Abstract
Microbes living in the mammalian gut exist in constant contact with immunity system that prevents infection and maintains homeostasis. Enteric alpha defensins play an important role in regulation of bacterial colonization of the gut, as well as in activation of pro- and anti-inflammatory responses of the adaptive immune system cells in lamina propria. This review summarizes currently available data on functions of mammalian enteric alpha defensins in the immune defense and changes in their secretion in intestinal inflammatory diseases and cancer.
Similar content being viewed by others
Introduction
Defensins are short, cysteine-rich, cationic peptides found in vertebrates, invertebrates and plants, which play an important role in innate immunity against bacteria, fungi, protozoa, and viruses [1]. Mammalian defensins are predominantly expressed in epithelial cells of skin, respiratory airways, gastrointestinal and genitourinary tracts, which form physical barriers to external infectious agents [2, 3], and also in leukocytes (mostly neutrophils), which kill microbes that have already penetrated the body [4]. Mature defensins contain six cysteine residues (Cys1-6) forming three intramolecular disulphide bonds. Depending on the bonds arrangement they are classified into alpha, beta and theta subfamilies. Alpha defensins secreted by leukocytes and intestinal Paneth cells of mammals [5] contain disulfide bridges between 1-6, 2-4, and 3-5 cysteine residues, while beta defensins produced by epithelial cells and leukocytes of most multicellular organisms [6] are distinguished by pairing of cysteine residues 1-5, 2-4, and 3-6 [7]. Members of the rare theta defensin subfamily (circular minidefensins) are expressed only in leukocytes and bone marrow cells of monkeys. They are produced by the head-to-tail ligation of two different C-terminally truncated pro-alpha defensins (demidefensins), each nine amino acids long [8].
The tertiary structure of mature alpha defensins consists of a triple-stranded β-sheet with two β-turns [9]. An amphipathic character of the peptide (i.e., mostly hydrophobic structure with a positively charged hydrophilic part) is essential for the insertion into the microbial membrane and the formation of a pore leading to membrane permeabilization and lysis of the microbe [10]. Initial recognition of numerous microbial targets is a consequence of electrostatic interactions between the defensins arginine residues and the negatively charged phospholipids of the microbial cytoplasmic membrane [2, 5]. However, the precise mechanism of target recognition and its putative effectors have not been studied in sufficient detail for all known defensin molecules [11]. The most sensitive targets of enteric alpha defensins are Gram-positive and Gram-negative bacteria [12]. Though viruses usually cannot be cleared by the innate immune system, some defensins are able to suppress replication of human immunodeficiency virus [13].
Since their discovery in rabbit neutrophils in 1966 [14], around fifty alpha defensin genes have been identified in primates, rodents, and equines, most of which haven't been analyzed in sufficient detail. The genes evolve extremely rapidly, so that their copy number in various organisms and even in individual genomes of the same species significantly varies [15]. Six best characterized alpha defensins, which are most abundantly expressed in the human body, include four peptides expressed in neutrophils (DEFA1 through 4, previously referred to as human neutrophil peptides HNP1-4) and two enteric defensins - DEFA5 and 6 (formerly designated as HD5 and 6). DEFA5 and 6 are secreted in the mucous layer by Paneth cells of small intestine and colon and to a smaller extent by cells of female reproductive tract and oropharyngeal mucosa [16, 17]. Besides, Paneth cells produce more than a dozen of other antimicrobials, including lysozyme, IgAs, angiogenins, and secreted phospholipase A2 [16].
Twenty-four murine alpha defensin genes are listed in the mouse genome informatics database http://www.informatics.jax.org, six of which (enteric alpha defensins 1 through 6, formerly referred to as cryptdins) have been thoroughly investigated in recent years. Unlike humans, rats, and rabbits genomes, mouse DNA does not contain Defa encoding genes that are expressed in neutrophils [18]. Recently, eleven murine alpha defensin related genes (formerly called cryptdin related sequences) with yet unknown functions have been identified. These genes encode prepro-sequences that are nearly identical to those of alpha defensins, but their mature peptides show no homology to defensins [19].
Gene expression and peptide processing
Human and murine enteric alpha defensin genes consist of two exons, whereas alpha defensin genes expressed in human neutrophils consist of three exons, two of which (second and third) are homologous to enteric defensins [1]. Search for orthologous sequences in promoters of these genes revealed presence of putative binding sites for transcription factors AP1, OCT1.4, and GCN4 [20, 21]. Upstream regions of human DEFA5 and murine Defa4 genes contain highly conserved cis-acting elements as evident from the expression of functionally active human peptide in small intestinal crypts of mice transgenic for human DEFA5 minigene construct [22].
Analysis of postnatal changes in concentration of murine enteric defensins in the small intestine of conventional and germ-free mice revealed two patterns of gene expression: gradual increase in production of defensins 1, 3 and 6 and rapid raise in secretion of defensins 2, 4 and 5 in the jejunum (but not in the ileum), which is presumably induced by the presence of luminal bacteria [23]. In adult mice alpha defensins are equally expressed along the small intestine except Defa4, which is more abundant in the ileum as compared to the duodenum [24]. Recently, it has been shown, that in human tropical populations secretion of enteric alpha defensins may decrease up to ten times, as compared to Europeans, due to down-regulation of gene expression in response to infections, inflammatory conditions, and malnutrition [25].
Human enteric alpha defensins are synthesized in vivo as precursor proteins, some of which have antimicrobial activity. For example human DEFA5 prepropeptide (aa 1-94), contains signal peptide (aa 1-19) and propeptide (aa 20-94), which is subsequently processed into major mature peptide (aa 63-94), and minor mature peptide (aa 56-94) [26]. The prosequence flanking the mature peptide is necessary for correct intracellular sorting and trafficking of the propeptide into the secretory vesicles, where it is proteolytically processed by trypsin during secretion [27, 28]. Conversely, murine defensins are stored in vesicles in a mature form after propeptide processing by matrilysin (also known as matrix metalloproteinase Mmp7) [29]. It has been shown that abrogation of murine alpha defensin processing by targeted disruption of the matrilysin gene increases susceptibility of Mmp7 knockout mice to oral challenges with enteric bacteria, whereas transgenic mice overexpressing human DEFA5 are markedly resistant to orally administered virulent Salmonella typhimurium, due to proper processing of human propeptide by murine matrilysin [22].
Structure of the intestinal epithelium
The mucosal surface of the small intestine consists of crypts of Lieberkühn and villi. Due to the constant shedding of gut epithelial cells in the lumen the whole epithelium renews once in four-five days, except for Paneth cells, which live approximately 70 days. Intestinal epithelium is derived from multipotent columnar stem cells located at the base of the crypt, which expess LGR5 receptor and other stem cell-specific protein markers [30]. An alternative pool of stem cells is positioned higher in the crypt wall [30–32]. Columnar stem cells give rise to actively proliferating transit amplifying cells differentiating into four major epithelial cell lineages: 1) enterocytes absorbing nutrients; 2) goblet cells producing mucus; 3) enteroendocrine cells secreting hormones in the capillaries of the underlying connective tissue (lamina propria); and 4) Paneth cells secreting enteric alpha defensins, as well as other antimicrobials in the mucous layer. Besides, stem cells generate less documented microfold cells (M-cells) responsible for the uptake of mucosal antigens [33] and the recently described tuft cells secreting endogenous intestinal opioids [34]. Paneth cells protect the adjacent stem cells and the whole gut epithelium from microbial infection and regulate bacterial colonization of the gut [35].
Functions of enteric alpha defensins
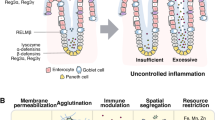
Enteric alpha defensins are the most abundant products secreted by Paneth cells [36]. The main inducers of their secretion are the products of degradation of Gram-positive and Gram-negative bacteria, inhabiting the gut including: muramyl dipeptide, bacterial lipopolysaccharide, flagellin, lipid A, and unmethylayed CpG sequences in bacterial DNA. Their presence in the mucous layer of the intestinal epithelium is constantly monitored by the three receptor types that are expressed in Paneth cells and enterocytes: 1) toll-like receptors (TLRs, including the most abundant cell surface receptors 2 and 4); 2) cytoplasmic nucleotide oligomerization domain-like receptors (NLRs, in particular NOD1 and 2); and 3) retinoic acid inducible gene 1-like receptors (RLRs, including the most abundant receptor RIG1) (Figure 1) [37]. Receptor signals transmitted by the MAP kinase signaling pathway induce translocation to the nucleus of transcription factors, which initiate transcription of genes that are involved in functioning of the innate and adaptive immune systems and stimulation of inflammation, wound healing, and angiogenesis in the adjacent connective tissues [38–40].
Schematic diagram of enteric alpha defensins functions in the immune defense of the gut. Paneth cell receptor activation by microbe's degradation products results in defensins secretion in the lumen (aimed at the regulation of bacterial colonization) and in lamina propria (in order to activate pro- and anti-inflammatory responses of the adaptive immune system cells). SC - stem cells, PC - Paneth cells, TAC - transit amplifying cells, which give rise to two partially differentiated cell lineages: progenitors of secreting cells (SEC) and progenitors of enterocytes (EC).
Detection of microbial presence in the mucous layer of the gut results in massive release of enteric alpha defensins by Paneth cells that leads to an enhanced killing of microbes in the lumen. Simultaneous secretion of alpha defensins in lamina propria [12] triggers two opposite defense mechanisms of pro- and anti-inflammatory response. According to the first mechanism, human enteric alpha defensins bind to as yet unidentified Gαi-protein coupled receptors located on the surface of macrophages and T lymphocytes of lamina propria (Figure 1) [41–43], which leads to proliferation of T lymphocytes and their chemotaxis to the site of inflammation [44]. Moreover, it was shown that murine defensins 2 and 3 (as well as beta defensins) may induce reversible formation of ion channels on apical membranes of undifferentiated Cl- secreting cells of the crypt. This results in salt and water secretion, causing the crypt lumen to be flushed after Paneth cell discharge [45].
At the same time enteric alpha defensins moderate intestinal anti-iflammatory response, as a consequence of suppression of IL1 beta release from bacterial lipopolysaccharide-activated monocytes [46] and inhibition of interleukin 17-producing T helper cell proliferation in lamina propria (Figure 1) [36]. In order to avoid the immune response, microbes inject effector proteins into intestinal epithelial cells that either block their immune and inflammatory response (mostly due to inactivation of transcription factors NF-κB or AP-1) or reprogram signaling pathways sensing and killing bacteria [47, 48]. Besides, microbes are able to block expression of enteric alpha defensins and other antimicrobial substances by Paneth cells using as yet unknown mechanisms [49].
Enteric defensins in pathology
Changes in secretion of enteric alpha defensins have been registered in the intestinal epithelium of patients with inflammatory bowel disease (IBD) - a large group of inflammatory illnesses of the gastrointestinal tract, which are caused by immune system over-activation due to a loss of tolerance to gut microflora [50–52]. Two major types of IBD are Crohn's disease (major location - terminal ileum) and ulcerative colitis (predominant lesions in colon and rectum). Susceptibility to Crohn's ileitis in European and North American populations is most strongly associated with mutations in the gene encoding cytoplasmic protein NOD2 - bacterial muramyl dipeptide receptor from NLR family, which is essential for activation of enteric alpha defensins secretion by Paneth cells (Figure 1) [53]. Accordingly, mutations in NOD2 encoding gene (most frequently a frame shift Leu1007fsX1008, caused by single nucleotide insertion 3020insC or "snp13") lead to a decreased level of enteric alpha defensins in the intestinal mucosal extracts [54]. Analysis of Nod2-deficient mice confirmed decreased secretion of alpha defensins 1-6 by Paneth cells and demonstrated inability of such mice to develop intestinal inflammation, which results in their susceptibility to bacterial infection by oral administration [55]. Part of the cases of Crohn's ileitis is independent of NOD2 genotype and is linked to changes in the WNT pathway, mostly due to a decreased activity of transcription factor TCF4 that binds to enteric defensins gene promoters [56].
The second major type of IBD - ulcerative colitis is associated with the infection of gastrointestinal tract by bacterium Helicobacter pylori [57]. Subsequent over-activation of the intestinal immune system results in elevated secretion of enteric alpha defensins (as well as TNF alpha and IL1 beta) suggesting that Helicobacter is able to avoid the innate and adaptive immune response in human intestine by as yet unknown mechanisms [58].
Similar to ulcerative colitis, 15% of gastrointestinal malignancies arise as a consequence of chronic microbial infections, as demonstrated by the raised chance of hepatocellular carcinoma in patients with chronic hepatitis, association between Helycobacter pylori infection and higher gastric cancer risk, and increased chance of colon cancer in patients with inflammatory bowel disease [59]. At initial stages of colon carcinogenesis, mutations in intestinal epithelial cells lead to constitutive activation of the Wnt pathway in early adenoma cells, which simultaneously follow differentiation programs of progenitor and Paneth cells [60]. This results in 60 fold increase of DEFA5 and DEFA6 production by tumors, as compared to normal colonic epithelium [61, 62]. Besides, both early adenomas and adenocarcinomas acquire the ability to secrete these proteins into the bloodstream. Thus, enteric alpha defensins are promising markers for early diagnosis of colon cancer provided that test sensitivity is sufficient for their robust detection in sera.
Conclusion
Enteric alpha defensins play an important role in regulation of bacterial colonization of the gut, as well as in activation of pro- and anti-inflammatory response of the adaptive immune system cells in lamina propria. Further studies of the defensins functions in norm and pathology can provide important clues for the development of new tools for diagnosis and treatment of gastrointestinal cancers and most widespread inflammatory illnesses of the small and large intestine.
References
Droin N, Hendra JB, Ducoroy P, Solary E: Human defensins as cancer biomarkers and antitumour molecules. J Proteomics. 2009, 72: 918-27. 10.1016/j.jprot.2009.01.002
Epand RM, Vogel HJ: Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta. 1999, 1462: 11-28. 10.1016/S0005-2736(99)00198-4
Lai Y, Gallo RL: AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30: 131-41. 10.1016/j.it.2008.12.003
Guani-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Teran LM: Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010, 135: 1-11. 10.1016/j.clim.2009.12.004
Salzman NH, Underwood MA, Bevins CL: Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol. 2007, 19: 70-83. 10.1016/j.smim.2007.04.002
Semple CA, Gautier P, Taylor K, Dorin JR: The changing of the guard: Molecular diversity and rapid evolution of beta-defensins. Mol Divers. 2006, 10: 575-84. 10.1007/s11030-006-9031-7
Bauer F, Schweimer K, Kluver E, Conejo-Garcia JR, Forssmann WG, Rosch P, Adermann K, Sticht H: Structure determination of human and murine beta-defensins reveals structural conservation in the absence of significant sequence similarity. Protein Sci. 2001, 10: 2470-9. 10.1110/ps.ps.24401
Garcia AE, Osapay G, Tran PA, Yuan J, Selsted ME: Isolation, synthesis, and antimicrobial activities of naturally occurring theta-defensin isoforms from baboon leukocytes. Infect Immun. 2008, 76: 5883-91. 10.1128/IAI.01100-08
Szyk A, Wu Z, Tucker K, Yang D, Lu W, Lubkowski J: Crystal structures of human alpha-defensins HNP4, HD5, and HD6. Protein Sci. 2006, 15: 2749-60. 10.1110/ps.062336606
Ganz T: Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003, 3: 710-20. 10.1038/nri1180
Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, Buchner J, Schaller M, Stange EF, Wehkamp J: Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature. 2011, 469: 419-23. 10.1038/nature09674
Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ: Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000, 1: 113-8. 10.1038/77783
Chang TL, Klotman ME: Defensins: natural anti-HIV peptides. AIDS reviews. 2004, 6: 161-8.
Zeya HI, Spitznagel JK: Antimicrobial specificity of leukocyte lysosomal cationic proteins. Science. 1966, 154: 1049-51. 10.1126/science.154.3752.1049
Linzmeier RM, Ganz T: Human defensin gene copy number polymorphisms: comprehensive analysis of independent variation in alpha- and beta-defensin regions at 8p22-p23. Genomics. 2005, 86: 423-30. 10.1016/j.ygeno.2005.06.003
Wehkamp J, Chu H, Shen B, Feathers RW, Kays RJ, Lee SK, Bevins CL: Paneth cell antimicrobial peptides: topographical distribution and quantification in human gastrointestinal tissues. FEBS Lett. 2006, 580: 5344-50. 10.1016/j.febslet.2006.08.083
Quayle AJ, Porter EM, Nussbaum AA, Wang YM, Brabec C, Yip KP, Mok SC: Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998, 152: 1247-58.
Eisenhauer PB, Lehrer RI: Mouse neutrophils lack defensins. Infect Immun. 1992, 60: 3446-7.
Amid C, Rehaume LM, Brown KL, Gilbert JG, Dougan G, Hancock RE, Harrow JL: Manual annotation and analysis of the defensin gene cluster in the C57BL/6J mouse reference genome. BMC Genomics. 2009, 10: 606- 10.1186/1471-2164-10-606
Ouellette AJ, Darmoul D, Tran D, Huttner KM, Yuan J, Selsted ME: Peptide localization and gene structure of cryptdin 4, a differentially expressed mouse paneth cell alpha-defensin. Infect Immun. 1999, 67: 6643-51.
Huttner KM, Selsted ME, Ouellette AJ: Structure and diversity of the murine cryptdin gene family. Genomics. 1994, 19: 448-53. 10.1006/geno.1994.1093
Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL: Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003, 422: 522-6. 10.1038/nature01520
Inoue R, Tsuruta T, Nojima I, Nakayama K, Tsukahara T, Yajima T: Postnatal changes in the expression of genes for cryptdins 1-6 and the role of luminal bacteria in cryptdin gene expression in mouse small intestine. FEMS Immunol Med Microbiol. 2008, 52: 407-16. 10.1111/j.1574-695X.2008.00390.x
Karlsson J, Putsep K, Chu H, Kays RJ, Bevins CL, Andersson M: Regional variations in Paneth cell antimicrobial peptide expression along the mouse intestinal tract. BMC Immunol. 2008, 9: 37- 10.1186/1471-2172-9-37
Kelly P, Feakins R, Domizio P, Murphy J, Bevins C, Wilson J, McPhail G, Poulsom R, Dhaliwal W: Paneth cell granule depletion in the human small intestine under infective and nutritional stress. Clin Exp Immunol. 2004, 135: 303-9. 10.1111/j.1365-2249.2004.02374.x
Rajabi M, de Leeuw E, Pazgier M, Li J, Lubkowski J, Lu W: The conserved salt bridge in human alpha-defensin 5 is required for its precursor processing and proteolytic stability. The Journal of biological chemistry. 2008, 283: 21509-18. 10.1074/jbc.M801851200
Ghosh D, Porter E, Shen B, Lee SK, Wilk D, Drazba J, Yadav SP, Crabb JW, Ganz T, Bevins CL: Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol. 2002, 3: 583-90. 10.1038/ni797
Elphick D, Liddell S, Mahida YR: Impaired luminal processing of human defensin-5 in Crohn's disease: persistence in a complex with chymotrypsinogen and trypsin. Am J Pathol. 2008, 172: 702-13. 10.2353/ajpath.2008.070755
Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC: Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999, 286: 113-7. 10.1126/science.286.5437.113
Simons BD, Clevers H: Stem cell self-renewal in intestinal crypt. Exp Cell Res. 2011, 317: 2719-24. 10.1016/j.yexcr.2011.07.010
Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ: A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011, 7368: 255-9.
Takeda N, Jain R, Leboeuf MR, Wang Q, Lu MM, Epstein JA: Interconversion Between Intestinal Stem Cell Populations in Distinct Niches. Science. 2011, ,
Roberts CL, Keita AV, Duncan SH, O'Kennedy N, Söderholm JD, Rhodes JM, Campbell BJ: Translocation of Crohn's disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut. 2010, 59: 1331-9. 10.1136/gut.2009.195370
Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C: Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011, 192: 767-80. 10.1083/jcb.201010127
Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D: Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010, 11: 76-83. 10.1038/ni.1825
Uematsu S, Fujimoto K: The innate immune system in the intestine. Microbiol Immunol. 2010, 54: 645-57. 10.1111/j.1348-0421.2010.00267.x
Choi YJ, Im E, Chung HK, Pothoulakis C, Rhee SH: TRIF mediates Toll-like receptor 5-induced signaling in intestinal epithelial cells. The Journal of biological chemistry. 2010, 285: 37570-8. 10.1074/jbc.M110.158394
Foureau DM, Mielcarz DW, Menard LC, Schulthess J, Werts C, Vasseur V, Ryffel B, Kasper LH, Buzoni-Gatel D: TLR9-dependent induction of intestinal alpha-defensins by Toxoplasma gondii. J Immunol. 2010, 184: 7022-9. 10.4049/jimmunol.0901642
Eckmann L: Innate immunity and mucosal bacterial interactions in the intestine. Curr Opin Gastroenterol. 2004, 20: 82-8. 10.1097/00001574-200403000-00006
Menendez A, Ferreira RB, Finlay BB: Defensins keep the peace too. Nat Immunol. 2010, 11: 49-50. 10.1038/ni0110-49
Lin PW, Simon PO, Gewirtz AT, Neish AS, Ouellette AJ, Madara JL, Lencer WI: Paneth cell cryptdins act in vitro as apical paracrine regulators of the innate inflammatory response. The Journal of biological chemistry. 2004, 279: 19902-7. 10.1074/jbc.M311821200
de Leeuw E, Burks SR, Li X, Kao JP, Lu W: Structure-dependent functional properties of human defensin 5. FEBS Lett. 2007, 581: 515-20. 10.1016/j.febslet.2006.12.036
Ishikawa C, Tanabe H, Maemoto A, Ito T, Watari J, Kono T, Fujiya M, Ashida T, Ayabe T, Kohgo Y: Precursor processing of human defensin-5 is essential to the multiple functions in vitro and in vivo. J Innate Immun. 2009, 2: 66-76.
Grigat J, Soruri A, Forssmann U, Riggert J, Zwirner J: Chemoattraction of macrophages, T lymphocytes, and mast cells is evolutionarily conserved within the human alpha-defensin family. J Immunol. 2007, 179: 3958-65.
Lencer WI, Cheung G, Strohmeier GR, Currie MG, Ouellette AJ, Selsted ME, Madara JL: Induction of epithelial chloride secretion by channel-forming cryptdins 2 and 3. Proc Natl Acad Sci USA. 1997, 94: 8585-9. 10.1073/pnas.94.16.8585
Shi J, Aono S, Lu W, Ouellette AJ, Hu X, Ji Y, Wang L, Lenz S, van Ginkel FW, Liles M: A novel role for defensins in intestinal homeostasis: regulation of IL-1beta secretion. J Immunol. 2007, 179: 1245-53.
Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, Rao AS, Madara JL: Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000, 289: 1560-3. 10.1126/science.289.5484.1560
Salzman NH, Chou MM, de Jong H, Liu L, Porter EM, Paterson Y: Enteric salmonella infection inhibits Paneth cell antimicrobial peptide expression. Infect Immun. 2003, 71: 1109-15. 10.1128/IAI.71.3.1109-1115.2003
Xavier RJ, Podolsky DK: Microbiology. How to get along--friendly microbes in a hostile world. Science. 2000, 289: 1483-4. 10.1126/science.289.5484.1483
Negroni A, Stronati L, Pierdomenico M, Tirindelli D, Di Nardo G, Mancini V, Maiella G, Cucchiara S: Activation of NOD2-mediated intestinal pathway in a pediatric population with Crohn's disease. Inflamm Bowel Dis. 2009, 15: 1145-54. 10.1002/ibd.20907
Wehkamp J, Stange EF: A new look at Crohn's disease: breakdown of the mucosal antibacterial defense. Ann N Y Acad Sci. 2006, 1072: 321-31. 10.1196/annals.1326.030
Ramasundara M, Leach ST, Lemberg DA, Day AS: Defensins and inflammation: the role of defensins in inflammatory bowel disease. J Gastroenterol Hepatol. 2009, 24: 202-8. 10.1111/j.1440-1746.2008.05772.x
Rehman A, Sina C, Gavrilova O, Hasler R, Ott S, Baines JF, Schreiber S, Rosenstiel P: Nod2 is essential for temporal development of intestinal microbial communities. Gut. 2011, 60: 1354-62. 10.1136/gut.2010.216259
Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R: Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci USA. 2005, 102: 18129-34. 10.1073/pnas.0505256102
Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA: Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005, 307: 731-4. 10.1126/science.1104911
Wehkamp J, Wang G, Kübler I, Nuding S, Gregorieff A, Schnabel A, Kays RJ, Fellermann K, Burk O, Schwab M: The Paneth cell alpha-defensin deficiency of ileal Crohn's disease is linked to Wnt/Tcf-4. J Immunol. 2007, 179: 3109-18.
Thomson JM, Hansen R, Berry SH, Hope ME, Murray GI, Mukhopadhya I, McLean MH, Shen Z, Fox JG, El-Omar E: Enterohepatic helicobacter in ulcerative colitis: potential pathogenic entities?. PloS one. 2011, 6: e17184- 10.1371/journal.pone.0017184
Stronati L, Negroni A, Pierdomenico M, D'Ottavio C, Tirindelli D, Di Nardo G, Oliva S, Viola F, Cucchiara S: Altered expression of innate immunity genes in different intestinal sites of children with ulcerative colitis. Dig Liver Dis. 2010, 42: 848-53. 10.1016/j.dld.2010.04.003
Quante M, Wang TC: Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology (Bethesda). 2008, 23: 350-9. 10.1152/physiol.00031.2008.
Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M, Laurent-Puig P, Kahn A, Robine S, Perret C: Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 2005, 132: 1443-51. 10.1242/dev.01700
Joo M, Shahsafaei A, Odze RD: Paneth cell differentiation in colonic epithelial neoplasms: evidence for the role of the Apc/beta-catenin/Tcf pathway. Hum Pathol. 2009, 40: 872-80. 10.1016/j.humpath.2008.12.003
Radeva MY, Jahns F, Wilhelm A, Glei M, Settmacher U, Greulich KO, Mothes H: Defensin alpha 6 (DEFA 6) overexpression threshold of over 60 fold can distinguish between adenoma and fully blown colon carcinoma in individual patients. BMC Cancer. 2010, 10: 588- 10.1186/1471-2407-10-588
Acknowledgements
This work was supported by the Ministry of education and science (State Contract No. 14.740.11.0757).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
NA drafted the manuscript. YA compiled the reference list. IG performed proofreading. GS designed the figure. Y contributed to the description of defensins functions. SF participated in the design and coordination. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Lisitsyn, N.A., Bukurova, Y.A., Nikitina, I.G. et al. Enteric alpha defensins in norm and pathology. Ann Clin Microbiol Antimicrob 11, 1 (2012). https://doi.org/10.1186/1476-0711-11-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-0711-11-1