Abstract
Background
CHROMagar Candida (CaC) is increasingly being reported as a medium used to differentiate Candida albicans from non-albicans Candida (NAC) species. Rapid identification of NAC can assist the clinician in selecting appropriate antifungal therapy. CaC is a differential chromogenic medium designed to identify C. albicans, C. krusei, and C. tropicalis based on colony color and morphology. Some reports have proposed that CaC can also reliably identify C. dubliniensis and C. glabrata.
Methods
We evaluated the usefulness of CaC in the identification of C. dubliniensis, C. famata, C. firmetaria, C. glabrata, C. guilliermondii, C. inconspicua, C. kefyr, C. lipolytica, C. lusitaniae, C. norvegensis, C. parapsilosis, and C. rugosa.
Results
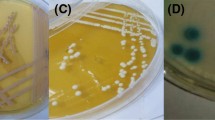
Most NAC produced colonies that were shades of pink, lavender, or ivory. Several isolates of C. firmetaria and all C. inconspicua produced colonies difficult to differentiate from C. krusei. Most C. rugosa isolates produced unique colonies with morphology like C. krusei except in a light blue-green color. C. glabrata isolates produced small dark violet colonies that could be differentiated from the pink and lavender colors produced by other species. All seventeen isolates of C. dubliniensis produced green colonies similar to those produced by C. albicans.
Conclusion
C. glabrata and C. rugosa appear distinguishable from other species using CaC. Some NAC, including C. firmetaria and C. inconspicua, could be confused with C. krusei using this medium.
Similar content being viewed by others
Background
Candida albicans once accounted for most serious nosocomial candidal infections. Over the last decade Candida infections, specifically candidemia, due to C. albicans have declined [1]. Other non-albicans Candida species (NAC) are now responsible for about half of candidemias and other deep Candida infections [2–4]. Most NAC infections are caused by C. glabrata, C. parapsilosis, or C. tropicalis, although in total over a dozen NAC have been reported to cause candidemia and other invasive infections [5–8]. In addition to deoxycholate and lipid formulations of amphotericin B, clinicians now must chose from azoles with differing spectrums of activity (fluconazole, itraconazole, and voriconazole) and echinocandin antifungal agents. In vitro testing has revealed that there are clear differences among the various NAC in their susceptibility to specific drugs. Rapid, reliable identification to species is now needed more than ever for clinicians to make treatment choices.
Identification of yeast pathogens by traditional methods requires several days and specific mycological media. Chromogenic media contain chromogenic substrates which react with enzymes secreted by the target microorganisms to yield colonies of varying colors. CHROMagar Candida (CaC) is one such medium that, per its manufacturer (CHROMagar Microbiology, Paris, France, http://www.chromagar.com), can identify three candidal yeasts, C. albicans (green colonies), C. tropicalis (steel blue colonies), and C. krusei (fuzzy, rose colored colonies) after 48 hours of incubation at 30–37°C. Independent groups have reported success with CaC in differentiating C. dubliniensis from C. albicans [9, 10]. Investigators have also reported the ability to distinguish C. glabrata from other species [11–14], although this has been contested by other authors [15–17].
We studied the appearance of 180 isolates of 12 Candida species on CaC to determine whether these more rare species could be reproducibly distinguished from each other with this medium.
Methods
Clinical yeast isolates were employed for all testing, predominately from our facility. Additional isolates were provided by the Fungus Testing Laboratory, University of Texas Health Science Center at San Antonio, Texas. Yeasts tested included C. dubliniensis (17 isolates), C. famata (11 isolates), C. firmetaria (12 isolates), C. glabrata (38 isolates), C. guilliermondii (10 isolates), C. inconspicua (6 isolates), C. kefyr (9 isolates), C. lipolytica (10 isolates), C. lusitaniae (15 isolates), C. norvegensis (3 isolates), C. parapsilosis (34 isolates), and C. rugosa (15 isolates). Five isolates each of C. albicans, C. krusei, and C. tropicalis were used as controls. All isolates were identified by standard clinical practices as previously described [18].
Inocula from clinical isolates stored at -70°C were transferred into yeast extract peptone (YEP) broth (Bacto Peptone, BD, Sparks, MD) and incubated at 30°C in a rocking incubator for up to 96 hours. Recovered yeasts were then streaked for isolation onto Sabouraud dextrose agar (SDA) plates (BD, Sparks, MD) and incubated at 30°C for 24–48 hours and subcultured a second time prior to inoculation in duplicate onto commercially prepared CHROMagar Candida (CaC) plates (BD, Sparks, MD). Inoculated CaC plates were incubated in parallel at 30°C and 37°C. Two independent readers, blinded to species inoculated, observed each set of plates for color and colony morphology daily for 7 days. Following the initial blinded study, select isolates were repeated in an unblinded fashion in parallel with the control isolates for direct comparison.
Results
Individual isolates produced the same colors at both incubation temperatures. In general, these colors appeared more intense at 37°C and intensified daily at both temperatures, peaking at 72 hours. Most of the NAC (C. famata, C. firmetaria, C. guilliermondii, C. inconspicua, C. kefyr, C. lipolytica, C. lusitaniae, C. norvegensis, and C. parapsilosis) tested produced variable shades of ivory, pink, and lavender (Table 1). C. parapsilosis isolates were the most varied of all species tested, in both color and colony morphology. C. kefyr commonly produced large colonies with centers that deepened to a brownish color with duration of incubation. C. firmetaria and C. inconspicua isolates produced large flat colonies. Most of the C. firmetaria and all of the C. inconspicua were pink with pale borders and rough in texture, indistinguishable from the color and morphology of C. krusei. C. rugosa most commonly (eleven of 15 isolates) produced light blue-green colonies with pale borders, similar in morphology (large, flat) to C. krusei. The minority of isolates grew as small to medium pink colonies not distinguishable from many other species.
C. glabrata was readily identifiable using CaC, producing small, convex, dark pink to violet colonies. Peak color intensity was noted at 72 hours when incubated at 30°C and at 48 hours when incubated at 37°C. C. glabrata isolates typically produced colonies with thin pale borders and a deep violet pigment that diffused into the medium. Unfortunately, neither of these features were universal. Our readers could not distinguish the medium to dark green colonies produced by C. dubliniensis from those of C. albicans at either temperature or any duration, except when directly compared to each other on repeat testing.
Discussion
Amphotericin B, fluconazole, and caspofungin are currently the three antifungal agents most commonly used to treat candidemia and invasive candidiasis. This practice is supported by FDA approval and the most recent Infectious Diseases Society of America guidelines [19]. While all these agents have been used effectively in clinical studies, these studies included mostly patients with C. albicans infections; thus their efficacy against NAC is not necessarily universal or well known. In vitro study has shown decreased susceptibility to amphotericin B in isolates of C. famata, C. guilliermondii, C. inconspicua, C. kefyr, C. krusei, C. lusitaniae, and C. rugosa and to fluconazole in isolates of C. famata, C. firmetaria, C. guilliermondii, C. inconspicua, C. krusei, and C. lusitaniae [7, 8]. The MICs of caspofungin have been demonstrated to be higher in C. famata, C. guilliermondii, and C. parapsilosis isolates [20]. The number of antifungal agents available continues to increase in the setting of a shift of candidal infections to those caused by non-albicans species. Because of this, identification to species and increased use of susceptibility testing has become necessary to appropriately select which agent to use [21].
CHROMagar Candida is a chromogenic medium that is advertised as able to identify C. albicans, C. krusei, and C. tropicalis. With the increasing incidence of human disease being produced by the less common Candida species, we were interested in testing the performance of this medium with the less common agents of candidiasis. We found, as previously reported, that most of these rarer Candida species and C. parapsilosis produced typical convex, creamy yeast colonies in shades of pink, lavender, and less commonly ivory, not distinguishable from each other (and often not consistent between isolates of the same species). C. parapsilosis, one of the most commonly recovered NAC, produced the widest range of colors and morphologies, making it impossible to identify using this medium.
Many of the rare NAC produced morphology and colors similar to those seen with C. krusei on this medium. We found individual isolates of C. lipolytica and C. norvegensis, many isolates of C. firmetaria, and all tested C. inconspicua were indistinguishable from C. krusei. Some that were distinguishable, produced similar color as that of C. krusei, with the pale border, but with waxy colonies. Most of these species share reduced susceptibility to fluconazole that is common with C. krusei.
We confirmed our previous observation that C. rugosa appears to typically produce a readily identifiable and unique color/colony type on CaC [12, 22]. Eleven of 15 isolates of this organism produced the same light blue-green color and a colonial morphology similar to that of C. krusei. C. rugosa has been shown in clinical reports and by in vitro testing to be less susceptible to amphotericin B [7, 8, 23]. Rapid identification of this NAC is of great importance to allow provision of appropriate therapy to patients.
As previously reported by our group and others, this study did find C. glabrata to be readily distinguishable from other species that produced pink to lavender colonies on CaC. With the use of larger numbers of isolates, we again noted that this species produced smaller colonies starting out as dark pink hues, becoming dark violet with time, commonly with a small diffusion of dark violet pigment into the surrounding agar and a thin pale border [12, 22]. This was most apparent after prolonged incubation (72 hours) at the lower temperature suggested by the manufacturer (30°C), or after 48 hours when incubated at 37°C.
Our readers could not distinguish C. dubliniensis from C. albicans when observed in a blinded fashion. The isolates we tested were somewhat darker, especially at the higher incubation temperature, but this was only obvious when CaC culture was performed in parallel to C. albicans controls. The report by Kirkpatrick et al. [9] that made the initial observation that C. dubliniensis produce darker green colonies with CaC did this with primary isolation of clinical materials onto the medium. Our isolates were not from primary samples and thus may have been affected by storage conditions and repeated subculturing. Odds and Davidson reported that they could differentiate C. dubliniensis from C. albicans using stored isolates, but this was best shown after 72 hours of incubation at 37°C [10].
Conclusion
In our hands CHROMagar Candida shows good potential as a screening medium to rapidly identify potentially azole-resistant as well as amphotericin B-resistant species, including C. krusei, C. glabrata. C. rugosa, and C. inconspicua. Identification of C. dubliniensis appears to have some limitations based on our study, but CaC appears (from other studies) to also hold this potential when used as a primary medium. We would suggest inclusion of a C. albicans control plate if this medium is used as a primary fungal recovery medium to help increase the presumptive identification of C. dubliniensis. Combined with confirmation of speciation by standard methods and knowledge of the local antifungal susceptibility patterns of these species, CaC may be a useful adjunctive medium for use in the clinical laboratory.
References
Trick WE, Fridkin SK, Edwards JR, Hejjeh RA, Gaynes RP, National Nosocomial Infections Surveillance System Hospitals : Secular trends of hospital-acquired candidemia among intensive care unit patients in the United States during 1989–1999. Clin Infect Dis. 2002, 35: 627-630. 10.1086/342300
Beck-Sague CM, Jarvis WR, : Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993, 167: 1247-1251.
Pfaller MA, Diekema DJ, Jones RN, Sader HS, Fluit AC, Hollis RJ, Messer SA, SENTRY participant group : International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J Clin Microbiol. 2001, 39: 3254-3259. 10.1128/JCM.39.9.3254-3259.2001
Rangel-Frausto MS, Wiblin T, Blumberg HM, Saiman L, Patterson J, Rinaldi M, Pfaller M, Edwards JE, Jarvis W, Dawson J, Wenzel RP, : National Epidemiology of Mycoses Survey (NEMIS): variations in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin Infect Dis. 1999, 29: 253-258.
Hazen KC: New and emerging yeast pathogens. Clin Microbiol Rev. 1995, 8: 462-478.
Krcmery V, Barnes AJ: Non-albicans Candida spp. causing fungaemia: pathogenicity and antifungal resistance. J Infect Control. 2002, 50: 243-260.
Pfaller MA, Diekema DJ, Messer SA, Boyken L, Hollis RJ, Jones RN: In vitro susceptibilities of rare Candida bloodstream isolates to ravuconazole and three comparative antifungal agents. Diagn Microbiol Infect Dis. 2004, 48: 101-105. 10.1016/j.diagmicrobio.2003.09.009
Pfaller MA, Diekema DJ, Messer SA, Boyken L, Hollis RJ, Jones RN, International Fungal Surveillance Participant Group : In vitro activities of voriconazole, posaconazole, and four licensed systemic antifungal agents against Candida species infrequently isolated from blood. J Clin Microbiol. 2003, 41: 78-83. 10.1128/JCM.41.1.78-83.2003
Kirkpatrick WR, Revankar SG, McAtee RK, Lopez-Ribot JL, Fothergill AW, McCarthy DI, Sanche SE, Cantu RA, Rinaldi MG, Patterson TF: Detection of Candida dubliniensis in oropharyngeal samples from human immunodeficiency virus-infected patients in North America by primary CHROMagar Candida screening and susceptibility testing of isolates. J Clin Microbiol. 1998, 36: 3007-3012.
Odds FC, Davidson A: "Room temperature" use of CHROMagar Candida. Diagn Microbiol Infect Dis. 2000, 38: 147-150. 10.1016/S0732-8893(00)00197-8
Bernal S, Martin Mazuelos E, Garcia M, Aller AI, Martinez MA, Gutierrez MJ: Evaluation of CHROMagar Candida medium for the isolation and presumptive identification of species of Candida of clinical importance. Diagn Microbiol Infect Dis. 1996, 24: 201-4. 10.1016/0732-8893(96)00063-6
Hospenthal DR, Murray CK, Beckius ML, Green JA, Dooley DP: Persistence of pigment production by yeast isolates grown on CHROMagar Candida medium. J Clin Microbiol. 2002, 40: 4768-4770. 10.1128/JCM.40.12.4768-4770.2002
Huang LU, Chen CH, Chou CF, Lu JJ, Chi WM, Lee WH: A comparison of methods for yeast identification including CHROMagar Candida, Vitek system YBC and a traditional biochemical method. Zhonghua Yi Xue Za Zhi (Taipei). 2001, 64: 568-74.
Pfaller MA, Houston A, Coffmann S: Application of CHROMagar Candida for rapid screening of clinical specimens for Candida albicans, Candida tropicalis, Candida krusei, and Candida (Torulopsis) glabrata. J Clin Microbiol. 1996, 34: 58-61.
Koehler AP, Chu K, Houang ETS, Cheng AFB: Simple, reliable, and cost-effective yeast identification scheme for the clinical laboratory. J Clin Microbiol. 1999, 37: 422-426.
Odds FC, Bernaerts R: CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994, 32: 1923-1929.
Powell HL, Sand CA, Rennie RP: Evaluation of CHROMagar Candida for presumptive identification of clinically important Candida species. Diagn Microbiol Infect Dis. 1998, 32: 201-204. 10.1016/S0732-8893(98)00096-0
Murray CK, Beckius ML, Green JA, Hospenthal DR: Use of chromogenic medium in the isolation of yeasts from clinical specimens. J Med Microbiol. 2005, 54: 981-985. 10.1099/jmm.0.45942-0
Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, Walsh TJ, Edwards JE: Guidelines for treatment of candidiasis. Clin Infect Dis. 2004, 38: 161-189. 10.1086/380796
Pfaller MA, Diekema DJ, Messer SA, Hollis RJ, Jones RN: In vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3, 959 clinical isolates of Candida spp., including 157 fluconazole-resistant isolates. Antimicrob Agents Chemother. 2003, 47: 1068-1071. 10.1128/AAC.47.3.1068-1071.2003
Hospenthal DR, Murray CK, Rinaldi MG: The role of antifungal susceptibility testing in the therapy of candidiasis. Diagn Microbiol Infect Dis. 2004, 48: 153-160. 10.1016/j.diagmicrobio.2003.10.003
Horvath LL, Hospenthal DR, Murray CK, Dooley DP: Direct isolation of Candida spp. from blood cultures on the chromogenic medium CHROMagar Candida. J Clin Microbiol. 2003, 41: 2629-2632. 10.1128/JCM.41.6.2629-2632.2003
Colombo AL, Melo ASA, Rosas RFC, Salomao R, Briones M, Hollis RJ, Messer SA, Pfaller MA: Outbreak of Candida rugosa candidemia: an emerging pathogen that may be refractory to amphotericin B therapy. Diagn Microbiol Infect Dis. 2003, 46: 253-257. 10.1016/S0732-8893(03)00079-8
Acknowledgements
Disclaimer: The views expressed herein are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government. The authors are employees of the US government. This work was prepared as part of their official duties and, as such, there is no copyright to be transferred.
This work was presented in part at the 104th Annual Meeting of the American Society for Microbiology, New Orleans, LA, 23–27 May 2004.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
DH and LL had primary responsibility for study design. MB maintained isolates and prepared plates for observation. DH and KF served as readers for the blinded plate readings. All authors assisted in the unblinded reading of the plates and preparation of the manuscript. All authors have read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hospenthal, D.R., Beckius, M.L., Floyd, K.L. et al. Presumptive identification of Candida species other than C. albicans, C. krusei, and C. tropicalis with the chromogenic medium CHROMagar Candida. Ann Clin Microbiol Antimicrob 5, 1 (2006). https://doi.org/10.1186/1476-0711-5-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-0711-5-1