Abstract
We studied the presence of extended spectrum beta lactamases (ESBLs) in 44 clinical isolates of Escherichia coli collected from out-patients in two university teaching hospitals in South-Eastern Nigeria. Species identification was performed by standard microbiology methods and re-confirmed by MALDI-TOF technology. Phenotypic characterization of ESBL enzymes was done by double disc synergy test and presence of ESBL genes was determined by specific PCR followed by sequencing. Transfer of plasmid DNA was carried out by transformation using E. coli DH5 as recipient strain. Phenotypic characterization identified all isolates to be ESBL positive. 77% of strains were from urine, 13.6% from vaginal swabs and 9.0% from wound swabs. 63.6% were from female patients, 68% were from outpatients and 95.5% from patients younger than 30 years. All ESBL producers were positive in a PCR for blaCTX-M-1 cluster, in exemplary strains blaCTX-M-15 was found by sequencing. In all strains ISEcp1 was found upstream and ORF477 downstream of blaCTX-M. PCR for blaTEM and blaOXA-1 was positive in 93.1% of strains, whereas blaSHV was not detected, aac(6′)-Ib-cr was found in 97.7% of strains. RAPD analysis revealed seven different clonal groups named A through G with the majority of the strains (65.9%) belonging to clone A. Transfer of an ESBL plasmid with co-resistance to gentamicin, kanamycin, tobramycin, doxycycline and trimethropim-sulfamethoxazole was successful in 19 (43.2%) strains. This study showed a high rate of CTX-M-1 cluster - ESBLs in South-Eastern Nigeria and further confirms the worldwide spread of CTX-M ESBL in clinical isolates.
Similar content being viewed by others
Introduction
Microbial resistance is a growing major public health issue and a strong concern for the medical community. Production of β-lactamases is a major means by which Gram-negative bacteria exhibit resistance to β-lactam antibiotics [1]. Extended spectrum β-lactamases (ESBLs) are a group of enzymes that can hydrolyze a variety of β-lactams including cephalosporins like ceftazidime, cefotaxime, ceftriaxone and monobactams like aztreonam in addition to penicillins but do not hydrolyze cephamycins like cefoxitin. Most of the ESBLs also have the ability to hydrolyze fourth generation cephalosporins like cefepime [2]. Until recently, ESBL-producing organisms were viewed as hospital-acquired or health care-associated pathogens, i. e. affecting patients who had typically been in hospitals or other health care facilities like nursing homes [3, 4]. In the last decades however, these infections have increasingly been recognized in patients who had no prior contact with the health care system [5, 6]. E. coli strains producing CTX-M type ESBLs drive this new epidemic. Reports on this newer group of ESBLs, coined CTX-M for their preferential hydrolysis of cefotaxime over ceftazidime started to emerge in E. coli in the late 1990s [7]. Studies have indicated that the genes for CTX-M type ESBLs were mobilized from the chromosomes of Kluvera spp. to E. coli plasmids through transposon-mediated events [8, 9]. Currently five clusters of CTX-M type ESBLs have been identified namely the CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9, and CTX-M-25 clusters. CTX-M-14 belonging to the CTX-M-9 cluster and CTX-M-15 belonging to the CTX-M-1 cluster are particularly associated with community-acquired isolates. In the present study, we investigated the molecular epidemiology of clinical isolates of ESBL producing Escherichia coli isolated from outpatients in South-Eastern Nigeria.
Materials and methods
A total of 44 clinical isolates of E. coli were isolated from patients attending University of Nigeria teaching hospital, Enugu (HA, n = 28) and Ebonyi State University teaching hospital Abakaliki (HB, n = 16). All isolates were characterized using standard microbiology methods and re-confirmed by MALDI-TOF [10, 11]. Extended spectrum beta lactamase enzymes were determined by double disc synergy test (DDST) method with disks of ceftazidime, cefotaxime, ceftriaxone, cefepime, and aztreonam (30 μg each) placed at a distance of 15 mm (center to center) from a disk containing amoxicillin plus clavulanic acid (20 and 10 μg, respectively) [12].
Isolation of genomic DNA of ESBL E. Coli
Genomic DNA of ESBL E. coli was prepared using the NucleoSpin Kit (Macherey & Nagel, Germany) following manufacturer’s instructions.
Detection of resistance gene using PCR method
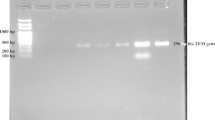
Primers used for amplification of resistance genes are shown in Table 1. Cycling conditions were as follows: initial denaturation at 94°C for 5 min, followed by 34 cycles of denaturation at 94°C for 30 s and a final extension step at 72°C for 5 min. Annealing temperatures differed according to the primer pair used and were 42°C for TEM, 47°C for SHV, 52°C for OXA-1, 58.8°C for CTX-M-1. Amplified PCR products were separated on 0.8% agarose gels, stained with ethidium bromide and visualized under UV illumination. Appropriate positive and negative controls were used in all cases [13–15]. Some PCR products were sequenced with 3730 sequencer (applied biosystem). The nucleotide and deduced amino acid sequence were analyzed and compared to sequence available over the Internet at the National Center for Biotechnology Information (http:IIwww.ncbi.nlm.gov). A PCR with specific primers for aac(6′)-Ib-cr was performed [16] with the primers shown in Table 1 and an annealing temperature of 45°C and followed by restriction with Bse GI. The genetic environment of blaCTX-M[17] was analyzed by PCR for ISEcp1 upstream and ORF477 downstream with the primers shown in Table 1.
Randomly amplified polymorphic DNA (RAPD) analysis
RAPD was performed with all the 44 ESBL positive strains using a single primer as showed in Table 1 with annealing temperature of 37°C [18].
Transformation studies
Plasmid DNA was extracted with the Qiagen Midi Kit PC100 (Qiagen, Germany). Transformation of plasmids was carried out by electroporation with E. coli DH5 cells as recipient strain at 2.5 kV, 25 F and 200 using a Gene–Pulser (Bio-Rad, Hemel hempstead, UK). Samples from donor and recipient strains were used as controls. Transformants growing on the selection plates (cefotaxime 2 mg/L, Sigma Aldrich Poole UK) were subjected to DDST to confirm the presence of ESBL genes and were examined for co-transfer of other antibiotic resistance determinants present in the donor clinical isolates by disk diffusion.
Results
The overall result showed that of 44 E. coli strains studied 34 (77.2%) were from urine, 6 (13.6%) from vaginal swabs and 4 (9.0%) from wound swabs, 28 (63.6%) were from female patients. Remarkably the proportion of outpatients was very high (68%). The majority of patients (n = 42; 95.5%) were younger than 30 years.
All 44 E. coli strains were phenotypically confirmed as ESBL, PCR for blaCTX-M and blaCTX-M-1 cluster was positive in all strains. Three exemplary isolates were chosen for sequencing of the full blaCTX-M gene and sequence analysis revealed them as blaCTX-M-15. PCRs for ISEcp1 upstream and ORF 477 downstream of blaCTX-M were positive in all strains. PCRs for TEM and OXA-1 beta lactamase genes were positive in 41 (93.1%) ESBL isolates. SHV could not be found in any isolate (Table 2). Also the presence of aac(6′)-Ib-cr could be demonstrated in 43 strains (97.7%) by PCR and subsequent restriction analysis. No significant difference regarding the frequency of blaOXA-1, blaTEM and aac(6′)-Ib-cr between ESBLs from hospital A and Hospital B could be found. RAPD analysis of ESBL strains for clonal relatedness grouped the strains into seven clonal groups (A-G). Clone A was by far the most frequent clone and found in 65.9% of all studied strains. Compared to other clones it is more frequent in hospital B (81%) than in hospital A (57.1%). PCR results in relation to clonal type are presented in Table 2. The association of CTX-M-1 cluster genes to TEM, OXA-1 and aac(6′)-Ib-cr is stronger in Clone A ESBLs than in other clonal types. ESBL plasmid could be successfully transformed in 19 (43.2%) out of 44 ESBL E. coli. Co-resistance to gentamicin, kanamycin, tobramycin, doxycycline and trimethropim-sulfamethoxazole was observed in most transformants (Table 3).
Discussions
This study was carried out in two major university teaching hospitals in South-Eastern Nigeria where there is no record of investigation of molecular epidemiology of ESBL E. coli. Nosocomial bacterial infections constitute a substantial cause of morbidity and mortality in developing countries such as Nigeria. E. coli is known to be one of the major organisms causing nosocomial infections within the hospital and has also been implicated in community acquired ESBL [19, 20]. The observation of ESBL producing E. coli strains in this study is very alarming and this could be attributed to the indiscriminate and widespread use of antibiotics, particularly beta lactam antibiotics that are sold over the counter in pharmacy shops without doctors’ prescription in Nigeria. This misuse of antibiotics might have contributed to the emergency of ESBL producing isolates. All 44 ESBL E. coli strains harbored ESBLs of the CTX-M-1 cluster. In three exemplary strains the CTX-M-15 ESBL could be confirmed which belongs to the CTX-M-1 cluster. These results further emphasize that this enzyme is now one of the most common CTX-M β- lactamases worldwide. In several countries CTX-M-15 is currently the most frequent ESBL in E. coli like in the UK, India, Spain, France, Latin America and Lebanon [20]. In Nigeria ESBL has been described in Enterobacter spp. from Western Nigeria several years ago [21] and CTX-M-15 ESBL in K. pneumoniae from the same region four years later [22] while ESBL was also reported in E. coli from South Eastern Nigeria this same year [23] but no description of CTX-M-15 ESBL from outpatients has been reported. Recently CTX-M-15 ESBL producing E. coli was reported from hospital patients in Osogbo in Western Nigeria [24]. CTX-M-15 has also been reported from other African countries like Egypt [25], Algeria [26], Tunisia [27], Tanzania [28], and Cameroon [29]. One of the most alarming findings of our study is that most ESBL isolates were detected in urine from young outpatients; urine was the source of 81.8% of ESBL strains, 68.2% of patients were of an age below 30 years. This strongly indicates that CTX-M-15 carrying E. coli strains can cause community-acquired urinary tract infections and that those ESBL strains are not only present in a hospital environment but also in the community population of this area of Nigeria. Similar observations have been made also in other parts of the world [5, 6].
Typing of isolates via RAPD grouped the isolates into seven different clones. Seven strains could not be typed by this method and the majority of ESBL E. coli strains studied in this work belongs to a single clone as determined by RAPD. Of particular interest is that this clone A was detected in two hospitals located more that 70 km away from each other. One possible explanation is patient-to-patient transmission in both hospitals and introduction of clone A into another hospital by patient transfer. Hospital A serves as referral hospital for patients in hospital B with serious medical problems. A certain degree of inter-hospital spread was discovered because two patients with clone A ESBLs were known to have visited both hospitals during our study period. Thus, it is of high importance to apply specific infection control measures like hand disinfection in hospitals. Considering that also clone A ESBL isolates were found in young outpatients, another possible explanation might be that clone A occurs rather frequently in the population of this area. If so, it would be worth the effort to exclude a possible common source like drinking water or food products.
As observed worldwide for CTX-M-15 ESBL E. coli strains, we found a high association of CTX-M-1 cluster ESBLs with ISEcp1 upstream and ORF 477 downstream of the gene, with the presence of TEM and OXA-1 beta lactamases and with the detection of aac(6′)-Ib-cr. The latter gene mediates resistance to tobramycin and decreased susceptibility to ciprofloxacin [30]. Thus it is one of several factors responsible for multi-drug resistance also to non-beta lactam antibiotics in ESBL strains.
Conclusions
For the first time ESBL E. coli strains could be demonstrated from Southeastern Nigeria with CTX-M-1 cluster enzymes present in all isolates. Most of the ESBL strains also carried TEM, OXA-1 and aac(6′)-Ib-cr and the majority of strains were isolated from outpatients below the age of 30. Based on our findings we suggest that antimicrobial resistance surveillance network be established in the two hospitals to monitor the trends and new types of resistance mechanism in Southeastern, Nigeria.
Ethics
Ethical committee of the two hospitals approved the use of their hospitals for this study and patients gave consent for the collection of their samples.
References
Medeiros AA: Evolution and dissemination of β- lactamases accelerated by generations of β- lactam antibiotics. Clin Infect Dis. 1997, 24 (Suppl1): S19-S45.
Bradford PA: Extended-spectrum β- lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistant threat. Clin Microbiol Rev. 2001, 14: 933-951. 10.1128/CMR.14.4.933-951.2001
Amber RP, Coulson AFW, Frere JM, Ghuysen JM, Joris B, Forsman M, Levesque RC, Tiraby G, Waley SG: A standard numbering scheme for the class A β- lactamases. Biochem J. 1991, 276: 269-272.
Kassis-Chikhani V, Vimont S, Asselat K, Trivalle C, Minassian B, Gautier V: CTX-M- β- lactamase producing E. coli in long term care facilities, France. Emerg Infect Dis. 2004, 10: 1697-1698. 10.3201/eid1009.030969
Borer A, Gilad J, Menasche G, Peied N, Riesenberg K, Schlaeffer F: Extended-spectrum beta lactamase-producing Enterobacteriaceae strains in community-acquired bacteremia in Southern Israel. Med Sci Monit. 2002, 8: CR44-CR47.
Pitout JDD, Normann P, Laupland KB, Poirel L: Emergence of Enterobactericeae producing extended-spectrum beta-lactamases (ESBLs) in the community. J Antimicrob Chemother. 2005, 56: 52-59. 10.1093/jac/dki166
Pitout JDD, Gregson DB, Church DL, Elsayed S, Laupland KB: Community-wide outbreaks of clonally related CTX-M-14 β- lactamase-producing Escherichia coli strains in the Calgary health region. J Clin Microbiol. 2005, 43: 2844-2849. 10.1128/JCM.43.6.2844-2849.2005
Paterson DL, Bonomo RA: Extended-spectrum β- lactamases: a clinical update. Clin Microbiol Rev. 2005, 18: 657-686. 10.1128/CMR.18.4.657-686.2005
Poirel L, Kamfer P, Nordmann P: Chromosomal-encoded Amber class A β- lactamases of Kluvera georgiana, a probable progenitor of a subgroup of CTX-M extended spectrum β- lactamases. Antimicrob Agents Chemother. 2002, 46: 4038-4040. 10.1128/AAC.46.12.4038-4040.2002
Murray PR: Manual of Clinical Microbiology. 2002, Washington DC USA: American Society for Microbiology Press, 7,
Pineda FJ, Lin LS, Pensalau C, Dinarov PA: Testing the significance of micro-organism identification by mass spectrometry and protein database search. Anal Chem. 2000, 72: 3739-3744. 10.1021/ac000130q
Pitout JDD, Ashfaque H, Hanson HD: Phenotypic and Molecular detection of CTX-M-β-lactamses produced by Escherichia coli and Klebsiella spp. J Clin Microbiol. 2004, 42 (12): 5715-5721. 10.1128/JCM.42.12.5715-5721.2004
Schlesinger J: Navon Venezia S, Chmelnistsky I, Hammer-Munz O, Leavitt A, Gold HS, Extended spectrum beta-lactamases among Enterobacter isolates Obtained in Tel Aviv, Israel, Antimicrob. Agents Chemother. 2005, 49 (3): 1150-1156. 10.1128/AAC.49.3.1150-1156.2005.
Empel J, Baraniak A, Literacka E, Mrowka A, Fiett J, Hryniewicz EW: Beta Lactamase study group: Molecular survey of beta-lactamases conferring resistance to newer beta-lactams in enterobacteriaceae isolates from Polish hospitals. Antimicrob Agents Chemother. 2008, 52 (7): 2449-2454. 10.1128/AAC.00043-08
Karisik E, Ellington MJ, Warren RE, Livermore DM, Woodford N: Molecular characterization of plasmids encoding CTX-M-15 beta-lactamases from Escherichia coli strains in the United Kingdom. J Antimicrob Chemother. 2006, 58 (3): 665-668. 10.1093/jac/dkl309
Park CH, Robicsek A, Jacoby GA, Sahm D, Hooper HC: Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzymes. Antimicrob Agents Chemother. 2006, 50: 3053-3055.
Eckert C, Gautier V, Arlet G: DNA sequence analysis of the genetic environment of various bla CTX-M genes. J Antimicrob Chemother. 2006, 57 (1): 14-23.
Pacheco AB, Guth BE, Soares KC, Nishimura L, de Almeida DF, Ferreira LC: Random amplification of polymorphic DNA reveals serotype-specific clonal clusters among enterotoxigenic Escherichia coli from humans. J Clin Microbiol. 1997, 35 (6): 1521-5).
Jacoby GA, Han P: Detection of extended-spectrum - lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J Clin Microbiol. 1996, 34: 908-911.
Woodford N, Ward ME, Kaufmann ME, Turton J, Fagan EJ, James D: Community and hospital spread of Escherichia coli producing CTX-M extended-spectrum β-lactamases in the UK. J Antimicrob Chemother. 2004, 54: 735-743. 10.1093/jac/dkh424
Aibinu IE, Ohaegbulam VC, Adenipekun EA, Ogunsola PT, Odugbemi TO, Mee BJ: Extended spectrm beta lactamases in clinical isolates of Enterobacter species from Lagos, Nigeria. J Clin Microbiol. 2003, 56: 241-249.
Soge OO, Queenan AM, Ojo KK, Adeniyi BA, Roberts MC: CTX-M-15 extended spectrum beta lactamases from Nigeria Klebsiella pneumoniae. J Antimicrob Chemother. 2006, 57: 24-30.
Iroha IR, Adikwu MU, Esimone CO, Aibinu I, Amadi ES: Extended spectrum beta lactamase (ESBL) in E. coli isolated from a tertiary hospital in Enugu State, Nigeria. Pak J Med Sci. 2009, 25 (2): 279-282.
Olowe OA, Grobbel M, Buchter B, Lubke-Becker A, fruth A, Wieler LU: Detection of bla CTX-M-15 extended-spectrum β- lactamase genes in Escherichia coli from hospital patients in Nigeria. Int J Antimicrob Agents. 2010, 35: 200-209. 10.1016/j.ijantimicag.2009.11.001
Khalaf NG, Eletreby ME, Hanson ND: Characterization of CTX-M ESBLs in Enterobacter cloacae, Escherichia coli and Klebsiella pneumoniae from Cairo, Egypt. BMC Infect Dis. 2009, 9 (84): 1-5.
Ramdani-Bouguessa N, Mendorica N, Levtao J, Ferreira E, Tazir M: CTX-M-3 and CTX-M-15 extended spectrum β- lactamases in isolates of Escherichia coli from a hospital in Algiers, Algeria. J Clin Microbio. 2006, 44 (12): 4584-4586. 10.1128/JCM.01445-06.
Boualiegue-Godet O, Bensalem Y, Fabre L, Demartin M, Grimont PAD, Mzoughi R: Nosocomial outbreak caused by Salmonella enteric serotype producing CTX-M-27 extended-spectrum β-lactamase in a neonatal unit in Sousse, Tunisia. J Clin Microbiol. 2005, 43: 1843-1849. 10.1128/JCM.43.4.1843-1845.2005
Blomberg B, Jureen R, Manji KP, Tamin BS, Mwakagile DSM: High rate of cases of pediatric septicemia caused by Gram-negative bacteria with extended spectrum β-lactamases in Dar es Salaam, Tanzania. J Clin Microbiol. 2005, 43: 745-749. 10.1128/JCM.43.2.745-749.2005
Gangoue-Pieboji J, Miriagou V, Vourli S, Tzelepi E, Ngassam P, Tzouvelekis LS: Emergence of CTX-M-15-producing enterobacteria in Cameroon and characterization of a bla CTX-M-15- carrying element. Antimicrob Agents Chemother. 2005, 49 (1): 441-443. 10.1128/AAC.49.1.441-443.2005
Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A: Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbio Rev. 2009, 22 (4): 664-689. 10.1128/CMR.00016-09.
Acknowledgments
This study was sponsored by Alexander von Humboldt Stiftung foundation (George-Foster).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
No interest to declare.
Authors’ contributions
MKa and IRI planed this study. IRI performed most of the experiments. MKo, LM and SM performed parts of the experiments. MKa, FS and SG revised the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Iroha, I.R., Esimone, C.O., Neumann, S. et al. First description of Escherichia coli producing CTX-M-15- extended spectrum beta lactamase (ESBL) in out-patients from south eastern Nigeria. Ann Clin Microbiol Antimicrob 11, 19 (2012). https://doi.org/10.1186/1476-0711-11-19
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-0711-11-19