Abstract
Background
Spices traditionally have been used as coloring agents, flavoring agents, preservatives, food additives and medicine in Bangladesh. The present work aimed to find out the antimicrobial activity of natural spices on multi-drug resistant Escherichia coli isolates.
Methods
Anti-bacterial potentials of six crude plant extracts (Allium sativum, Zingiber officinale, Allium cepa, Coriandrum sativum, Piper nigrum and Citrus aurantifolia) were tested against five Escherichia coli isolated from potable water sources at kushtia, Bangladesh.
Results
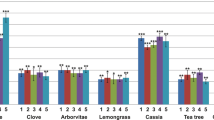
All the bacterial isolates were susceptible to undiluted lime-juice. None of them were found to be susceptible against the aqueous extracts of garlic, onion, coriander, pepper and ginger alone. However, all the isolates were susceptible when subjected to 1:1:1 aqueous extract of lime, garlic and ginger. The highest inhibition zone was observed with lime (11 mm).
Conclusion
Natural spices might have anti-bacterial activity against enteric pathogens and could be used for prevention of diarrheal diseases. Further evaluation is necessary.
Similar content being viewed by others
Introduction
Multi-drug resistant strains of Escherichia coli are widely distributed in hospitals and are increasingly being isolated from community [1]. Thus, it is urgent need to find out new antimicrobial agents. However, new families of antimicrobial agents will have a short life expectancy [2]. For this reason, researchers are increasingly turning their attention to herbal products, looking for new leads to develop better drugs against multidrug resistant microbe strains [3].
Spice plants and essential oils extracted from them have become important due to their potential antimicrobial [4–6] and stimulating effects in the animal digestive system. The antimicrobial effectiveness of mustard, clove, cinnamon and their essential oils were reported for the first time around 1880's. Antimicrobial effectiveness of spices depend on the kind of spice, its composition and concentration, type and concentrations of the target microorganism, substrate composition, and processing and food storage conditions [7]. Spice plants have been used traditionally as coloring agents, flavoring agents, preservatives, food additives and as well as antiparasitic, antihelmintic, analgesic, expectorant, sedative, antiseptic and anti-diabetic substances in many parts of the world [8]. In addition, they possess biological activities such as that of antioxidants [9] and hypocholesterolemics [10]. In this study, we aimed to explore the antibacterial effect of natural spices on multiple drug resistant E. coli isolates.
Materials and methods
Multi-drug resistant E. coli used in present study were isolated from drinking water of various region of kushtia town by standard methods [11]. Pure cultures of isolates were preserved at 4°C on nutrient agar slants. In order to confirm the identity of the test bacterial isolates morphological characteristics and conventional biochemical tests were performed according to Harley and Prescott [11]. The spices were bought from local market. Garlic and onion were peeled, cut into pieces and sun-dried for one week. These were then grounded using a blender. Twenty gram of the ground material (garlic, onion, ginger, pepper and coriander seed) were soaked in 100 ml of hot sterile water and allowed to stand for 72 h. The lime fruits were washed with sterile water then cut open with a sterile knife and the juice pressed out. The crude extracts were obtained by filtration [12]. Sterile paper disks of 5 mm diameter were impregnated with 10 μl of crude extracts and mixtures of different crude extracts (1:1, 1:1:1), then dried in a hot air oven at 60°C for five min. Each disk contains approximately 2 μg of crude extract. All extracts were stored at 4°C when not in use.
Susceptibility testing
Standard norfloxacin (NOR, 10 μg/disc), clindamycin (CC, 2 μg/disc), neomycin (N, 30 μg/disc), cefoxitin (FOX, 30 μg/disc), streptomycin (S, 10 μg/disc), ciprofloxacin (CPX, 5 μg/disc), chloramphenicol (C, 30 mcg/disc), tetracycline (T, 30 mcg/disc), penicillin G (P, 10 units/disc), erythromycin (E, 15 mcg/disc), cefotaxime (CTX, 30 μg/disc), nalidixic acid (NA, 30 mcg/disc), cloxacillin (CX, 1 mcg/disc), kanamycin (K, 30 mcg/disc), amoxicillin (AM, 30 mcg/disc) were used for comparison of the antibacterial activity. In vitro antibacterial susceptibility of the test samples was carried out by disc diffusion method [13, 14]. The antibacterial activities were determined by measuring the diameter of the zone in mm.
Results and discussion
Table 1 shows the antibiotic resistance pattern of the isolates. All strains showed resistance against the activity of clindamycin and cloxacillin. Penicillin G is also not quite effective against those isolates as four of the isolates are resistant against it. Highest growth inhibition zones were found with ciprofloxacin followed by cefotaxime and norfloxacin. The multiple antibiotic resistance of E. coli demonstrated in this study accord with those found in other studies [15–17].
Table 2 shows that the aqueous extracts of Allium sativum and Zingiber officinale alone and in combination (1:1) did not exhibit any in vitro inhibition on the growth of test organisms except in case of one isolate for later cases (7 mm). Similarly Allium cepa, Piper nigrum, and Coriandrum sativum seed alone did not exhibit any in vitro anti-bacterial effect, however the combination of Allium cepa + Allium sativum (1:1) and Citrus aurantifolia + Zingiber officinale + Allium sativum (1:1:1) showed inhibition zones. Citrus aurantifolia juice showed the highest effect on the test organisms. These findings are in good accord with others [12, 18–22].
A. sativum has traditional dietary and medicinal applications as an anti-infective agent [23]. In vitro evidence of the antimicrobial activity of fresh and freeze-dried garlic extracts against many bacteria [24], fungi and viruses [25] supports these applications. Allicin, the active ingredient of A. sativum, acts by partially inhibiting DNA and protein synthesis and also totally inhibiting RNA synthesis as a primary target [26]. Like A. sativum, A. cepa is another medicinally important anti-infective agent [12]. Raw A. cepa can completely sterilize mouth and throat. Organosulfur compounds (OSCs) and phenolic compounds have been reported to be involved in the A. cepa antimicrobial activity [27]. Z. officinale has been used widely as herbal medicine. In particular, its gingerol-related components have been reported to possess antimicrobial and anti fungal properties, as well as several pharmaceutical properties [28]. C. sativum is considered both herb and spice since both its leaves and seeds are used as a seasoning condiment [18]. It has traditionally been referred to as antimicrobial [29]. The seeds of C. sativum contain 0.5-1% essential oil and are rich in beneficial phytonutrients including carvone, geraniol, limonene, borneol, camphor, elemol and linalool [30]. Ferrous sequestering activity of these compounds may play role in inhibiting microbial growth [31]. P. nigrum is used to treat asthma, chronic indigestion, colon toxins, obesity, sinus, congestion, fever, intermittent fever, cold extremities, colic, gastric ailments and diarrhoea [32]. It has shown to have antimicrobial activity [33]. From HPTLC analysis it is reported that the major phytochemical present in the crude extract of P. nigrum was found to be piperine thus the inhibitor effect of crude extract of P. nigrum and its active constituent was found to be piperine [34]. C. aurantifolia is popular as fruit and food ingredient for flavoring and adding acidity, its juices have been reported to exhibit antibacterial activity against wide range of microbes including Klebsiella pneumoniae, Shigella flexnerii, E. coli ATCC 25922 and Vibrio cholerae[35, 36].
The antimicrobial potency of plants is believed to be due to tannins, saponins, phenolic compounds, essential oils and flavonoids [37]. It is interesting to note that even crude extracts of these plants showed good activity against multidrug resistant strains where modern antibiotic therapy has limited effect.
The effect of these spices on these organisms in vivo cannot be predicted from this study. And though paper disk assays a practical approach to study potential antibacterial compounds, using the size of inhibition zone to indicate relative antibacterial activity is not adequate. The zone must be affected by the solubility and rate of diffusion in agar medium or its volatilization; and thus the results could be affected.
Thus, there is a need for detailed scientific study of traditional medical practices to ensure that valuable therapeutic knowledge of some plants is preserved and also to provide scientific evidence for their efficacies.
Conclusions
The extracts of these spices could be a possible source to obtain new and effective herbal medicines to treat diarrheal diseases caused by multi-drug resistant strains of E. coli in community. However, it is necessary to isolate the active constituents, and determine their toxicity, side effects and pharmaco-kinetic properties.
References
Akram M, Shahid M, Khan AU: Etiology and Antibiotics Resistance Pattern of Community Acquired Urinary Infections in J N M C Hospital Aligarh India. Ann Clin Microbiol Antimicrob. 2007, 6: 4- 10.1186/1476-0711-6-4
Coates A, Hu Y, Bax R, Page C: The future challenges facing the developement of new antimicrobial drugs. Nat Rev Drug Discov. 2002, 1: 895-910. 10.1038/nrd940
Braga LC, Leite AAM, Xavier KGS, Takahashi JA, Bemquerer MP, Chartone-Souza E, Nascimento AMA: Synergic interaction between pomegranate extracts and antibiotics against Staphylococcus aureus. Can J Microbiol. 2005, 51: 541-547. 10.1139/w05-022
Elgayyar M, Draughon FA, Golden DA, Mount JR: Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. J Food Prot. 2001, 64: 1019-1024.
Singh G, Kapoor IP, Pandey SK, Singh UK, Singh RK: Studies on essential oils: part 10; antibacterial activity of volatile oils of some spices. Phytother Res. 2002, 16: 680-682. 10.1002/ptr.951
Valero M, Salmeron MC: Antibacterial activity of 11 essential oils against Bacillus cereus in tyndallized carrot broth. Int J Food Microbiol. 2003, 85: 73-81. 10.1016/S0168-1605(02)00484-1
Leite de Souza E, Guerr NB, Stamford TLM, Lima EO: Spices: alternative sources of antimicrobial compounds to use in food conservation. Rev Bras Farm. 2006, 87: 22-25.
Lee KW, Everts H, Beynen AC: Essential oils in broiler nutrition. Int J Poult Sci. 2004, 3: 738-752. 10.3923/ijps.2004.738.752.
Miura K, Kikuzaki H, Nakatani N: Antioxidant activity of chemical components from sage (Salvia officinalis L.) and thyme (Thymus vulgaris L.) measured by the oil stability index method. J Agric Food Chem. 2002, 50: 1845-1851. 10.1021/jf011314o
Craig WJ: Health-promoting properties of common herbs. Am J Clin Nutr. 1999, 70: 491-499.
Harley JP, Prescott LM: Laboratory Exercises in Microbiology. 2002, McGraw-Hill Publishers, 5
Onyeagba RA, Ugbogu OC, Okeke CU, Iroakasi O: Studies on the antimicrobial effects of garlic (Allium sativum Linn), ginger (Zingiber officinale Roscoe) and lime (Citrus aurantifolia Linn). African Journal of Biotechnology. 2004, 3: 552-554.
Barry AL: Procedure for testing antimicrobial agent in agar media. Antibiotica in laboratory medicines. Edited by: Lorian V. 1980, 1-23. Baltimiore: Williams and Wilkins Co,
Bauer AW, Kibry WMM, Sherris JC, Turck M: Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol. 1966, 45: 493-496.
Kinge NW, Ateba CN, Kawadza DT: Antibiotic resistance profiles of Escherichia coli isolated from different water sources in the Mmabatho locality, Northwest Province, South Africa. South African Journal of Science. 2010, 106: 44-49.
Ebrahimi A, Lotfalian S: Isolation and antibiotic resistance pattern of Escherichia coli and couagulase possative Staphylococcus aureus from honey bees digestive tract. Iranian Journal of Veterinary Research. 2005, 6: 1-3.
White DG, Zhao S, Sinijee S: Antimicrobial resistance of foodborne pathogens. Microbes Infect. 2002, 4: 405-412. 10.1016/S1286-4579(02)01554-X
Chaudhry NMA, Tariq P: Bactericdal activity of black pepper, bay leaf, aniseed and coriander against oral isolates. Pak J Pharm Sci. 2006, 19: 214-218.
Indu MN, Hatha AAM, Abirosh C, Harsha U, Vivekanandan G: Antibacterial Activity of some of the south- Indian spices againest serotypea of Escherichia coli, Salmonella, Listeria monocytogenes and Aeromonas hydrophila. Brazilian Journal of Microbiology. 2006, 37: 153-158. 10.1590/S1517-83822006000200011.
Olonisakin A, Oladimeji MO, Lajide L: Chemical composition and antibacterial activity of steam distilled oil of Ashanti pepper (Piper guineense) fruits (berries). Electron. J Environ Agric Food Chem. 2006, 5: 1531-1535.
Oboh PA, Abula EO: The antimicrobial activities of extracts of Sidium guajava and Citrus aurantifolia. Niger J Biotechnol. 1997, 8: 25-29.
Oboh PA, Agbonlahor DE, Ekundayo AO, Owen-Ureghe B: Antibacterial activity of Citrus aurantifolia (lime) juice against some Gram positive and Gram negative bacteria. Ann Nat Sci. 1992, 2: 1-6.
Ross ZM, O'gara EA, Hill DJ, Sleightholme HV, Maslin DJ: Antimicrobial Properties of Garlic Oil against Human Enteric Bacteria: Evaluation of Methodologies and Comparisons with Garlic Oil Sulfides and Garlic Powder. Applied and Enviromnental Microbiology. 2001, 67: 475-480. 10.1128/AEM.67.1.475-480.2001.
Rees LP, Minney SF, Plummer NT, Slater JH, Skyrme DA: A quantitative assessment of the antimicrobial activity of garlic (Allium sativum). World J Microbiol Biotechnol. 1993, 9: 303-307. 10.1007/BF00383068.
Weber ND, Anderson DO, North JA, Murray BK, Lawson LD, Hughes BG: In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992, 58: 417-423. 10.1055/s-2006-961504
Eja ME, Asikong BE, Abriba C, Arikpo GE, Anwan EE, Enyi-Idoh KH: A comparative assessment of the antimicrobial effects of garlic (Allium sativum) and antibiotics on diarrheagenic organisms. Southeast Asian J Trop Med Public Health. 2007, 38: 343-348.
Griffiths G, Trueman L, Crowther T, Thomas B, Smith B: Onions - A global benefit to health. Phytotherapy Research. 2002, 16: 603-615. 10.1002/ptr.1222
Park M, Bae J, Lee D: Antibacterial Activity of Gingerol isolated from Ginger Rhizome Against Periodontal Bacteria. Phytotherapy Research. 2008, 22: 1446-1449. 10.1002/ptr.2473
Kubo I, Fujita K, Kubo A, Nihei K, Ogura T: Antibacterial Activity of Coriander Volatile Compounds against Salmonella choleraesuis. J Agric Food Chem. 2004, 52: 3329-3332. 10.1021/jf0354186
Isao K, Ken-Ichi F, Aya K, Ken-Ichi N, Tetsuya A: Antibacterial activity of coriander volatile compounds against Salmonella choleraesuits. J Agric Food Chem. 2004, 52: 3329-3332. 10.1021/jf0354186
Wong PYY, Kitts DD: Studies on the dual antioxidant and antibacterial properties of parsley (Petroselenium crispum) and cilantro (Coriandrum sativum) extracts. Food Chemistry. 2006, 97: 505-515. 10.1016/j.foodchem.2005.05.031.
Ao P, Hu S, Zhao A: Essential oil analysis and trace element study of the roots of Piper nigrum L. Zhongguo Zhong Yao Za Zhi. 1998, 23: 42-63.
Dorman HJD, Deans SG: Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Applied Microbiology. 2000, 88: 308-316. 10.1046/j.1365-2672.2000.00969.x.
Reshmi SK, Sathya E, Devi PS: Isolation of piperdine from Piper nigrum and its antiproliferative activity. African Journal of Pharmacy and Pharmacology. 2010, 4: 562-573.
Castillo MC, Allori CG, Gutierrez RC, Saab OA, Fernandez NP, Ruiz CS, Ruiz Holgado AP, Nader OM: Bactericidal activity of lemon juice and lemon derivatives against Vibrio cholerae. Biol Pharm Bull. 2000, 23: 1235-1238.
Aibinu I, Adenipekun T, Adelowotan T, Ogunsanya T, Odugbemi T: Evaluation of the antimicrobial properties of different parts of Citrus aurantifolia (lime fruit) as used locally. Afr J Trad CAM. 2007, 4: 185-190.
Aboaba O, Efuwape BM: Antibacterial Properties of Some Nigerian Species. Bio Res Comm. 2001, 13: 183-188.
Acknowledgements
We thank staffs and kind support of the Department of Biotechnology and Genetic Engineering, Islamic University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
MSR conducted laboratory analysis and drafted the manuscript. MSR and MAKP contributed equally to this work. MAKP, MRI and MMHK participated in the design of the study and supervise the work. All authors were involved in the interpretation of the data and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Shahedur Rahman, Anowar Khasru Parvez contributed equally to this work.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Rahman, S., Parvez, A.K., Islam, R. et al. Antibacterial activity of natural spices on multiple drug resistant Escherichia coli isolated from drinking water, Bangladesh. Ann Clin Microbiol Antimicrob 10, 10 (2011). https://doi.org/10.1186/1476-0711-10-10
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-0711-10-10