Abstract
Background
Plague, caused by the bacterium Yersinia pestis, is a public and wildlife health concern in California and the western United States. This study explores the spatial characteristics of positive plague samples in California and tests Maxent, a machine-learning method that can be used to develop niche-based models from presence-only data, for mapping the potential distribution of plague foci. Maxent models were constructed using geocoded seroprevalence data from surveillance of California ground squirrels (Spermophilus beecheyi) as case points and Worldclim bioclimatic data as predictor variables, and compared and validated using area under the receiver operating curve (AUC) statistics. Additionally, model results were compared to locations of positive and negative coyote (Canis latrans) samples, in order to determine the correlation between Maxent model predictions and areas of plague risk as determined via wild carnivore surveillance.
Results
Models of plague activity in California ground squirrels, based on recent climate conditions, accurately identified case locations (AUC of 0.913 to 0.948) and were significantly correlated with coyote samples. The final models were used to identify potential plague risk areas based on an ensemble of six future climate scenarios. These models suggest that by 2050, climate conditions may reduce plague risk in the southern parts of California and increase risk along the northern coast and Sierras.
Conclusion
Because different modeling approaches can yield substantially different results, care should be taken when interpreting future model predictions. Nonetheless, niche modeling can be a useful tool for exploring and mapping the potential response of plague activity to climate change. The final models in this study were used to identify potential plague risk areas based on an ensemble of six future climate scenarios, which can help public managers decide where to allocate surveillance resources. In addition, Maxent model results were significantly correlated with coyote samples, indicating that carnivore surveillance programs will continue to be important for tracking the response of plague to future climate conditions.
Similar content being viewed by others
Background
Plague, caused by the bacterium Yersinia pestis, is a disease that has played an important role in human history, most notably through the demographic impacts of three major historical pandemics [1]. Plague was introduced to the United States during the third pandemic (ca. 1900), and spread from the Pacific coast to its current distribution in the western states. Plague is maintained among wild rodents in distinct geographic foci in the western United States [2]. Although the mechanisms by which plague is maintained between epizootic cycles are not well understood, it is generally accepted that the disease cycles between enzootic infections and occasional epizootic outbreaks among susceptible hosts [2]. Humans are presumably at greatest risk of infection during epizootics, when infectious rodent fleas seek a new host. Plague transmission to humans may also occur through contact with infected pets or other animals, through exposure to infected tissue, or via respiratory exposure to infectious air-borne droplets [3, 4].
The incidence of human plague cases is relatively low in the United States: for example, a total of 107 cases occurred in the United States from 1990 – 2005 [5], compared to over 240,000 cases of Lyme disease, another vector-borne disease, during roughly the same period (1992 – 2006) [6]. Because of this low incidence, plague surveillance in the western United States is often conducted on a limited budget. However, in contrast to Lyme disease, the case-fatality ratio of plague can be high. If antibiotic treatment is not initiated promptly, plague is fatal in 40–70% of bubonic cases and nearly 100% of pneumonic cases [1]. The combination of low incidence with high mortality presents unique surveillance and public health challenges, because early detection through surveillance may not always be feasible and infrequent clinical cases may be misdiagnosed.
In addition, there is concern that certain factors [2, 7–10] could increase the occurrence of plague epizootics as well as the risk of exposure and infection to humans. In particular, the direct and indirect effects of climate change on land use, population distribution, and ecologic character are projected to contribute to an increase in the emergence and incidence of infectious diseases [11], including plague. Climate change may drive plague activity through several pathways (Figure 1), including influences on flea burden, rodent population dynamics, and plague transmission [12–19]. A spatially explicit understanding of how plague risk may shift with changing climate patterns can help not only to direct prevention and control efforts, but can also alert health care providers toward quicker recognition of exposure potential and initiation of appropriate treatment of patients [20], which is critical for improving the health outcome of the individual infected as well as reducing secondary transmission to other people.
A conceptual model of the mechanisms by which climate influences plague transmission and maintenance. Precipitation and temperature have been linked to plague outbreaks in prairie dogs, and to human cases in the United States. A proposed model for this relationship suggests that precipitation and temperature may influence rodent abundance (by influencing rodent survival and food abundance), and that increased rodent populations may affect flea abundance and/or plague transmission rates. In addition to having a positive effect on rodent population dynamics, certain soil moisture, humidity and temperature variables may influence flea ecology and the transmission of the plague pathogen.
Recent studies describing the relationships between future climatic and environmental factors and plague activity in the United States have focused on human cases, as well as animal cases in the Southwestern United States and Colorado plateau [12, 17, 19, 21–24]; here, we focus on the potential distribution of plague in California. The point inputs to the models developed in this study were derived from plague serology data collected by the California Department of Public Health (CDPH) and other agencies. Because active surveillance had most often been conducted in areas with a known history of plague-positive rodents or human cases, we used ecological niche modeling (ENM) to identify the potential distribution of plague throughout California (including in previously unsampled areas). Niche modeling has most often been applied to predict the potential for plant and animal species occurrences [for example, [25, 26]], and is increasingly being used to identify and map the distribution of diseases, such as Chagas disease [27], filovirus disease [28], Marburg hemorrhagic fever [29], avian influenza [30], and plague [15, 31]. In this study we evaluated Maxent, a presence-only niche modeling technique, to describe the potential distribution of plague foci in California under recent and future climatic conditions.
Methods
Data
The point inputs to the models developed in this study were derived from plague surveillance data collected by the California Department of Public Health (CDPH) and other agencies [32]. Records of approximately 37,000 animals (33 different genera) collected throughout the state of California during 1984–2004 were entered into an Access database by public health researchers.
Rodent point data
Rodent samples were obtained most often by active surveillance, which was conducted in areas with a known history of plague-positive rodents or human cases [32]. Rodent sera were tested by passive hemagglutination to F1 antigen of Y. pestis; specimens with antibody titer ≥ 1:32 were considered positive [33–35].
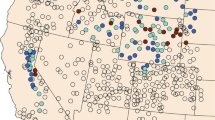
Rodent samples were geocoded based on an address or campsite name, which allowed for location of rodent case point at a <1-km2 spatial resolution. All rodent records were geo-located using National Geographic TOPO software (National Geographic Society 2001). Locations that could not be reliably located to a campground or address were excluded from this analysis. All geocoded points were projected to Teale Albers, NAD 1983 projection. The geocoded locations of the rodent case points are located along the north-south transect of the Sierra Nevada range, and along the southern coast and inland areas of Southern California. No positive rodents were collected in the Modoc plateau, eastern Mojave, or Colorado Desert bioregions during the surveillance period.
We identified a total of 166 unique locations for positive rodent samples (Figure 2a). The California ground squirrel (Spermophillus beecheyi) was the rodent species with the largest total number of specimens (12,546; Table 1) and number of positive specimens (559; Table 1), representing 105 of these unique geocoded locations. Because California ground squirrels are a key indicator species for plague epizootics [36] and human disease risk in California [10], we also ran models for this subset of data only.
Rodent samples. Study area and geocoded data for (a) 995 positive rodent samples (166 unique locations) and (b) 3,788 negative rodent samples (905 unique locations). Lines designate California bioregions (NC = Klamath/North Coast; BD = Bay Area/Delta; CC = Central Coast; SC = South Coast; MOD = Modoc Plateau; SRA = Sierra; SAV = Sacramento Valley; SJV = San Joaquin Valley; MOJ = Mojave Desert; CD = Colorado Desert).
Only records of positive rodents were included for niche modeling, as negative samples (Figure 2b) were frequently obtained from areas that had also yielded positive samples, or from which too few specimens had been collected to be considered representative. 3,788 sampling events had yielded negative samples, but 2,296 of these were at locations where positive samples had also been collected. Of the remaining 1,492 sampling events, only five locations had been sampled more than 20 times, which we estimated as the minimum number of samples that would need to be taken to confirm a location as a true absence.
Coyote point data
Sampling for plague in coyotes (Canis latrans) was conducted independently from sampling for plague in rodents. Unlike the rodent data, coyote blood specimens were collected opportunistically as part of a depredation control and state-wide plague surveillance partnership between the California Department of Health and the United States Department of Agriculture/Wildlife Services. Because coyotes can occupy a home range of up to 80 km2 [37], the location of capture may not be the location of infection; however, the opportunistic sampling program provides a more complete description of general plague activity throughout the state, albeit at a coarser spatial resolution. Coyote sera were tested by passive hemagglutination to F1 antigen of Y. pestis; specimens with antibody titer ≥ 1:32 were considered positive [33–35]. The plague surveillance partnership program and the diagnostic tests that were used are described in detail by [7].
In order to compare environmental niche model results based on rodent/ground squirrel data to data on positive and negative samples of California coyotes, records for 477 positive and 2,250 negative coyotes were identified from the database (Figure 3). Collection sites for coyote samples were geocoded using National Geographic TOPO software (National Geographic Society 2001) based on field records that indicated distance and direction from a town [7, 33, 38]. Collection sites that could not be reliably located were excluded from this analysis. All geocoded points were projected to Teale Albers, NAD 1983 projection. The geocoded locations of the coyote case points were distributed in the northern and southern Sierra Nevada, along the Pacific coast, and across the Modoc plateau.
Environmental variables
We downloaded the full set of 19 Worldclim bioclimatic variables http://www.worldclim.org (Table 2). These products are derived from monthly weather station measurements of altitude, temperature, and rainfall. They are biologically meaningful variables that capture annual ranges, seasonality, and limiting factors useful for niche modeling (such as monthly and quarterly temperature and precipitation extremes) [39]. The Worldclim data are at ~1-km2 spatial resolution and have been averaged over a 50-year time period from 1950–2000. Elevation was not explicitly used in model construction because it is already used as a covariate in the Worldclim data production. For modeling purposes, all environmental variable layers were masked to fit the extent of the California state outline. These layers were projected to Teale Albers, NAD 1983 projection.
Because the Worldclim variables are derived from a common set of temperature and precipitation data, they can exhibit multicollinearity [39]. A Spearman rank correlation matrix was created in JMP (SAS Institute) to explore the relationships between the Worldclim bioclimatic variables. We removed the four mean temperature variables (Bio8 – Bio11) because they were significantly correlated with minimum and/or maximum temperature variables, and were less likely to be biologically significant in contributing to or limiting plague activity. Of the remaining 15 variables, those that were correlated (Spearman rho > 0.60, p < 0.001) were not used together in the same model. During model runs, a jackknife manipulation was used to assess the relative contribution of each variable, and to remove variables that did not contribute significantly to the model predictions.
Modeling current and future distribution of plague in California using Maxent
Models of the current potential (i.e. based on climate conditions) distribution of plague in California were run in Maxent (version 3.1.0). Maxent is a machine learning program that uses presence-only data to predict distributions based on the principle of maximum entropy [40]. Maximum entropy [41] is a method to provide the probability distribution which incorporates the minimum amount of information. Given a set of constraints determined by environmental variables or functions thereof, Maxent outputs the maximum entropy distribution that satisfies these constraints. Among species distribution models, Maxent has been shown to provide better identification of suitable versus unsuitable areas when compared to other presence-only modeling methods [40, 42]. In place of true absences, Maxent uses background points (pseudo-absences) to evaluate commission.
Maxent does not need multiple model runs to be averaged together [40]; thus, for each set of variables, we ran Maxent once. For each Maxent run, 75% of the points were randomly selected for model training and cross-validation, and 25% of the data were set aside for model testing and independent validation. 10,000 random background points (pseudo-absences) were used to evaluate commission. A regularization setting of 2 was used for data smoothing and to address spatial autocorrelation. Model results were compared and validated using area under the ROC curve (AUC) statistics. The AUC statistic is similar to the Mann-Whitney U test and compares the likelihood that a random presence site will have a higher predicted value in the model than a random absence site [42, 43]. One of the appeals of ROC curves is that they do not depend on a user-defined threshold for determining presence versus absence. However, because using a geographical extent that goes beyond the presence environmental domain can lead to inflated AUC scores [44, 45], we limited the study area to the rough geographic extent of the sampling distribution (i.e. the California state boundary). The four most predictive models were used as the final models, and mapped as a cumulative probability output.
To explore the spatial relationship between model predictions and serologic samples of carnivores, we compared the final model results to data on positive and negative specimens from California coyotes. We used prediction values extracted for negative and positive coyote specimens using Hawth's point intersect tool [46]. A one-tailed t-test was performed using JMP (SAS Institute) to test the hypothesis that model predictions at positive coyote points would be significantly higher than model predictions at negative coyote points.
In order to simulate the distribution of plague under possible future climate conditions, we ran Maxent using coupled global climate model data from the IPCC 3rd Assessment (available at http://www.worldclim.org/futdown.htm). These data were originally produced by three different global climate models: CCCma [47], HadCM3 [48, 49], and CSIRO [50], and had been further processed using downscaling procedures in order to match current climate data from Worldclim [39]. We implemented an ArcInfo AML script (freely available at http://www.worldclim.org/mkBCvars.aml) to reformat and substantively convert these future temperature and precipitation data into the same bioclimatic variables that had been used as inputs for current-conditions modeling.
For each model we tested for two different time horizons, 2020 and 2050, and two different emissions scenarios (A2 and B2). The A2 scenario assumes that population growth does not slow down and reaches 15 billion by 2100 [51], with an associated increase in emissions and implications for climate change. The B2 scenario assumes a slower population growth (10.4 billion by 2100) and that precautionary environmental practices are implemented [51], yielding more conservative predictions of anthropogenic emissions. To simulate plague response to climate change, we used the final models that had been developed based on the rodent/ground squirrel data, and ran them with the future climate data.
Results
Four models were selected as the final candidate models predicting plague distribution based on climate variables (Table 3). In all four cases, models based only on California ground squirrel specimens had higher AUC values than their counterpart models that used all rodent samples as case points. Biologically meaningful variables used in these models included two temperature variables (Maximum Temperature of Warmest Month, and Temperature Annual Range) and four precipitation variables (Precipitation Seasonality, Precipitation of Wettest Quarter, Precipitation of Driest Quarter, and Precipitation of Warmest Quarter). The log response charts for the two most important variables used in models of plague in California ground squirrels (Precipitation in the Wet Quarter and Maximum Temperature of the Warmest Month) reflect a quadratic response to increasing temperatures and precipitation (Table 3).
Models of plague activity in all rodent species (AUC of 0.835 to 0.88) and in California ground squirrels (AUC of 0.913 to 0.948) based on recent climate conditions accurately identified case locations. All models predicted the highest plague activity in the Sierra Nevada and along the southern coast under recent climate conditions (Figure 4 and Figure 5). Models using environmental variables based on squirrel data performed well at predicting plague presence in coyotes. All four Maxent models predicted significantly higher values for pixels that overlapped with positive coyote specimens (Table 3).
Maxent model results, using all plague positive rodent samples as case points. a) Model using Precipitation of Warmest Quarter, Precipitation of Wettest Quarter, Precipitation Seasonality, Temperature Annual Range, and the Maximum Temperature of Warmest Month as predictor variables; b) Model using Precipitation of Driest Quarter, Precipitation of Wettest Quarter, Precipitation Seasonality, Temperature Annual Range, and the Maximum Temperature of Warmest Month as predictor variables: c) Model using Precipitation of Warmest Quarter, Precipitation of Wettest Quarter, Temperature Annual Range, and the Maximum Temperature of Warmest Month as predictor variables; and d) Model using Precipitation of Wettest Quarter, Precipitation of Warmest Quarter and the Maximum Temperature of Warmest Month as predictor variables.
Maxent model results, using positive California ground squirrel samples as case points. a) Model using Precipitation of Warmest Quarter, Precipitation of Wettest Quarter, Precipitation Seasonality, Temperature Annual Range, and the Maximum Temperature of Warmest Month as predictor variables; b) Model using Precipitation of Driest Quarter, Precipitation of Wettest Quarter, Precipitation Seasonality, Temperature Annual Range, and the Maximum Temperature of Warmest Month as predictor variables: c) Model using Precipitation of Warmest Quarter, Precipitation of Wettest Quarter, Temperature Annual Range, and the Maximum Temperature of Warmest Month as predictor variables; and d) Model using Precipitation of Wettest Quarter, Precipitation of Warmest Quarter and the Maximum Temperature of Warmest Month as predictor variables.
Under future emissions scenarios, our models indicated that climate conditions will drive a) an overall decrease in the probability of plague in the state, b) a subtle shift to higher elevations as well as c) a subtle shift to higher latitudes. Future climate conditions will support increased plague activity in the northern Sierra and central/north coast counties. However, plague risk associated with climate conditions may decrease in the southern Sierras and southern inland counties (Figure 6).
Discussion
Climate variables, such as temperature, precipitation, and humidity, can play important roles in vector-borne disease transmission by affecting vector and pathogen development, and by influencing the distribution of disease hosts and habitats [11, 52]. The biologically meaningful variables that were used in the final models we developed included two temperature variables (Maximum Temperature of Warmest Month, Temperature Annual Range) and four precipitation variables (Precipitation Seasonality, Precipitation of Wettest Quarter, Precipitation of Driest Quarter, and Precipitation of Warmest Quarter). We also found that plague presence exhibits a quadratic response to temperature increases. These results are consistent with other studies [12–14] that have examined the role of temperature and precipitation variables on plague outbreaks in human and animal populations. In addition to having a positive effect on rodent population dynamics, certain soil moisture, humidity and temperature variables may influence flea ecology and the transmission of the plague pathogen [53]. Specifically, while warmer temperatures may in general stimulate plague activity, temperatures above 35 degrees Celsius are associated with a negative effect on flea fecundity, survival, and behavior [13, 18, 54].
Under future emissions scenarios, our models indicate that climate conditions will drive a) an overall decrease in the probability of plague the state, b) a subtle shift to higher elevations as well as c) a subtle shift to higher latitudes. These results are generally consistent with other climate modeling studies that show species movement to higher latitudes and elevations in response to warming [55], and with studies that have examined the historical record of plague response to climate and show a shift to higher latitudes [16, 22]. Several other recent studies have also projected a potential decrease in plague activity in certain areas of the United States in response to more frequent hot days [19, 23, 24].
In addition, these results provide insight into the relationship between plague maintenance in carnivore and rodent populations. Carnivores, and particularly coyotes, have been implicated in plague transmission and serve as sentinel species for the disease [7, 56]. Recent studies [57, 58] conducted on the Central Plains Experimental Range and Pawnee National Grasslands (which collectively cover a ~80,000 ha area) link the prevalence of carnivores and rodent hosts in a spatially explicit manner. Our results expand these analyses to a larger scale, by exploring the overlap in predicted plague-positive rodent distributions with positive and negative coyote samples derived through an independent sampling program. Model results demonstrate a link between positive coyote samples and areas of predicted rodent infection, providing additional support for rodent surveillance and follow-up in areas where the carnivore surveillance program identifies plague-positive animals.
California ground squirrels are the rodents that have been the most frequently sampled for plague in California. However, six other species (Douglas' squirrel, Lodgepole chipmunk, Merriam's chipmunk, Shadow chipmunk, Yellow-pine chipmunk, and Belding's ground squirrel) often had higher serum titers than California ground squirrels. This suggests these species may be of interest for further sampling and surveillance, and that additional modeling of these species' distributions could be conducted to explore the spatial heterogeneity of plague foci in California [59]. Maxent models of California ground squirrels fit better than models that used all rodent specimens as training points. Because California ground squirrels occupy a narrower ecologic zone than all rodents collectively, with less variable climatic conditions, these models described a more precise climatic niche for plague.
Current model results matched areas with historical and recent plague activity, including the San Francisco peninsula and San Bruno Mountain, the San Jacinto mountains, and the Los Padres National Forest area [32]. Models did not yield high prediction values for the Modoc plateau region, which has historically been a focus of plague [10]. Because the low population density and rural nature of this area does not readily lend itself to observation of epizootic events, it is not surprising that no positive rodents were collected in the Modoc plateau during the study period. Thus, no model input points were used from this area, which can present a challenge to niche modeling techniques in terms of extrapolating results to new conditions in geographic and ecologic space [60]. In addition, the low prediction values for the Modoc plateau may be related to the extreme climate profile and characteristics of the plague system in this area, where plague maintenance and transmission is driven by a climate regime and rodent-host complex that differ from the rest of California. Many areas of the Modoc plateau experience plague, but in wood rats (Neotoma spp.), as well as in yellow pine chipmunks (Tamias amoenus) and their associated fleas.
It is important to keep in mind that by modeling the climatic niche for plague in California, we have modeled a potential distribution for plague that is not the actual or realized distribution. Other important factors, including landscape configuration, biotic variables, and barriers to dispersal likely limit the actual distribution of plague to smaller areas than those predicted using a climatic niche modeling approach [40, 59, 61]. Secondly, a number of studies have demonstrated that different modeling approaches can yield substantially different predictions [42, 62]. Thus, future work could include modeling plague potential distributions under a suite of different modeling approaches. Additionally, using niche models to predict distributions into expanded temporal and/or spatial domains can result in significant variance inflation [62]. We have attempted to dampen this variability by averaging future model outputs based on three different global climate models. However, the use of global climate models (as opposed to local or regional climate models) may itself be another source of error in niche modeling studies, and thus a potential area of research could explore the effects of different modeling datasets on disease distributions (for example, see [63]). Finally, averaged climate variables dampen seasonal effects and do not capture climatic anomalies, which may be important drivers of plague epizootics [11–13]. Thus multi-temporal modeling is required to elucidate the effects that increased climatic variability will have on vector-borne disease dynamics.
Conclusion
Because different modeling approaches can yield substantially different results, care should be taken when interpreting future model predictions. Nonetheless, niche modeling can provide general trends in response to climate conditions. Models of plague activity in California ground squirrels, based on recent climate data, accurately identified plague-positive rodent locations, as well as areas of historical and recent plague activity. Maxent model results were significantly correlated with coyote samples, and suggest that carnivore and rodent plague surveillance programs should be more tightly coupled in California. The final models were used to identify potential plague risk areas based on an ensemble of six future climate scenarios, which can help public managers decide where to allocate scarce surveillance resources.
References
Stenseth NC, Atshabar BB, Begon M, Belmain SR, Bertherat E, Carniel E, Gage KL, Leirs H, Rahalison L: Plague: Past, present, and future. PLoS Med. 2008, 5: e3-10.1371/journal.pmed.0050003.
Gage KL, Kosoy MY: Natural history of plague: Perspectives from more than a century of research. Annu Rev Entomol. 2005, 50: 505-528. 10.1146/annurev.ento.50.071803.130337.
Oyston P: Plague virulence. J Med Microbiol. 2001, 50: 1015-1017.
Dennis DT, Gage KL, Gratz N, Poland JD, Tikhomirov E: Plague manual: epidemiology, distribution, surveillance and control. 1999, Geneva: World Health Organization (WHO/CDS/CSR/EDC/992)
CDC: Human Plague – Four States, 2006. MMWR. 2006, 55: 940-943.
Bacon RM, Kugeler KJ, Mead PS: Surveillance for Lyme Disease – United States, 1992–2006. MMWR. 2008, 57: 1-9.
Hoar BR, Chomel BB, Rolfe DL, Chang CC, Fritz CL, Sacks BN, Carpenter TE: Spatial analysis of Yersinia pestis and Bartonella vinsonii subsp. berkhoffi seroprevalance in California coyotes (Canis latrans). Prev Vet Med. 2003, 56: 299-311. 10.1016/S0167-5877(02)00194-0.
Doll JM, Zeitz PS, Ettestad P, Bucholtz AL, Davis T, Gage K: Cat-transmitted fatal pneumonic plague in a person who traveled from Colorado to Arizona. Am J Trop Med Hyg. 1994, 51: 109-114.
Bertherat E, Bekhoucha S, Chougrani S, Razik F, Duchemin JB, Houti L, Deharib L, Fayolle C, Makrerougrass B, Dali-Yahia R, Bellal R, Belhabri L, Chaieb A, Tikhomirov E, Carniel E: Plague reappearance in Algeria after 50 Years, 2003. Emerg Infect Dis. 2007, 13: 1459-1462.
Nelson BC: Plague studies in California – The roles of various species of sylvatic rodents in plague ecology in California. Proc 9th Vert Pest Conf. 1980, 9: 89-96.
Gage KL, Burkot TR, Eisen RJ, Hayes EB: Climate and vectorborne diseases. Am J Preven Med. 2008, 35: 436-450. 10.1016/j.amepre.2008.08.030.
Collinge SK, Johnson WC, Ray C, Matchett R, Grensten J, Jack F, Cully J, Gage KL, Kosoy MY, Loye JE, Martin AP: Testing the generality of a trophic-cascade model for plague. EcoHealth. 2005, 2: 102-112. 10.1007/s10393-005-3877-5.
Parmenter RR, Yadav EP, Parmenter CA, Ettestad P, Gage KL: Incidence of plague associated with increased winter-spring precipitation in New Mexico. Am J Trop Med Hyg. 1999, 61: 814-821.
Stapp P, Antolin MF, Ball M: Patterns of extinction in prairie dog metapopulations: plague outbreaks follow El Nino events. Front Ecol Environ. 2004, 2: 235-240. 10.1890/1540-9295(2004)002[0235:POEIPD]2.0.CO;2.
Adjemian JCZ, Girvetz H, Beckett L, Foley J: Analysis of Genetic Algorithm for Rule-Set Production (GARP) modeling approach for predicting distributions of fleas implicated as vectors of plague, Yersinia pestis, in California. J Med Entomol. 2006, 43: 93-103. 10.1603/0022-2585(2006)043[0093:AOGAFR]2.0.CO;2.
Stenseth NC, Samia NI, Viljugrein H, Kausrud KL, Begon M, Davis S, Leirs H, Dubyanskiy VM, Esper J, Ageyev VS, Klassovskiy NL, Pole SB, Chan K-S: Plague dynamics are driven by climate variation. Proc Natl Acad Sci U S A. 2006, 103 (35): 13110-13115. 10.1073/pnas.0602447103.
Eisen RJ, Reynolds PJ, Ettestad P, Brown T, Enscore RE, Biggerstaff BJ, Cheek J, Bueno R, Targhetta J, Montenieri JA, Gage KL: Residence-linked human plague in New Mexico: A habitat-suitability model. Am J Trop Med Hyg. 2007, 77: 121-125.
Enscore RE, Biggerstaff BJ, Brown TL, Fulgham RE, Reynolds PJ, Engelthaler DM, Levy CE, Parmenter RR, Montenieri JA, Cheek JE, Grinnell RK, Ettestad PJ, Gage KL: Modeling relationships between climate and the frequency of human plague cases in the southwestern United States. Am J Trop Med Hyg. 2002, 66: 186-196.
Ben-Ari T, Gershunov A, Gage K, Snall T, Ettestad P, Kausrud KL, Stenseth NC: Human plague in the USA: The importance of local and regional climate. Biol Lett. 2008, 4: 737-740. 10.1098/rsbl.2008.0363.
Eisen L, Eisen RJ: Need for improved methods to collect and present spatial epidemiologic data for vectorborne diseases. Emerg Infect Dis. 2007, 13: 1816-1820.
Eisen RJ, Glass GE, Eisen L, Cheek J, Enscore RE, Ettestad P, Gage KL: A spatial model of shared risk for plague and hantavirus pulmonary syndrome in the Southwestern United States. Am J Trop Med Hyg. 2007, 77: 999-1004.
Nakazawa Y, Williams R, Peterson AT, Mead P, Staples E, Gage KL: Climate change effects on plague and tularemia in the United States. Vector Borne Zoonotic Dis. 2007, 7 (4): 529-540. 10.1089/vbz.2007.0125.
Snall T, Benestad RE, Stenseth NC: Expected future plague levels in a wildlife host under different scenarios of climate change. Global Change Biology. 2009, 15: 500-507. 10.1111/j.1365-2486.2008.01725.x.
Snall T, O'Hara RB, Ray C, Collinge SK: Climate-Driven Spatial Dynamics of Plague among Prairie Dog Colonies. The American Naturalist. 2008, 171: 238-248. 10.1086/525051.
Raxworthy CJ, Martinez-Meyer E, Horning N, Nussbaum RA, Schneider GE, Ortega-Huerta MA, Peterson AT: Predicting distributions of known and unknown reptile species in Madagascar. Nature. 2003, 426: 837-841. 10.1038/nature02205.
Loarie SR, Carter BE, Hayhoe K, McMahon S, Moe R, Knight CA, Ackerly DD: Climate change and the future of California's endemic flora. PLoS ONE. 2008, 3: e2502-10.1371/journal.pone.0002502.
Peterson AT, Sánchez-Cordero V, Beard CB, Ramsey JM: Ecologic niche modeling and potential reservoirs for Chagas disease, Mexico. Emerg Infect Dis. 2002, 8: 662-667.
Peterson AT, Bauer JT, Mills JN: Ecologic and geographic distribution of Filovirus disease. Emerg Infect Dis. 2004, 10: 40-47.
Peterson AT, Lash RR, Carroll DS, Johnson KM: Geographic potential for outbreaks of Marburg hemorrhagic fever. Am J Trop Med Hyg. 2006, 75: 9-15.
Williams R, Fasina FO, Peterson AT: Predictable ecology and geography of avian influenza (H5N1) transmission in Nigeria and West Africa. Trans R Soc Trop Med Hyg. 2008, 102: 471-479. 10.1016/j.trstmh.2008.01.016.
Neerinckx SB, Peterson AT, Gulinck H, Deckers J, Leirs H: Geographic distribution and ecological niche of plague in sub-Saharan Africa. Int J Health Geogr. 2008, 7:
CDHS: California compendium of plague control. 2008, California Department of Health Services
Smith CR: Wild carnivores as plague indicators in California – A cooperative interagency disease surveillance program. Proc 16th Vert Pest Conf. 1994, 16: 192-199.
Wolff KL, Hudson BW: Paper-strip blood sampling techniques for the detection of antibody to the plague organism Yersinia pestis. Applied Microbiology. 1974, 28: 323-325.
Hopkins D, Gresbrink R: Surveillance of sylvatic plague in Oregon by serotesting carnivores. American Journal of Public Health. 1982, 72: 1295-1297. 10.2105/AJPH.72.11.1295.
Williams JE, Cavanaugh DC: Differential signs of plague in young and old California ground squirrels (Spermophilus beecheyi). Journal of Wildlife Diseases. 1983, 19: 54-55.
Nowak RM: Walker's Mammals of the World. 1999, Baltimore, MD: Johns Hopkins University Press, 6
Smith CR, Nelson BC, Barnes AM: The use of wild carnivore serology in determining patterns of plague activity in rodents in California. Proceedings of the 11th Vertebrate Pest Conference. 1984, 11: 71-76.
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A: Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005, 25: 1965-1978. 10.1002/joc.1276.
Phillips SJ, Anderson RP, Schapire RE: Maximum entropy modeling of species geographic distributions. Ecol Model. 2006, 190: 231-259. 10.1016/j.ecolmodel.2005.03.026.
Jaynes ET: On the rationale of maximum entropy methods. Proc IEEE. 1982, 70: 939-952. 10.1109/PROC.1982.12425.
Elith J, Graham CH, Anderson RP, Dudik M, Ferrier S, Guisan A, Hijmans RJ, Huettmann F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, McCoverton J, Peterson AT, Phillips SJ, Richardson K, Scachetti-Pereira R, Schapire RE, Soberon J, Williams S, MS W, Zimmermann NE: Novel methods improve prediction of species' distributions from occurrence data. Ecography. 2006, 29: 129-151. 10.1111/j.2006.0906-7590.04596.x.
DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988, 44: 837-845. 10.2307/2531595.
Lobo JM, Jimenez-Valverde A, Real R: AUC: a misleading measure of the performance of predictive distribution models. Global Ecol Biogeog. 2008, 17: 145-151. 10.1111/j.1466-8238.2007.00358.x.
Peterson AT, Papes M, Soberon J: Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol Model. 2008, 213: 63-72. 10.1016/j.ecolmodel.2007.11.008.
Beyer HL: Hawth's Analysis Tools for ArcGIS. 2004,http://www.spatialecology.com/htools
Flato GM, Boer GJ, Lee WG, McFarlane NA, Ramsden D, Reader MC, Weaver AJ: The Canadian Centre for Climate Modeling and Analysis global coupled model and its climate. Climate Dyn. 2000, 16: 451-467. 10.1007/s003820050339.
Gordon C, Cooper C, Senior CA, Banks H, Gregory JM, Johns TC, Mitchell JFB, Wood RA: The simulation of SST, sea ice extents and ocean heat transports in a version of the Hadley Centre coupled model without flux adjustments. Climate Dyn. 2000, 16: 147-168. 10.1007/s003820050010.
Pope VD, Gallani ML, Rowntree PR, Stratton RA: The impact of new physical parameterizations in the Hadley Centre climate model – HadAM3. Climate Dyn. 2000, 16: 123-146. 10.1007/s003820050009.
Gordon HB, Rotstayn LD, McGregor JL, Dix MR, Kowalczyk EA, O'Farrell SP, Waterman LJ, Hirst AC, Wilson SG, Collier MA, Watterson IG, Elliott TI: The CSIRO Mk3 climate system model. CSIRO Atmospheric Research Technical Paper. 2002, 60: 1-130.
IPCC: Climate Change 2007: The Physical Science Basis – Summary for Policymakers. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. 2007, Cambridge, UK: Cambridge University Press
Gubler DJ, Reiter P, Ebi KL, Yap W, Nasci R, Patz JA: Climate variability and change in the United States: Potential impacts on vector- and rodent-borne diseases. Environ Health Persp. 2001, 109: 223-233. 10.2307/3435012.
Silverman J, Rust MK: Some abiotic factors affecting the survival of the cat flea, Ctenocephalides felis (Siphonaptera: Pulicidae). Environ Entomol. 1983, 12: 490-495.
Hinnebusch BJ, Fischer ER, Schwan TG: Evaluation of the role of the Yersinia pestis plasminogen activator and other plasmid-encoded factors in temperature-dependent blockage of the flea. J Infect Dis. 1998, 178: 1406-1415. 10.1086/314456.
Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F: Ecological responses to recent climate change. Nature. 2002, 416: 389-395. 10.1038/416389a.
Salkeld DJ, Stapp P: Seroprevalence rates and transmission of plague (Yersinia pestis) in mammalian carnivores. Vector Borne Zoonotic Dis. 2006, 6 (3): 231-239. 10.1089/vbz.2006.6.231.
Boone A, Kraft JP, Stapp P: Scavenging by mammalian carnivores on prairie dog colonies: Implications for the spread of plague. Vector Borne Zoonotic Dis. 2009, 9 (2): 185-189. 10.1089/vbz.2008.0034.
Salkeld DJ, Eisen RJ, Stapp P, Wilder AP, Lowell J, Tripp DW, Albertson D, Antolin MF: The potential role of swift foxes (Vuples velox) and their fleas in the plague outbreaks in prairie dogs. J Wildl Dis. 2007, 43: 425-431.
Wimberly MC, Yabsley MJ, Baer AD, Dugan VG, Davidson WR: Spatial heterogeneity of climate and land-cover constraints on distributions of tick-borne pathogens. Global Ecol Biogeog. 2008, 17: 189-2002. 10.1111/j.1466-8238.2007.00353.x.
Peterson AT, Pape M, Eaton M: Transferability and model evaluation in ecological niche modeling: A comparison of GARP and Maxent. Ecography. 2007, 30: 550-560.
Peterson AT: Biogeography of diseases: a framework for analysis. Naturwissenschaften. 2008, 95: 483-491. 10.1007/s00114-008-0352-5.
Araújo MB, Whittaker RJ, Ladle RJ, Erhard M: Reducing uncertainty in projections of extinction risk from climate change. Global Ecol Biogeog. 2005, 14: 529-538. 10.1111/j.1466-822X.2005.00182.x.
Peterson AT, Nakazawa Y: Environmental data sets matter in ecological niche modeling: An example with Solenopsis invicta and Solenopsis richteri. Global Ecol Biogeog. 2008, 17: 135-144.
Acknowledgements
The authors would like to thank Lynette Yang for assistance with geocoding. We are grateful to the two anonymous reviewers for their comments, and to Mark Novak, Kenneth Gage, and Charles Smith for providing guidance and feedback at various stages of this project. Funding for open-access publication fees was provided by a grant from the Berkeley Research Impact Initiative (BRII).
Disclaimer: The findings and conclusions of this article are those of the author(s) and do not necessarily reflect the views of the California Department of Public Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ACH, CLF, JRT, and PG designed the study. ACH conducted spatial analysis and Maxent modeling, and contributed to the writing of the manuscript. DJS contributed to the analysis of carnivore data and to the interpretation of model results. CLF and JRT conducted field sampling and collated the data used in this study, and were instrumental in analyzing model results in the context of recent trends in plague activity in California. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Holt, A.C., Salkeld, D.J., Fritz, C.L. et al. Spatial analysis of plague in California: niche modeling predictions of the current distribution and potential response to climate change. Int J Health Geogr 8, 38 (2009). https://doi.org/10.1186/1476-072X-8-38
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-072X-8-38