2935. 10.1158/0008-5472.CAN-12-3876
Cho KN, Sukhthankar M, Lee SH, Yoon JH, Baek SJ: Green tea catechin (−)-epicatechin gallate induces tumour suppressor protein ATF3 via EGR-1 activation. Eur J Cancer. 2007, 43: 2404-2412. 10.1016/j.ejca.2007.07.020
Gibbs JD, Liebermann DA, Hoffman B: Egr-1 abrogates the E2F-1 block in terminal myeloid differentiation and suppresses leukemia. Oncogene. 2008, 27: 98-106. 10.1038/sj.onc.1210627
Bar-Shavit R, Turm H, Salah Z, Maoz M, Cohen I, Weiss E, Uziely B, Grisaru-Granovsky S: PAR1 plays a role in epithelial malignancies: transcriptional regulation and novel signaling pathway. IUBMB Life. 2011, 63: 397-402. 10.1002/iub.452
Raimondi C, Falasca M: Targeting PDK1 in cancer. Curr Med Chem. 2011, 18: 2763-2769. 10.2174/092986711796011238
Lu Z, Cox-Hipkin MA, Windsor WT, Boyapati A: 3-phosphoinositide-dependent protein kinase-1 regulates proliferation and survival of cancer cells with an activated mitogen-activated protein kinase pathway. Mol Cancer Res. 2010, 8: 421-432. 10.1158/1541-7786.MCR-09-0179
Liu Y, Wang J, Wu M, Wan W, Sun R, Yang D, Sun X, Ma D, Ying G, Zhang N: Down-regulation of 3-phosphoinositide-dependent protein kinase-1 levels inhibits migration and experimental metastasis of human breast cancer cells. Mol Cancer Res. 2009, 7: 944-954. 10.1158/1541-7786.MCR-08-0368
Hann SS, Zheng F, Zhao S: Targeting 3-phosphoinositide-dependent protein kinase 1 by N-acetyl-cysteine through activation of peroxisome proliferators activated receptor alpha in human lung cancer cells, the role of p53 and p65. J Exp Clin Cancer Res. 2013, 32: 43- 10.1186/1756-9966-32-43
Gagliardi PA, di Blasio L, Orso F, Seano G, Sessa R, Taverna D, Bussolino F, Primo L: 3-phosphoinositide-dependent kinase 1 controls breast tumor growth in a kinase-dependent but Akt-independent manner. Neoplasia. 2012, 14: 719-731.
Wu J, Wu JJ, Yang LJ, Wei LX, Zou DJ: Rosiglitazone protects against palmitate-induced pancreatic beta-cell death by activation of autophagy via 5′-AMP-activated protein kinase modulation. Endocrine. 2013, 44: 87-98. 10.1007/s12020-012-9826-5
Morrison A, Yan X, Tong C, Li J: Acute rosiglitazone treatment is cardioprotective against ischemia-reperfusion injury by modulating AMPK, Akt, and JNK signaling in nondiabetic mice. Am J Physiol Heart Circ Physiol. 2011, 301: H895-H902. 10.1152/ajpheart.00137.2011
Ramer R, Heinemann K, Merkord J, Rohde H, Salamon A, Linnebacher M, Hinz B: COX-2 and PPAR-gamma confer cannabidiol-induced apoptosis of human lung cancer cells. Mol Cancer Ther. 2013, 12: 69-82.
Peifer C, Alessi DR: Small-molecule inhibitors of PDK1. ChemMedChem. 2008, 3: 1810-1838. 10.1002/cmdc.200800195
Plissonnier ML, Fauconnet S, Bittard H, Lascombe I: The antidiabetic drug ciglitazone induces high grade bladder cancer cells apoptosis through the up-regulation of TRAIL. PLoS One. 2011, 6: e28354- 10.1371/journal.pone.0028354
Moss PE, Lyles BE, Stewart LV: The PPARgamma ligand ciglitazone regulates androgen receptor activation differently in androgen-dependent versus androgen-independent human prostate cancer cells. Exp Cell Res. 2010, 316: 3478-3488. 10.1016/j.yexcr.2010.09.015
Al-Alem L, Southard RC, Kilgore MW, Curry TE: Specific thiazolidinediones inhibit ovarian cancer cell line proliferation and cause cell cycle arrest in a PPARgamma independent manner. PLoS One. 2011, 6: e16179- 10.1371/journal.pone.0016179
Pestereva E, Kanakasabai S, Bright JJ: PPARgamma agonists regulate the expression of stemness and differentiation genes in brain tumour stem cells. Br J Cancer. 2012, 106: 1702-1712. 10.1038/bjc.2012.161
Han S, Roman J: Rosiglitazone suppresses human lung carcinoma cell growth through PPARgamma-dependent and PPARgamma-independent signal pathways. Mol Cancer Ther. 2006, 5: 430-437. 10.1158/1535-7163.MCT-05-0347
Hazra S, Batra RK, Tai HH, Sharma S, Cui X, Dubinett SM: Pioglitazone and rosiglitazone decrease prostaglandin E2 in non-small-cell lung cancer cells by up-regulating 15-hydroxyprostaglandin dehydrogenase. Mol Pharmacol. 2007, 71: 1715-1720. 10.1124/mol.106.033357
Yang C, Jo SH, Csernus B, Hyjek E, Liu Y, Chadburn A, Wang YL: Activation of peroxisome proliferator-activated receptor gamma contributes to the survival of T lymphoma cells by affecting cellular metabolism. Am J Pathol. 2007, 170: 722-732. 10.2353/ajpath.2007.060651
Wang P, Dharmaraj N, Brayman MJ, Carson DD: Peroxisome proliferator-activated receptor gamma activation inhibits progesterone-stimulated human MUC1 expression. Mol Endocrinol. 2010, 24: 1368-1379. 10.1210/me.2009-0221
Han EJ, Im CN, Park SH, Moon EY, Hong SH: Combined treatment with peroxisome proliferator-activated receptor (PPAR) gamma ligands and gamma radiation induces apoptosis by PPARgamma-independent up-regulation of reactive oxygen species-induced deoxyribonucleic acid damage signals in non-small cell lung cancer cells. Int J Radiat Oncol Biol Phys. 2013, 85: e239-e248. 10.1016/j.ijrobp.2012.11.040
Kim BM, Maeng K, Lee KH, Hong SH: Combined treatment with the Cox-2 inhibitor niflumic acid and PPARgamma ligand ciglitazone induces ER stress/caspase-8-mediated apoptosis in human lung cancer cells. Cancer Lett. 2011, 300: 134-144. 10.1016/j.canlet.2010.09.014
Tsai JS, Chen CY, Chen YL, Chuang LM: Rosiglitazone inhibits monocyte/macrophage adhesion through de novo adiponectin production in human monocytes. J Cell Biochem. 2010, 110: 1410-1419. 10.1002/jcb.22657
Okami N, Narasimhan P, Yoshioka H, Sakata H, Kim GS, Jung JE, Maier CM, Chan PH: Prevention of JNK phosphorylation as a mechanism for rosiglitazone in neuroprotection after transient cerebral ischemia: activation of dual specificity phosphatase. J Cereb Blood Flow Metab. 2013, 33: 106-114. 10.1038/jcbfm.2012.138
Awuah PK, Rhieu BH, Singh S, Misse A, Monga SP: beta-Catenin loss in hepatocytes promotes hepatocellular cancer after diethylnitrosamine and phenobarbital administration to mice. PLoS One. 2012, 7: e39771- 10.1371/journal.pone.0039771
Shimoyamada H, Yazawa T, Sato H, Okudela K, Ishii J, Sakaeda M, Kashiwagi K, Suzuki T, Mitsui H, Woo T, Tajiri M, Ohmori T, Ogura T, Masuda M, Oshiro H, Kitamura H: Early growth response-1 induces and enhances vascular endothelial growth factor-A expression in lung cancer cells. Am J Pathol. 2010, 177: 70-83. 10.2353/ajpath.2010.091164
Myung DS, Park YL, Kim N, Chung CY, Park HC, Kim JS, Cho SB, Lee WS, Lee JH, Joo YE: Expression of early growth response-1 in colorectal cancer and its relation to tumor cell proliferation and apoptosis. Oncol Rep. 2014, 31: 788-794.
Ma Y, Cheng Q, Ren Z, Xu L, Zhao Y, Sun J, Hu S, Xiao W: Induction of IGF-1R expression by EGR-1 facilitates the growth of prostate cancer cells. Cancer Lett. 2012, 317: 150-156. 10.1016/j.canlet.2011.11.021
Chbicheb S, Yao X, Rodeau JL, Salamone S, Boisbrun M, Thiel G, Spohn D, Grillier-Vuissoz I, Chapleur Y, Flament S, Mazerbourg S: EGR1 expression: a calcium and ERK1/2 mediated PPARgamma-independent event involved in the antiproliferative effect of 15-deoxy-Delta12, 14-prostaglandin J2 and thiazolidinediones in breast cancer cells. Biochem Pharmacol. 2011, 81: 1087-1097. 10.1016/j.bcp.2011.02.006
Di-Poi N, Tan NS, Michalik L, Wahli W, Desvergne B: Antiapoptotic role of PPARbeta in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol Cell. 2002, 10: 721-733. 10.1016/S1097-2765(02)00646-9
Kim JB, Wright HM, Wright M, Spiegelman BM: ADD1/SREBP1 activates PPARgamma through the production of endogenous ligand. Proc Natl Acad Sci U S A. 1998, 95: 4333-4337. 10.1073/pnas.95.8.4333
Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, Caponigro G, Hieronymus H, Murray RR, Salehi-Ashtiani K, Hill DE, Vidal M, Zhao JJ, Yang X, Alkan O, Kim S, Harris JL, Wilson CJ, Myer VE, Finan PM, Root DE, Roberts TM, Golub T, Flaherty KT, Dummer R, Weber BL: COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010, 468: 968-972. 10.1038/nature09627
Moon Y, Bottone FG, McEntee MF, Eling TE: Suppression of tumor cell invasion by cyclooxygenase inhibitors is mediated by thrombospondin-1 via the early growth response gene Egr-1. Mol Cancer Ther. 2005, 4: 1551-1558. 10.1158/1535-7163.MCT-05-0213
Zheng Y, Ritzenthaler JD, Sun X, Roman J, Han S: Prostaglandin E2 stimulates human lung carcinoma cell growth through induction of integrin-linked kinase: the involvement of EP4 and Sp1. Cancer Res. 2009, 69: 896-904. 10.1158/0008-5472.CAN-08-2677
Acknowledgments
We are grateful to Drs. Michalik and Desvergne (Center for Intergrative Genomics at University of Lausanne in Switzerland) for providing the PDK1 promoter constructs, Dr. William Hahn (Dana-Farber Cancer Institute, Boston, Massachusetts, USA.) for providing PDK1 expression vector and Dr. Thomas E Eling (NIEHS, USA) for providing the Egr-1 expression vector, and finally, Dr. Bruce M. Spiegelman for providing PPRE X3-Tk-luc reporter (Dana-Farber Cancer Institute, Massachusetts, USA). This work was supported in part by the Special Science and Technology Join fund from Guangdong Province-Guangdong Academy of Traditional Chinese Medicine (2012A032500011) and grant from the National Nature Scientific Foundation of China (81272614).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
There is no potential conflict of interest or financial dependence regarding this publication.
Authors’ contributions
SSH conceived of the study, participated in its design and coordination, and draft the manuscript. QT carried out the cell growth, siRNA, Western Blot assays, transfection and luciferase report assays. FZ participated in performed the cell viability, siRNA, transfection assays and protein expression experiments, SYZ involved in cell viability, protein expression and statistical analysis. JPC and ZYW provided agents, coordinated and critical read the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hann, S.S., Tang, Q., Zheng, F. et al. Repression of phosphoinositide-dependent protein kinase 1 expression by ciglitazone via Egr-1 represents a new approach for inhibition of lung cancer cell growth. Mol Cancer 13, 149 (2014). https://doi.org/10.1186/1476-4598-13-149
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-4598-13-149