Abstract
Background
Despite clinical descriptions of severe vivax malaria cases having been reported, data regarding immunological and inflammatory patterns are scarce. In this report, the inflammatory and immunological status of both mild and severe vivax malaria cases are compared in order to explore immunopathological events in this disease.
Methods and Results
Active and passive malaria case detections were performed during 2007 in Buritis, Rondônia, in the Brazilian Amazon. A total of 219 participants enrolled the study. Study individuals were classified according to the presence of Plasmodium vivax infection within four groups: non-infected (n = 90), asymptomatic (n = 60), mild (n = 50) and severe vivax infection (n = 19). A diagnosis of malaria was made by microscopy and molecular assays. Since at present no clear criteria define severe vivax malaria, this study adapted the consensual criteria from falciparum malaria. Patients with severe P. vivax infection were younger, had lived for shorter time in the endemic area, and recalled having experienced less previous malaria episodes than individuals with no malaria infection and with mild or asymptomatic infection. Strong linear trends were identified regarding increasing plasma levels of C reactive protein (CRP), serum creatinine, bilirubins and the graduation of disease severity. Plasma levels of tumour necrosis factor (TNF), interferon-gamma(IFN-gamma) and also IFN-gamma/interleukin-10 ratios were increased and exhibited a linear trend with gradual augmentation of disease severity. Both laboratory parameters of organ dysfunction and inflammatory cytokines were reduced during anti-parasite therapy in those patients with severe disease.
Conclusion
Different clinical presentations of vivax malaria infection present strong association with activation of pro-inflammatory responses and cytokine imbalance. These findings are of utmost importance to improve current knowledge about physiopathological concepts of this serious widespread disease.
Similar content being viewed by others
Background
Plasmodium vivax infection has been considered for a long time a benign and self-limited disease, mainly when compared to the burden of Plasmodium falciparum infection in African countries [1]. Nevertheless, P. vivax is responsible for up to 400 million infections each year, representing the most widespread Plasmodium species [2]. Plasmodium vivax accounts for the majority of malaria cases within the Brazilian Amazon [3], and the prevalence of asymptomatic infection is very high [4, 5]. Historically, cases of complicated P. vivax malaria have been rare, and documented almost exclusively by case reports or small case series [6–8]. Recent evidence from larger studies performed in Melanesian populations has however reinforced the association between vivax malaria, severe complications, and death [9–11]. Severe complications associated with vivax malaria have also been reported in the Amazon region [12]. Together with rising documentation of drug resistance worldwide, the complications of P. vivax infection represent a global health menace which needs focused efforts to its resolution.
Major severe P. vivax clinical syndromes documented include important thrombocytopaenia [13, 14], cerebral malaria [15, 16], and acute renal [7, 17], hepatic [6] and pulmonary [18, 19] dysfunctions. In severe falciparum malaria syndromes, as in many other systemic infections, most of the pathology described seems to be a consequence of an intense inflammatory burst, favoured by a pathological activation of the immune system and cytokine release [20–22]. Despite clinical descriptions of the illness caused by P. vivax infection, data regarding immunological and inflammatory patterns are scarce. In the present report, inflammatory and immunological status of both mild and severe vivax malaria cases were compared in order to explore immunopathological events in this disease.
Methods
Study localities
A study investigating determinant factors for vivax malaria severity was performed during 2007 in Buritis (10°12'43" S; 63°49'44" W), a recent urbanized municipality of the Rondônia State, in the south-western part of Brazilian Amazon. Within this region, malaria transmission is unstable, with increased number of cases being detected annually between April to September, and the risk of infection is high [23], with an Annual Parasite Incidence of 77.5 per 1,000 inhabitants in 2005 [3]. The prevalence of P. falciparum infection in the Brazilian Amazon is 23.7% [3], and Plasmodium malariae case detection reaches 10% in Rondônia [24].
Participants and sampling
Active and passive malaria case detections were performed. These included home visits in areas of high disease transmission, and study of individuals who seek care at the diagnostic centers of Brazilian National Foundation of Health (FUNASA), responsible for malaria control in the country. In addition, patients admitted to the Buritis municipal Hospital (Hospital São Gabriel) presenting clinical signs of mild or complicated malaria were also included in the study. All individuals from fifteen to seventy years, of both sexes, who had been residing in the endemic area for more than six months, were invited to be included in the study. Exclusion criteria were: documented or strong clinical suspecting of viral hepatitis (HAV, HBV, HCV, HDV), chronic alcoholism, HIV infection, yellow fever, dengue, leptospirosis, tuberculosis, Hansen's disease, visceral leishmaniasis, documented or referred cancer and/or other chronic degenerative disease, sickle cell trait, and the use of hepatotoxic and immunessupressive drugs. All participants or legal responsible gave written informed consent before entering the study. This study was approved by the Ethical Committee of the São Lucas University, Rondônia, Brazil, for the human subject protocol.
Individuals were examined and interviewed and their blood samples (20 mL) were collected for serological experiments. In hospitalized participants, two venous blood collections were performed: one at the hospital admission and other seven days after malaria treatment initiation. All malaria diagnoses were performed using two methods. First, patients were screened by thick smear examination using field microscopy and the parasitaemia (parasites/uL) was quantified in positive cases. Further, nested PCR was performed in all whole blood samples to confirm the diagnosis. Two individuals presenting P. malariae infection and 16 people infected with P. falciparum (uncomplicated forms) were identified and excluded from the study. Hence, all the volunteers selected were negative for P. falciparum and/or P. malariae infection by both microscopic examination and nested PCR.
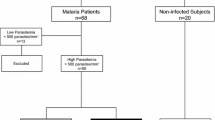
A total of 219 individuals enrolled in the study. All positive cases were followed for 30 days for the evaluation of malaria symptoms. Individuals who were positive for P. vivax infection and remained without fever (axillary temperature >37.8°C) and/or chills, sweats, strong headaches, myalgia, nausea, vomiting, jaundice, asthenia, and arthralgia for 30 days were considered asymptomatic P. vivax-infected cases. Cases showing positive parasitological tests in the presence of any symptom listed above were classified as symptomatic infections. Patients presenting any sign of acute severe organ dysfunction [25] were considered severe cases. Until today there are no clear criteria defining what a severe vivax malaria case is. Despite the absence of a consensus, this study used the previously defined criteria for severe falciparum infection [25]. Study individuals were then classified within four groups: non-infected (n = 90), asymptomatic (n = 60), mild (n = 50) and severe vivax infection (n = 19). The baseline characteristics of the volunteers are listed in the Table 1.
Nested PCR for malaria diagnosis
The molecular diagnosis of malaria infection was performed in all subjects using the nested PCR technique described previously [26, 27], with minimal alterations [28]. To control for cross-contamination, one uninfected blood sample was included for every twelve samples processed. Fifteen percent of positive PCR samples were retested to confirm the amplification of plasmodial DNA. All tests were performed and confirmed at the Centro de Pesquisas Gonçalo Moniz, Salvador, Bahia, Brazil.
Plasma cytokine levels detection
Plasma levels of IL-10, IFN-gamma, and TNF were measured using the Cytometric Bead Array - CBA (BD Biosciences Pharmingen, USA) according to the manufacturer's protocol, with all samples running in a single assay. The flow cytometric assay was performed and analysed by a single operator, and standard curves were derived from cytokine standards.
Laboratory assessment of organ dysfunction
Plasma measurements of creatinin, aspartate amino-transferase (AST), alanine amino-transaminase (ALT), total and direct bilirubins, haemoglobin, and CRP were made at the clinical laboratory of Faculdade São Lucas, at the Pharmacy School (Federal University of Bahia, Brazil) and at the Laboratório LPC (Salvador, Bahia. Brazil).
The Hepatic-Inflammatory Parasitic score
The hepatic-inflammatory parasitic (HIP) score was created to standardize a reproducible evaluation of severity in malaria cases. This score was developed by analysing data from another study conducted in 2006 with a sample size of 580 individuals from the Buritis Municipality, Rondônia State, Brazil. This group was composed of non-infected individuals (n = 183) and those infected with Plasmodium presenting malaria-related symptoms (n = 195) or asymptomatic infection (n = 202) composed this sample. In addition, this group was very similar to the one in 2007 with regard to age, gender, time of residence in endemic area and referred previous malaria episodes (data not shown). Optimal threshold plasma values of AST, ALT, total bilirubin, fibrinogen, CRP, and parasitaemia able to discriminate asymptomatic from symptomatic malaria infection were calculated using the Receiver Operator Characteristics (ROC) curves (Figure 1). For each variable measured, the cut-off values presenting the higher sensitivity and specificity, as well as the highest likelihood ratio, were established (Figure 1A-F). Further, one point was attributed to each variable that presented higher than the established cut-off value. Consequently, the minimum score was zero and maximum was five, and it reflected both parasitaemia and organ dysfunction aspects of symptomatic disease. Once the score was established, it was tested by applying to the sample constituted by the 219 participants approached in this study (Figure 1G). Additionally, the relationship between the HIP score and the IFN-gamma/IL-10 was assessed, since this ratio has been used as indicator of inflammatory activity in malaria [21, 22, 29].
The Hepatic-Inflammatory Parasitic score. In a primary investigation, 580 individuals from Buritis, Rondonia, Brazil were evaluated to standardize the Hepatic-Inflammatory Parasitic (HIP) score. This sample included non-infected individuals (n = 183) and those infected with Plasmodium presenting malaria-related symptoms (n = 195) or asymptomatic infection (n = 202). The threshold plasma values of (A) aspartate aminotransferase (AST), (B) alanino amino-transaminase (ALT), (C) total bilirubin, (D) fibrinogen, (E) C reactive protein (CRP) and (F) parasitaemia were established in order to categorize the individuals according to the HIP score. Once the HIP score was created, it was applied in another sample from the same endemic area composed by 219 individuals: non-infected (n = 90), asymptomatic (n = 60), mild (n = 50) and severe vivax infection (n = 19). Area under the curve (AUC) was calculated, together with the cut-off value, which presents the higher likelihood ratio, and P values are plotted. The HIP score is described in Methods.
Statistical analysis
Data were analyzed using the GraphPad Prism 5.0 (GraphPad Software Inc.). For the ordinal variables, differences between groups were calculated using the non-parametric Kruskal-Wallis test with Dunn's multiple comparisons or trend analysis. The chi-square test was used to compare differences in categorized variables. The correlations were assessed using the Spearman test. Non-linear curve fit was also plotted to illustrate the general trend of the correlations. The statistical analyses used are illustrated in each figure or table. Differences presenting P ≤ 0.05 were considered statistically significant.
Results and Discussion
Baseline characteristics and laboratory assessment of P. vivax infection severity
The majority of the participants were male, with no gender differences among groups (P = 0.78). As previously described [4, 28], individuals with asymptomatic P. vivax infection were older, had experienced more previous malaria episodes and presented lower parasitaemia than had symptomatic cases (Table 1). Patients with severe P. vivax infection were younger, having lived for a shorter time in the endemic area, and had experienced fewer previous malaria episodes than individuals with no malaria infection and with mild or asymptomatic infection (Table 1). Moreover, patients with severe disease displayed higher parasitaemias than those with uncomplicated infection (P < 0.0001). Haemoglobin levels were also decreased in patients with severe disease (P = 0.02). All patients with severe disease were admitted to the municipal hospital presenting with fever, tachycardia and tachypnea. Moreover, five out of nineteen individuals with severe infection developed jaundice and six presented with splenomegaly. Six infected patients died within 72 h of hospitalization, four presenting with acute respiratory failure and two with anuric renal failure, despite the haemodynamic support and anti-parasite therapy. These severe complications have been commonly implicated as major death causes in severe vivax infections [7, 18]. The other thirteen individuals with complicated disease received specific treatment with intravenous quinine and achieved total clinical recovery after 10-15 days. Clinical characteristics and outcomes of the patients with severe malaria are summarized in Table 2. All patients with mild disease recovered totally and no drug resistance was identified within individuals studied.
The further step was to assess whether the spectrum of vivax malaria clinical presentation could be associated with laboratory parameters of organ dysfunction. In a primary analysis, strong linear trends were identified regarding increasing plasma levels of CRP, serum creatinine, bilirubins and the graduation of disease severity (Table 2; P < 0.0001 for all trends analyzed). The individuals presenting higher HIP scores also displayed elevated IFN-gamma/IL-10 ratios (Figure 1G). These data indicate that a high grade of general inflammation-mediated systemic damage is occurring in some vivax malaria cases, explaining the severity of their clinical presentations.
Inflammatory balance according to P. vivax infection severity
Furthermore, a possible link between the differences in clinical presentation and laboratory parameters of organ damage and specific patterns of immune responses or inflammatory mediators profile was evaluated. Plasma TNF, which is related to P. vivax paroxysms [30], was higher according to infection severity (Figure 2A). IFN-gamma is also implicated in both resistance to malaria [31] and disease immunopathology [32]. In the present series, IFN-gamma levels were higher in patients with increased severity (Figure 2B). Interestingly, the increasing levels of all these inflammatory markers also presented a linear trend with the gradual augmentation of infection severity (P < 0.0001 for each parameter). Conversely, plasma levels of IL-10, a cytokine that down-regulates inflammation, were lower with increased disease severity (P < 0.0001, for linear trend; Figure 2C). Thus, IFN-gamma/IL-10 ratio values were higher in patients with increased disease severity (P < 0.0001, for linear trend; Figure 2D).
General trend of the Inflammatory profile in vivax malaria. Plasma levels of (A) TNF, (B) IFN-gamma, (C) IL-10 and (D) IFN-gamma/IL-10 ratios were estimated in non-infected individuals and those presenting different manifestations of the vivax malaria clinical spectrum. Study participants were stratified in groups as follows: non-infected (NI; n = 90); asymptomatic infection (Asy; n = 60); mild infection (Mild; n = 50); and severe infection (Sev; n = 19). One-Way ANOVA with trend analysis was performed to check the statistical significance between the groups studied. P values are plotted in each graph.
Kinetics of inflammatory responses during the treatment of severe vivax infection
In thirteen patients, who clinically recovered out of nineteen with severe vivax infection, there was an important reduction in the levels of all laboratory parameters of organ damage screened, including plasma CRP (P = 0.002; Figure 3A), creatinine (P = 0.005; Figure 3B), ALT (P = 0.001; Figure 3C) and total bilirubin (P = 0.016; Figure 3D) during anti-parasite treatment. This observation suggests that clinical recovery resulted from a reduction in systemic inflammatory aggression. Regarding the immune markers of pro-inflammatory responses, an important decrease in both IFN-gamma/IL-10 ratios (P = 0.0005; Figure 4A) and TNF levels (P = 0.001; Figure 4B) was noticed during anti-malarial treatment.
Kinetic of organ damage indicators during antimalarial treatment in individuals with severe vivax disease. Plasma levels of (A) CRP, (B) creatinine, (C), ALT and (D) total bilirubin were estimated before treatment (at admission to the Hospital) and after seven days of inhospital care in individuals with severe vivax infection who achieved cure (n = 13). Wilcoxon matched pairs test was performed to calculate the statistical significance. P values are plotted in each graph.
Kinetic of immunologic indicators during antimalarial treatment in individuals with severe vivax disease. (A) IFN-gamma/IL-10 ratios and (B) TNF plasma levels were estimated before treatment (at admission to the Hospital) and after seven days of in-hospital care in individuals with severe vivax infection who achieved cure (n = 13). Wilcoxon matched pairs test was performed to calculate the statistical significance. P values are plotted in each graph.
Conclusions
These investigations suggest that different clinical presentations of vivax malaria infection are strongly associated with a potent activation of pro-inflammatory responses and cytokine imbalance. These results are of utmost importance to improve current knowledge about physiopathological concepts of this serious, widespread disease.
References
Anstey NM, Russell B, Yeo TW, Price RN: The pathophysiology of vivax malaria. Trends Parasitol. 2009, 25: 220-227. 10.1016/j.pt.2009.02.003.
Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM: Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007, 77: 79-87.
da Silva J: National System in Health Surveillance: situation report: Rondônia. 2006, Brasília: Ministério da Saúde, 24.
Alves FP, Durlacher RR, Menezes MJ, Krieger H, Silva LH, Camargo EP: High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg. 2002, 66: 641-648.
Ladeia-Andrade S, Ferreira MU, de Carvalho ME, Curado I, Coura JR: Age-dependent acquisition of protective immunity to malaria in riverine populations of the Amazon Basin of Brazil. Am J Trop Med Hyg. 2009, 80: 452-459.
Nautiyal A, Singh S, Parmeswaran G, DiSalle M: Hepatic dysfunction in a patient with Plasmodium vivax infection. Med Gen Med. 2005, 7: 8.
Prakash J, Singh AK, Kumar NS, Saxena RK: Acute renal failure in Plasmodium vivax malaria. J Assoc Physicians India. 2003, 51: 265-267.
Rodriguez CN, Campos R, Pastran B, Jimenez I, Garcia A, Meijomil P, Rodriguez-Morales AJ: Sepsis due to extended-spectrum beta-lactamase-producing Aeromonas hydrophila in a pediatric patient with diarrhea and pneumonia. Clin Infect Dis. 2005, 41: 421-422. 10.1086/431773.
Barcus MJ, Basri H, Picarima H, Manyakori C, Sekartuti , Elyazar I, Bangs MJ, Maguire JD, Baird JK: Demographic risk factors for severe and fatal vivax and falciparum malaria among hospital admissions in northeastern Indonesian Papua. Am J Trop Med Hyg. 2007, 77: 984-991.
Genton B, D'Acremont V, Rare L, Baea K, Reeder JC, Alpers MP, Muller I: Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med. 2008, 5: e127-10.1371/journal.pmed.0050127.
Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, Lampah DA, Price RN: Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 2008, 5: e128-10.1371/journal.pmed.0050128.
Daniel-Ribeiro CT, Lacerda MV, Oliveira-Ferreira J: [Plasmodium vivax malaria in the Brazilian Amazon: some aspects of its epidemiology, clinical spectrum and naturally induced immune responses]. Bull Soc Pathol Exot. 2008, 101: 243-248.
Makkar RP, Mukhopadhyay S, Monga A, Gupta AK: Plasmodium vivax malaria presenting with severe thrombocytopenia. Braz J Infect Dis. 2002, 6: 263-265. 10.1590/S1413-86702002000500008.
Rodriguez-Morales AJ, Sanchez E, Vargas M, Piccolo C, Colina R, Arria M, Franco-Paredes C: Occurrence of thrombocytopenia in Plasmodium vivax malaria. Clin Infect Dis. 2005, 41: 130-131. 10.1086/430837.
Beg MA, Khan R, Baig SM, Gulzar Z, Hussain R, Smego RA: Cerebral involvement in benign tertian malaria. Am J Trop Med Hyg. 2002, 67: 230-232.
Ozen M, Gungor S, Atambay M, Daldal N: Cerebral malaria owing to Plasmodium vivax: case report. Ann Trop Paediatr. 2006, 26: 141-144. 10.1179/146532806X107494.
Das BS: Renal failure in malaria. J Vector Borne Dis. 2008, 45: 83-97.
Anstey NM, Handojo T, Pain MC, Kenangalem E, Tjitra E, Price RN, Maguire GP: Lung injury in vivax malaria: pathophysiological evidence for pulmonary vascular sequestration and posttreatment alveolar-capillary inflammation. J Infect Dis. 2007, 195: 589-596. 10.1086/510756.
Tanios MA, Kogelman L, McGovern B, Hassoun PM: Acute respiratory distress syndrome complicating Plasmodium vivax malaria. Crit Care Med. 2001, 29: 665-667. 10.1097/00003246-200103000-00037.
Francischetti IM, Seydel KB, Monteiro RQ: Blood coagulation, inflammation, and malaria. Microcirculation. 2008, 15: 81-107. 10.1080/10739680701451516.
Clark IA, Budd AC, Alleva LM, Cowden WB: Human malarial disease: a consequence of inflammatory cytokine release. Malar J. 2006, 5: 85-10.1186/1475-2875-5-85.
Day NP, Hien TT, Schollaardt T, Loc PP, Chuong LV, Chau TT, Mai NT, Phu NH, Sinh DX, White NJ, Ho M: The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J Infect Dis. 1999, 180: 1288-1297. 10.1086/315016.
Rodrigues Ade F, Escobar AL, Souza-Santos R: Spatial analysis and determination of malaria control areas in the State of Rondonia. Rev Soc Bras Med Trop. 2008, 41: 55-64.
Cavasini MT, Ribeiro WL, Kawamoto F, Ferreira MU: How prevalent is Plasmodium malariae in Rondonia, western Brazilian Amazon?. Rev Soc Bras Med Trop. 2000, 33: 489-492. 10.1590/S0037-86822000000500011.
WHO: Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000, 94: 1-90.
Snounou G: Detection and identification of the four malaria parasite species infecting humans by PCR amplification. Methods Mol Biol. 1996, 50: 263-291.
Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN: High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993, 61: 315-320. 10.1016/0166-6851(93)90077-B.
Andrade BB, Rocha BC, Reis-Filho A, Camargo LM, Tadei WP, Moreira LA, Barral A, Barral-Netto M: Anti-Anopheles darlingi saliva antibodies as marker of Plasmodium vivax infection and clinical immunity in the Brazilian Amazon. Malar J. 2009, 8: 121-10.1186/1475-2875-8-121.
Iriemenam NC, Okafor CM, Balogun HA, Ayede I, Omosun Y, Persson JO, Hagstedt M, Anumudu CI, Nwuba RI, Troye-Blomberg M, Berzins K: Cytokine profiles and antibody responses to Plasmodium falciparum malaria infection in individuals living in Ibadan, southwest Nigeria. Afr Health Sci. 2009, 9: 66-74.
Karunaweera ND, Wijesekera SK, Wanasekera D, Mendis KN, Carter R: The paroxysm of Plasmodium vivax malaria. Trends Parasitol. 2003, 19: 188-193. 10.1016/S1471-4922(03)00036-9.
D'Ombrain MC, Robinson LJ, Stanisic DI, Taraika J, Bernard N, Michon P, Mueller I, Schofield L: Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin Infect Dis. 2008, 47: 1380-1387. 10.1086/592971.
Wroczynska A, Nahorski W, Bakowska A, Pietkiewicz H: Cytokines and clinical manifestations of malaria in adults with severe and uncomplicated disease. Int Marit Health. 2005, 56: 103-114.
Acknowledgements
The authors would like to thank Mr. João Gambati and Imbroinise Neto for technical and logistic support in field study area, Mr. Jorge Tolentino, Ms. Natali Alexandrino, and Mrs. Adorielze Leite for logistic support. We are also grateful to Lucas Nogueira and Kiyoshi Fukutani for assistance with the molecular experiments. A special thank to Dr. Fabio Costa, from Universidade de Campinas for critical review of the data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
Wrote the paper: BBA and ARF; Performed the field study and clinical examinations: BBA, SMSN and LMAC; Performed the laboratory experiments and data analysis: BBA and JC; Participated in the design of the study and helped with the manuscript: LMAC and AB; Coordinated the study helped to draft the manuscript: MBN. All authors have read and approved the final version of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Andrade, B.B., Reis-Filho, A., Souza-Neto, S.M. et al. Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar J 9, 13 (2010). https://doi.org/10.1186/1475-2875-9-13
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-9-13