Abstract
Background
In animal studies, perfluorinated alkyl substances affect growth and neuro-behavioural outcomes. Human epidemiological studies are sparse. The aim was to investigate the association between pregnancy serum concentrations of perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) and offspring behaviour and motor development at 5–9 years of age.
Methods
Maternal sera from the INUENDO cohort (2002–2004) comprising 1,106 mother-child pairs from Greenland, Kharkiv (Ukraine) and Warsaw (Poland) were analysed for PFOS and PFOA, using liquid-chromatography-tandem-mass-spectrometry. Exposures were grouped into country specific as well as pooled tertiles as well as being used as continuous variables for statistical analyses. Child motor development and behaviour at follow-up (2010–2012) were measured by the Developmental Coordination Disorder Questionnaire 2007 (DCDQ) and Strength and Difficulties Questionnaire (SDQ), respectively. Exposure-outcome associations were analysed by multiple logistic and linear regression analyses.
Results
In the pooled analysis, odds ratio (OR) (95% confidence interval (CI)) for hyperactivity was 3.1 (1.3, 7.2) comparing children prenatally exposed to the highest PFOA tertile with those exposed to the lowest PFOA tertile. Comparing children in the highest PFOS tertile with those in the lowest PFOS tertile showed elevated but statistically non-significant OR of hyperactivity (OR (95% CI) 1.7 (0.9, 3.2)). In Greenland, elevated PFOS was associated with higher SDQ-total scores indicating more behavioural problems (β (95% CI) =1.0 (0.1, 2.0)) and elevated PFOA was associated with higher hyperactivity sub-scale scores indicating more hyperactive behaviour (β (95% CI) = 0.5 (0.1, 0.9)). Prenatal PFOS and PFOA exposures were not associated with motor difficulties.
Conclusions
Prenatal exposure to PFOS and PFOA may have a small to moderate effect on children’s neuro-behavioural development, specifically in terms of hyperactive behaviour. The associations were strongest in Greenland where exposure contrast is largest.
Similar content being viewed by others
Introduction
Perfluorinated alkyl substances (PFAS) are man-made persistent chemicals, which are being widely used due to their water and oil-repellent properties. Biomonitoring studies have shown that they are found globally in a variety of living organisms, including humans [1–4]. Exposure pathways are mainly through diet and drinking water [5, 6]. Perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) are widespread in the environment in spite of a phase out since year 2000. They have long serum half-lives in humans [7] and are known to cross the placenta [8], which is worrying as fetal and early life is considered to be the vulnerable period of normal brain development [9].
Some of the most prevalent neuro-developmental disorders are developmental coordination disorder and attention deficit hyperactivity disorder (ADHD) and they may be related through common causal pathways [10]. The world prevalence of developmental coordination disorder is 5-6% [11–13] and the world prevalence of ADHD is 5-10% [14]. The disorders are related to various predictors such as fetal alcohol exposure [15] and fetal smoking exposure [16] although newer studies report little evidence of a causal relation between prenatal smoking exposure and development of ADHD [17]. Twin studies of ADHD suggest a high level of heritability, but fetal environmental pollutant exposures are also likely to play a role [18].
Animal studies suggest associations between prenatal exposure to PFOS and PFOA and neuro-behavioural deficits [19–21]. Human observational studies on the relation between PFAS exposures and behaviour are sparse and results are inconsistent, which may be due to different exposure levels and study designs. One cross-sectional study reported a positive association between PFOS and PFOA and ADHD [22], while another study observed an inverse J-shaped association between PFOA and ADHD across quartiles of exposure [23], indicating elevated risk of ADHD among those exposed to medium exposure level compared to low exposure level. No association was observed between relatively high fetal PFOS and PFOA exposure and behaviour at 7 years of age [24], while another follow-up study with lower PFAS exposures observed a relation between higher levels of PFOS (but not PFOA) and worse gross motor function in 2-year old children [25].
The present longitudinal study with a relatively large number of participants and relatively large exposure spans investigated the associations between pregnancy concentrations of PFOS and PFOA at background exposure levels and motor development and behaviour in 5-9-year old children.
Methods
Study population and data collection
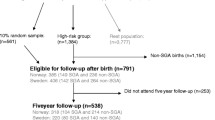
During the period from May 2002 to February 2004, 1,441 pregnant women from Greenland, Warsaw (Poland) and Kharkiv (Ukraine) were enrolled in the INUENDO (Biopersistent organochlorines in diet and human fertility) birth cohort from antenatal health care clinics and provided a blood sample at any stage of pregnancy. To be eligible for the study, the woman had to be born in the country of study, be pregnant and at least 18 years of age. At baseline, 2,478 women were eligible in Ukraine of whom 612 (24.7%) participated, and non-participants were slightly younger than participants but were otherwise similar. In Greenland, 665 were eligible and 571 (85.9%) participated, and participants and non-participants were very similar. In Poland, 690 were eligible and 258 (37.4%) participated. Unfortunately, we have no information available for the non-participants from Poland, limiting our ability to compare participants and non-participants. Further details on the baseline study population are available elsewhere [26]. A follow-up was conducted from January 2010 to May 2012, when the children were between 5 and 9 years old. Parents or guardians responded to questions concerning lifestyle, motor development, behaviour and other characteristics in a face-to-face interview or by filling in a questionnaire themselves.
At follow-up, a total of 1,113 mother-child-pairs (singleton births) with measured exposure information participated of the 1,441 pregnant women enrolled at baseline. After exclusion of mother-child-pairs with missing information on all 15 DCDQ items (n = 7) the study population consisted of 1,106 children, distributed between Greenland (n = 526 (47.6%)), Poland (n = 89 (8.0%)) and Ukraine (n = 491 (44.4%)).
Determination of PFOS and PFOA and cotinine
Plasma concentrations of PFOA, PFOS and cotinine were analysed, using liquid chromatography-tandem-mass-spectrometry (LC/MS/MS) at The Department of Occupational and Environmental Medicine in Lund, Sweden. A detailed description of the method for PFOS and PFOA is presented elsewhere [27]. Cotinine was analysed by the same method. Briefly, aliquots of 100 μl serum were added 25 μl of a water:acetonitrile (50:50) solution containing labelled internal standards. Proteins were precipitated with acetonitrile and vigorously shaking for 30 minutes. The samples were then centrifuged and the supernatant analyzed using a LC (UFLCXR, SHIMADZU Corporation, Kyoto, Japan) connected to a hybrid triple quadrupole linear ion trap mass spectrometer (QTRAP 5500, AB Sciex, Foster City, CA, USA). All samples were above limits of detection, which were 0.2 ng/ml and 0.04 ng/ml for PFOS and PFOA, respectively. Coefficient of variation of duplicate samples worked-up and analyzed on different days were 9% for PFOS and 11% for PFOA. The analyses of PFOA and PFOS are part of the Round Robin inter-comparison program (Professor Dr. Med. Hans Drexler, Institute and Out-patient Clinic for Occupational-, Social- and Environmental Medicine, University of Erlangen-Nuremberg, Germany) with results within the tolerance limits.
Assessment of child behaviour and motor abilities and covariate data
Behaviour was assessed using the parent version of the standardized questionnaire “The Strength and Difficulties Questionnaire” (SDQ), comprising 25 items on five scales (emotional, conduct, hyperactivity, peer and pro-social behaviour) [28]. SDQ is a screening tool used to identify common mental disorders in children 4 to 16 years of age. The items were coded 0 “not true”, 1 “somewhat true” or 2 “certainly true”. Each scale had a summed score ranging from 0 to 10. A SDQ-total score was calculated by summing four of the scales (emotional, conduct, hyperactivity and peer) with a score range of 0 to 40. Cut-offs on the SDQ were set according to standard (SDQ-total:0 to 13 = normal, 14 to16 = borderline and 17 to 40 = abnormal; Emotional symptoms: 0 to 5 = normal, 6 = borderline and 7 to 10 = abnormal; Conduct problems: 0 to 3 = normal, 4 = borderline and 5 to 10 = abnormal; Hyperactivity: 0 to 5 = normal, 6 = borderline and 7 to 10 = abnormal; Peer problems: 0 to 3 = normal, 4 to 5 = borderline and 6 to 10 = abnormal; Pro-social behaviour: 6 to 10 = normal, 5 = borderline and 1 to 4 = abnormal) [29]. When outcomes were dichotomised, the cut-offs were normal/borderline versus abnormal. In all scales, except the pro-social subscale, a high score indicated problems.
Parents were asked to assess their child’s behaviour during the past six months.
To evaluate the motor development, parents compared their child’s motor abilities to that of his or her peers using “Developmental Coordination Disorder Questionnaire 2007” (DCDQ). DCDQ is a screening tool to help identify motor difficulties in children 5-15-years of age, comprising 15 items on a 5-point Likert scale with a total score range of 15 to 75 [30]. A low score indicates problems. Only continuous DCDQ scores were used in this study as scores in the three populations were heterogeneous and the validity of DCDQ has not been examined specifically in the countries where the present study takes place.
Covariate data were collected from the pregnancy and follow-up questionnaires and included information about e.g. lifestyle, health and personal characteristics.
Statistical analysis
Missing information
The number of missing values on behaviour, motor development and covariates ranged from 0 to 55%. One of the 15 items (question 12; “your child learns new motor tasks easily”) was erroneously lacking in the Greenlandic version of the DCDQ (but not in the Danish version of the questionnaire used for some participants in Greenland), which caused 55% missing of the DCDQ in Greenland, as all items are needed to generate a total score. In the Greenlandic population, the median of missing answers of the DCDQ were 2%, and 85% had answered at least 14 of 15 items of the DCDQ. To increase power and overcome the risk of introducing selection bias by analysing only the complete case dataset, we used chained multiple imputation, allowing us to maintain participants with incomplete data [31]. Briefly, multiple different imputed datasets (m > 1) are created, and a set of random plausible values replace each missing value, based on known subject characteristics and other predictors in the complete dataset. This incorporates an appropriate variability across the m datasets. The new m complete datasets are analysed, producing a single set of results accounting for the variability of the missing data [31].
We generated 100 imputed datasets in a combined imputation that included all three countries. The predictors were: PFOA, PFOS, the 15 items of the DCDQ, the 5 subscales of the SDQ, maternal cotinine level during pregnancy, maternal alcohol consumption before conception, maternal educational level, maternal age at pregnancy, birth weight, gestational age at birth, gestational age at blood sampling, parity, breastfeeding duration, child age at follow-up and child sex. The robustness of the imputation model was examined creating fewer (m = 20) and more (m = 150) datasets and using less and more predictors, and furthermore, complete case analyses were performed as sensitivity analyses.
Data analysis
A non-response analysis was performed to check for inconsistencies between responders (n = 1,113) and non-responders (n = 328). Spearman’s rank correlation was used to assess the correlation between maternal pregnancy levels of PFOS and PFOA. We used logistic regression models in the primary analyses of PFOS and PFOA levels and behavioural problems (abnormal SDQ-total and sub-scale scores) and linear regression in the investigation of PFOS and PFOA exposure and motor development. Exposures were a priori decided to be categorized into tertiles per country as well as pooled, using the lowest tertile as reference group. Moreover, trends in exposure-outcome associations were further explored, using continuous, natural logarithm transformed exposures in linear regression models. To increase power, the associations between exposures and behavioural outcomes were also examined using linear regression models with exposures categorized into tertiles as well as a continuous variable. All estimates were adjusted for the most important potential confounders among the available data, which were identified a priori and included maternal cotinine level during pregnancy (serum cotinine ≤10 (non-smoker)/ >10 ng/ ml (smoker)); maternal alcohol consumption at conception (0, <7, ≥7 drinks per week); maternal age at pregnancy (continuous); child sex and gestational age at blood-sampling (continuous) [15, 16, 32, 33]. In addition, we explored possible interactions between the exposures and sex and exposures and country, adding interaction terms to the model. In a sensitivity analysis of the SDQ, we used the top 10 percentile cut-off from this sample as a marker of behavioural problems instead of the standard cut-offs suggested by Youth in Mind [29] as also used in another study [24]. Furthermore, duration of breastfeeding was added as covariate to adjust for postnatal PFAS exposures. Complete case results are presented as supplementary material.
All analyses were performed stratified by population as well as combined (adjusted for population). Not all analyses could be performed in the Polish dataset since only very few cases were present. A p-value less than 0.05 was considered statistically significant. Statistical analyses were performed using the Stata statistical package (version 12.1, StataCorp, College Station, Texas, USA).
Ethics
The study was approved by local ethical committees; Polish Bioethical Committee (approval no. 6/2002 of 3.07.2002), Ethical Committee for Human Research in Greenland (approval no. 2010–13) and the Commission on Ethics and Bioethics Kharkiv National Medical University in Ukraine (protocol number 7, October 7 2009). All participating parents signed informed consent.
Results
The non-response analysis showed no differences between responders and non-responders at follow-up concerning exposure levels, maternal educational level, pregnancy smoking status and maternal age at baseline (data not shown). The median (10–90 percentile) age of the children at follow-up was 8 (7 to 9) years in Greenland compared to 7 (7 to 8) and 8 (7 to 8) years in Ukraine and Poland, respectively. Personal characteristics of the study population are presented in Table 1.
In comparison with women from Greenland, women from Poland were older, had lower body mass index (BMI), more often expected their first child, were non-smokers and better educated. Women from Ukraine were younger at recruitment, had lower BMI, were more often non-smokers and drank less alcohol compared to women from Greenland (Table 1). The level of missing data is presented in Table 1, showing highest level of missing data on the DCDQ in Greenland.
The median serum concentrations of PFOS and PFOA are presented in Table 2 alongside the exposure tertiles across the three countries and pooled. Median PFOS exposure levels were highest in Greenland (median (10th–90th percentile) PFOS 20.3 ng/ml (12.0, 37.0)), which is approximately 4 times higher than the median PFOS exposure level in Ukraine. The median PFOA levels were highest in Poland (median (10th-90th percentile) 2.7 ng/ml (1.5, 4.3)).
The Spearman’s correlation coefficient between maternal pregnancy levels of PFOS and PFOA was 0.5 in Greenland, 0.6 in Poland and 0.5 in Ukraine.
In Table 3, results of the linear regression analyses between PFOS and PFOA and motor skills (DCDQ) are presented. In the combined analysis of all three populations, PFOS and PFOA were associated with a minor decrease in DCDQ score. All associations between PFOS and PFOA and motor development were clinically minor and statistically non-significant. No interaction was observed between exposures and sex and exposures and country.
The associations between PFOA and PFOS and behavioural problems (continuous SDQ-total and hyperactivity) are presented in Table 4. In the combined analyses of all three countries, high PFOA and PFOS exposures compared with low exposures were associated with a 0.5 point higher hyperactivity scores (more hyperactive behaviour). Higher PFOS levels were associated with higher SDQ-total scores (more behavioural problems) in Greenland (Table 4). No associations were observed in Poland and Ukraine. PFOA was positively associated with hyperactive scores in Greenland (β (95% CI) = 0.5 (0.1, 0.9)) but not in Poland and Ukraine. PFOA was not associated with SDQ-total in any of the populations. There was no evidence of interaction between exposures and sex or between exposures and country.
Table 5 presents results of the adjusted logistic regression analyses of the associations between PFOS and PFOA and dichotomized (normal/borderline versus abnormal) SDQ-total and hyperactivity. In the combined analysis, OR (95% CI) for abnormal SDQ-total was 2.7 (1.2, 6.3) comparing highest PFOA tertile to lowest PFOA tertile. In Greenland, the adjusted OR for hyperactive behaviour was higher comparing medium and high PFOA exposures to low PFOA exposure, although the 95% CIs were wide (OR (95% CI) = 5.4 (1.1, 25.6) and 6.3 (1.3, 30.1), respectively). In Ukraine, no associations were observed. PFOS was not associated with abnormal SDQ-total in any of the analyses.
Few associations were found for the other SDQ sub-scales (emotional, conduct, peer and pro-social) (See Additional file 1). The OR for abnormal pro-social behaviour was also above 1 in Ukraine but not in Greenland.
Results from complete-case analyses were generally similar to the imputation-based analyses presented above (See Additional files 2, 3 and 4).
In the sensitivity analysis using top ten percentile cut-offs results were somewhat attenuated but the overall pattern did not change (Additional file 5). Adding duration of breastfeeding as a covariate to the models did not change direction or significance of results.
Results of sensitivity analyses of smaller and larger imputation models showed similar results, indicating a robust imputation model.
Discussion
We observed a small to moderate positive association between pregnancy levels of PFOA and child hyperactive behaviour in the total sample, which was mainly attributable to associations in Greenland. In Greenland, PFOS was associated with behavioural problems (SDQ-total) (not in Poland and Ukraine), whereas PFOA was associated with hyperactivity. Only minor associations appeared in relation to other SDQ sub-scales and none to motor development, as measured using DCDQ.
The mechanism related to the association between prenatal PFAS exposure and behavioural and motor problem is unclear. However, an association between prenatal PFOS exposure and thyroid hormone disruption has been reported in mice [34]. In humans, higher pregnancy levels of PFOS have been associated with higher pregnancy levels of thyroid-stimulating hormone (TSH) [35], and higher pregnancy levels of some PFAS (perfluorononanoic acid (PFNA), perfluoroundecanoic acid (PFUnDA) and perfluorododecanoic acid (PFDoDA) have been associated with lower infant cord levels of triiodothyronine (T3) and total free thyroxine (T4) [36]. Since thyroid hormones are essential for fetal and early life neurologic development, a disruption by prenatal PFAS exposures could possibly impair healthy neurodevelopment.
The results of epidemiological studies examining the neurodevelopmental effects of PFAS are few and inconclusive. Fei et al. reported no associations between high pregnancy levels of PFOA (median (inter-quartile-range): 5.4 (4.0-7.1 ng/ml)) and PFOS (34.4 (26.6-44.5 ng/ml)) and developmental coordination disorder (assessed by DCDQ’07) at 7 years among a sub-sample of 537 participants from the Danish National Birth Cohort [24], which is in accordance with our results. In the same study, no associations were observed between PFOS and PFOA and SDQ-total or any of the SDQ sub-scales, using top 10 percentile cut-offs [24]. This is in accordance with our sensitivity analysis using top 10 percentile cut-off, whereas we observed associations between PFOA and hyperactivity in Greenland and in the combined analysis of the three countries in our main analysis using standard cut-offs. Furthermore, we observed a minor positive association between PFOS and SDQ-total in the combined analysis, however, statistically non-significant, whereas the country specific results were more heterogeneous. In the present study, we used the standard cut-offs suggested by Youth in Mind [29] and 804 of the 1,106 included children were older than 7 years, which may explain the difference in our results.
A small Taiwanese birth cohort study with exposure levels equivalent to exposure levels in this study observed associations between prenatal PFOS and gross motor functions at 2 years of age, whereas no association was reported between PFOA and any neuro-behavioural outcome [25]. Direct comparison with this study is, however, hampered since their outcome measure was assessed by use of a questionnaire developed specifically for Chinese populations. Some inconsistencies were present in our results of prenatal PFAS exposure and motor development, and we did not observe any consistent and clinically relevant associations which could be due to older age at follow-up or lack of sensitivity of the DCDQ, as the original version of the questionnaire has high sensitivity in populations with high prevalence of motor difficulties and lower sensitivity in a background population [37].
The C8 Health Project (which reports health outcomes in relation to PFOA contamination of drinking water from the Dupont factory in the United States) reported only minor associations between high levels of childhood PFOA measured at 2–8 years of age and a range of neuropsychological outcomes assessed at 6–12 years among a sub-sample of 321 children [38, 39], however, an interaction between PFOA and sex was reported in the analysis of ADHD-like behaviour, indicating high PFOA exposure to be protective among boys and adverse among girls [38]. When in utero PFOA exposures were estimated in a pharmacokinetic model, higher PFOA levels were associated with fewer signs of ADHD (using the clinical confidence index of Conners’ continuous performance test- II) [39]. In the study by Stein et al. [38], the exposures were measured postnatally, and in the study by Stein et al., [39], the prenatal exposures were estimated in a toxicokinetic model, whereas we measured the prenatal exposures in maternal blood. Furthermore, different study designs and exposure and outcomes assessments were used in these studies and also, the offspring had different ages at examination, which may explain their findings as we saw no signs of interaction between PFOA and sex.
We acknowledge that this study has some limitations. In the analysis of categorical exposures stratified by country, we used country specific tertiles as exposure levels differed considerably between countries. This could in part explain the inconsistencies in our findings. Furthermore, PFAS levels and SDQ and DCDQ results did vary in the three countries included in this study which further may account for the inconsistent findings between countries.
There is a risk of exposure misclassification since blood samples were collected throughout pregnancy in our study and PFAS tend to decrease during pregnancy [40]. However, we addressed this issue by adjusting for gestational age at blood sampling, although we recognize that this may not have completely eliminated the risk of information bias.
The DCDQ is developed as a screening tool and should not be used for diagnostics [41]. The questionnaire has not been validated in the three countries represented in this study, and thus there is a risk of misclassification of motor problems. However, we believe the questionnaire is suitable for detecting a difference in motor abilities according to PFOS and PFOA exposure levels within the three countries, and since we did not use the cut-offs for indication of motor difficulties, this should not be a large concern.
We chose to use the standard cut-offs for behavioral problems as no country specific cut-offs exist for Greenland, Ukraine and Poland. This enabled us to compare results between countries but may have resulted in misclassification of the SDQ outcome. SDQ as a screening tool has a high validity as well as reliability [42, 43], and our sensitivity analysis, using top ten percentile cut-offs in general suggested consistent results.
Missing data can cause selection bias and to overcome this challenge, we performed several multiple imputation analyses, and results were consistent. A lack of demographic information concerning the Polish non-participants at baseline limited our ability to compare participants and non-participants and selection bias in the Polish results can not be ruled out. Furthermore, the relatively low participation rate of 34% at follow-up in Poland poses a risk of selection bias. However, the non-response analysis at follow-up showed no evidence of difference between responders and non-responders, indicating low risk of selection bias by loss to follow-up.
It is difficult to estimate to what extent postnatal PFAS exposure may have influenced the results. In a sensitivity analysis, we adjusted for duration of breastfeeding, which did not materially change the results. However, residual confounding by postnatal PFAS exposure can not be completely ruled out.
We measured PFAS levels at all periods during pregnancy as a measure of fetal exposure. However, it is not clear to what extent this reflects the vulnerable window of exposure. Others have reported high correlation between 1st and 2nd trimester PFOA levels and because PFOA and PFOS have long half-lives, we believe blood-sampling throughout pregnancy is of minor concern.
The Polish results should be interpreted with caution, since the Polish sample is small. However, samples from Greenland and Ukraine and the pooled analysis are of considerable size and, thus, results should be robust.
Conclusions
Prenatal exposure to PFOS and PFOA may have a small to moderate effect on children’s neuro-behavioural development at the age of 5–9 years, specifically in terms of hyperactive behaviour. The associations were largest in Greenland, where the exposure contrast is largest. No association was observed in relation to motor skills. Standardized measures of behavioural- and motor problems were used and results were consistent across numerous sensitivity analyses.
Abbreviations
- ADHD:
-
attention deficit hyperactivity disorder
- CI:
-
confidence interval
- DCDQ:
-
Developmental Coordination Disorder Questionnaire 2007
- OR:
-
odds ratio
- PFAS:
-
Perfluorinated alkyl substances
- PFDoDA:
-
perfluorododecanoic acid
- PFNA:
-
perfluorononanoic acid
- PFOA:
-
perfluorooctanoate
- PFOS:
-
perfluorooctane sulfonate
- PFUnDA:
-
perfluoroundecanoic acid
- SDQ:
-
Strength and Difficulties Questionnaire
- T3:
-
triiodothyronine
- T4:
-
total free thyroxine
- TSH:
-
thyroid-stimulating hormone.
References
Butenhoff JL, Olsen GW, Pfahles-Hutchens A: The applicability of biomonitoring data for perfluorooctanesulfonate to the environmental public health continuum.Environ Health Perspect 2006,114(11):1776–1782.
Harada KH, Koizumi A: Environmental and biological monitoring of persistent fluorinated compounds in Japan and their toxicities.Environ Health Prev Med 2009,14(1):7–19. 10.1007/s12199-008-0058-5
Roosens L, D'Hollander W, Bervoets L, Reynders H, Van Campenhout K, Cornelis C, Van Den Heuvel R, Koppen G, Covaci A: Brominated flame retardants and perfluorinated chemicals, two groups of persistent contaminants in Belgian human blood and milk.Environ Pollut 2010,158(8):2546–2552. 10.1016/j.envpol.2010.05.022
Sundstrom M, Ehresman DJ, Bignert A, Butenhoff JL, Olsen GW, Chang SC, Bergman A: A temporal trend study (1972–2008) of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in pooled human milk samples from Stockholm, Sweden.Environ Int 2011,37(1):178–183. 10.1016/j.envint.2010.08.014
Vestergren R, Cousins IT: Tracking the pathways of human exposure to perfluorocarboxylates.Environ Sci Technol 2009,43(15):5565–5575. 10.1021/es900228k
Halldorsson TI, Fei C, Olsen J, Lipworth L, McLaughlin JK, Olsen SF: Dietary predictors of perfluorinated chemicals: a study from the Danish National Birth Cohort.Environ Sci Technol 2008,42(23):8971–8977. 10.1021/es801907r
Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR: Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers.Environ Health Perspect 2007,115(9):1298–1305. 10.1289/ehp.10009
Midasch O, Drexler H, Hart N, Beckmann MW, Angerer J: Transplacental exposure of neonates to perfluorooctanesulfonate and perfluorooctanoate: a pilot study.Int Arch Occup Environ Health 2007,80(7):643–648. 10.1007/s00420-006-0165-9
Rice D, Barone S Jr: Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models.Environ Health Perspect 2000,108(Suppl 3):511–533.
Gillberg C: Deficits in attention, motor control, and perception: a brief review.Arch Dis Child 2003,88(10):904–910. 10.1136/adc.88.10.904
Blank R, Smits-Engelsman B, Polatajko H, Wilson P, European Academy for Childhood Disability: European Academy for Childhood Disability (EACD): recommendations on the definition, diagnosis and intervention of developmental coordination disorder (long version).Dev Med Child Neurol 2012,54(1):54–93. 10.1111/j.1469-8749.2011.04171.x
Barnhart RC, Davenport MJ, Epps SB, Nordquist VM: Developmental coordination disorder.Phys Ther 2003,83(8):722–731.
Zwicker JG, Missiuna C, Harris SR, Boyd LA: Developmental coordination disorder: a review and update.Eur J Paediatr Neurol 2012,16(6):573–581. 10.1016/j.ejpn.2012.05.005
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA: The worldwide prevalence of ADHD: a systematic review and metaregression analysis.Am J Psychiatry 2007,164(6):942–948. 10.1176/ajp.2007.164.6.942
Bhatara V, Loudenberg R, Ellis R: Association of attention deficit hyperactivity disorder and gestational alcohol exposure: an exploratory study.J Atten Disord 2006,9(3):515–522. 10.1177/1087054705283880
Pauly JR, Slotkin TA: Maternal tobacco smoking, nicotine replacement and neurobehavioural development.Acta Paediatr 2008,97(10):1331–1337. 10.1111/j.1651-2227.2008.00852.x
Obel C, Olsen J, Henriksen TB, Rodriguez A, Jarvelin MR, Moilanen I, Parner E, Linnet KM, Taanila A, Ebeling H, Heiervang E, Gissler M: Is maternal smoking during pregnancy a risk factor for hyperkinetic disorder?–Findings from a sibling design.Int J Epidemiol 2011,40(2):338–345. 10.1093/ije/dyq185
de Cock M, Maas YG, van de Bor M: Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review.Acta Paediatr 2012,101(8):811–818. 10.1111/j.1651-2227.2012.02693.x
Johansson N, Fredriksson A, Eriksson P: Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice.Neurotoxicology 2008,29(1):160–169. 10.1016/j.neuro.2007.10.008
Fuentes S, Vicens P, Colomina MT, Domingo JL: Behavioral effects in adult mice exposed to perfluorooctane sulfonate (PFOS).Toxicology 2007,242(1–3):123–129.
Fuentes S, Colomina MT, Vicens P, Franco-Pons N, Domingo JL: Concurrent exposure to perfluorooctane sulfonate and restraint stress during pregnancy in mice: effects on postnatal development and behavior of the offspring.Toxicol Sci 2007,98(2):589–598. 10.1093/toxsci/kfm121
Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM: Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in U.S. children 12–15 years of age.Environ Health Perspect 2010,118(12):1762–1767. 10.1289/ehp.1001898
Stein CR, Savitz DA: Serum perfluorinated compound concentration and attention deficit/hyperactivity disorder in children 5–18 years of age.Environ Health Perspect 2011,119(10):1466–1471. 10.1289/ehp.1003538
Fei C, Olsen J: Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7 years.Environ Health Perspect 2011,119(4):573–578.
Chen MH, Ha EH, Liao HF, Jeng SF, Su YN, Wen TW, Lien GW, Chen CY, Hsieh WS, Chen PC: Perfluorinated compound levels in cord blood and neurodevelopment at 2 years of age.Epidemiology 2013,24(6):800–808. 10.1097/EDE.0b013e3182a6dd46
Toft G, Axmon A, Giwercman A, Thulstrup AM, Rignell-Hydbom A, Pedersen HS, Ludwicki JK, Zvyezday V, Zinchuk A, Spano M, Manicardi GC, Bonefeld-Jorgensen EC, Hagmar L, Bonde JP, INUENDO: Fertility in four regions spanning large contrasts in serum levels of widespread persistent organochlorines: a cross-sectional study.Environ Health 2005, 4:26. 10.1186/1476-069X-4-26
Lindh CH, Rylander L, Toft G, Axmon A, Rignell-Hydbom A, Giwercman A, Pedersen HS, Goalczyk K, Ludwicki JK, Zvyezday V, Vermeulen R, Lenters V, Heederik D, Bonde JP, Jonsson BA: Blood serum concentrations of perfluorinated compounds in men from Greenlandic Inuit and European populations.Chemosphere 2012,88(11):1269–1275. 10.1016/j.chemosphere.2012.03.049
Goodman R: The Strengths and Difficulties Questionnaire: a research note.J Child Psychol Psychiatry 1997,38(5):581–586. 10.1111/j.1469-7610.1997.tb01545.x
Youth in Mind. SDQ - Strength and Difficulties Questionnaire [http://www.sdqinfo.com/py/sdqinfo/c0.py]
Wilson BN, Crawford SG, Green D, Roberts G, Aylott A, Kaplan BJ: Psychometric properties of the revised Developmental Coordination Disorder Questionnaire.Phys Occup Ther Pediatr 2009,29(2):182–202. 10.1080/01942630902784761
Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR: Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls.BMJ 2009, 338:b2393. 10.1136/bmj.b2393
Fergusson DM, Woodward LJ: Maternal age and educational and psychosocial outcomes in early adulthood.J Child Psychol Psychiatry 1999,40(3):479–489. 10.1111/1469-7610.00464
Zahn-Waxler C, Shirtcliff EA, Marceau K: Disorders of childhood and adolescence: gender and psychopathology.Annu Rev Clin Psychol 2008, 4:275–303. 10.1146/annurev.clinpsy.3.022806.091358
Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, Stevenson LA: Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation.Toxicol Sci 2003,74(2):382–392. 10.1093/toxsci/kfg122
Wang Y, Starling AP, Haug LS, Eggesbo M, Becher G, Thomsen C, Travlos G, King D, Hoppin JA, Rogan WJ, Longnecker MP: Association between perfluoroalkyl substances and thyroid stimulating hormone among pregnant women: a cross-sectional study.Environ Health 2013,12(1):76. -069X-12–76 10.1186/1476-069X-12-76
Wang Y, Rogan WJ, Chen PC, Lien GW, Chen HY, Tseng YC, Longnecker MP, Wang SL: Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study.Environ Health Perspect 2014,122(5):529–534.
Schoemaker MM, Flapper B, Verheij NP, Wilson BN, Reinders-Messelink HA, de Kloet A: Evaluation of the Developmental Coordination Disorder Questionnaire as a screening instrument.Dev Med Child Neurol 2006,48(8):668–673. 10.1017/S001216220600140X
Stein CR, Savitz DA, Bellinger DC: Perfluorooctanoate exposure in a highly exposed community and parent and teacher reports of behaviour in 6–12-year-old children.Paediatr Perinat Epidemiol 2014,28(2):146–156. 10.1111/ppe.12097
Stein CR, Savitz DA, Bellinger DC: Perfluorooctanoate and neuropsychological outcomes in children.Epidemiology 2013,24(4):590–599. 10.1097/EDE.0b013e3182944432
Fei C, McLaughlin JK, Tarone RE, Olsen J: Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort.Environ Health Perspect 2007,115(11):1677–1682. 10.1289/ehp.10506
DCDQ - Developmental Co-ordination Disorder Questionnaire (DCDQ) [http://www.dcdq.ca/]
Goodman A, Goodman R: Strengths and difficulties questionnaire as a dimensional measure of child mental health.J Am Acad Child Adolesc Psychiatry 2009,48(4):400–403. 10.1097/CHI.0b013e3181985068
Obel C, Heiervang E, Rodriguez A, Heyerdahl S, Smedje H, Sourander A, Guethmundsson OO, Clench-Aas J, Christensen E, Heian F, Mathiesen KS, Magnusson P, Njarethvik U, Koskelainen M, Ronning JA, Stormark KM, Olsen J: The Strengths and Difficulties Questionnaire in the Nordic countries.Eur Child Adolesc Psychiatry 2004,13(Suppl 2):II32–9.
Acknowledgements
The CLEAR (Climate change, environmental contaminants and reproductive health) and INUENDO (Biopersistent organochlorines in diet and human fertility) studies were funded by the European Commission’s 7th and 5th Framework Programmes, respectively (grants FP7-ENV-2008-1-226217 and QLK4-CT-2001-00202). Skilful technical assistance with PFC analyses was performed by Åsa Amilon and Agneta Kristensen. Agneta Kristensen was paid by ReproHigh, a network funded by Interreg.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JPB designed and initiated the INUENDO cohort. JPB and GT designed and initiated the CLEAR project. GT, HSP, AH and VO were responsible for collecting blood samples and interview data. BAGJ and CL was responsible for the chemical analyses of the perfluorinated alkyl substances. JPB and GT coordinated the execution of the INUENDO and the CLEAR projects. GT had main responsibility for creating the INUENDO and CLEAR databases. CHR-H, CO, LR and ARH contributed to the design, analyses and interpretation of data. BBH contributed to the design and data collection and were responsible for statistical analyses and writing the draft version of the manuscript. All authors revised the manuscript and approved the final version for publication.
Electronic supplementary material
12940_2014_821_MOESM1_ESM.doc
Additional file 1: Table S1: Associationsa between pregnancy levels of PFOS/PFOA (ng/ml) and offspring behavioural problems. Imputation-based results. (DOC 87 KB)
12940_2014_821_MOESM2_ESM.doc
Additional file 2: Table S2: Associationsa between pregnancy levels of PFOS/PFOA (ng/ml) and offspring DCDQ-score. Complete-case results. (DOC 43 KB)
12940_2014_821_MOESM3_ESM.doc
Additional file 3: Table S3: Associationsa between pregnancy levels of PFOS/PFOA (ng/ml) and offspring SDQ-score. Complete-case results. (DOC 52 KB)
12940_2014_821_MOESM4_ESM.doc
Additional file 4: Table S4: Associationsa between pregnancy levels of PFOS/PFOA (ng/ml) and offspring behavioural and hyperactivity problems. Complete-case results. (DOC 57 KB)
12940_2014_821_MOESM5_ESM.doc
Additional file 5: Table S5: Associationsa between pregnancy levels of PFOS/PFOA (ng/ml) and offspring behavioural and hyperactivity problems. Imputation-based results. (DOC 58 KB)
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Høyer, B.B., Ramlau-Hansen, C.H., Obel, C. et al. Pregnancy serum concentrations of perfluorinated alkyl substances and offspring behaviour and motor development at age 5–9 years – a prospective study. Environ Health 14, 2 (2015). https://doi.org/10.1186/1476-069X-14-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-069X-14-2