Abstract
Certain distinctive components of the severe systemic inflammatory syndrome are now well-recognized to be common to malaria, sepsis, viral infections, and post-trauma illness. While their connection with cytokines has been appreciated for some time, the constellation of changes that comprise the syndrome has simply been accepted as an empirical observation, with no theory to explain why they should coexist. New data on the effects of the main pro-inflammatory cytokines on the genetic control of sickness behaviour can be extended to provide a rationale for why this syndrome contains many of its accustomed components, such as reversible encephalopathy, gene silencing, dyserythropoiesis, seizures, coagulopathy, hypoalbuminaemia and hypertriglyceridaemia. It is thus proposed that the pattern of pathology that comprises much of the systemic inflammatory syndrome occurs when one of the usually advantageous roles of pro-inflammatory cytokines – generating sickness behaviour by moderately repressing genes (Dbp, Tef, Hlf, Per1, Per2 and Per3, and the nuclear receptor Rev-erbα) that control circadian rhythm – becomes excessive. Although reversible encephalopathy and gene silencing are severe events with potentially fatal consequences, they can be viewed as having survival advantages through lowering energy demand. In contrast, dyserythropoiesis, seizures, coagulopathy, hypoalbuminaemia and hypertriglyceridaemia may best be viewed as unfortunate consequences of extreme repression of these same genetic controls when the pro-inflammatory cytokines that cause sickness behaviour are produced excessively. As well as casting a new light on the previously unrationalized coexistence of these aspects of systemic inflammatory diseases, this concept is consistent with the case for a primary role for inflammatory cytokines in their pathogenesis across this range of diseases.
Similar content being viewed by others
Common ground in inflammatory disease, sickness behaviour and hibernation
The idea of acute infectious illness being caused by rampant overproduction of inflammatory cytokines that, in lower concentrations, mediate innate immunity, was first argued a quarter of a century ago [1], and has generated a large literature. Once recombinant tumour necrosis factor (TNF) and interleukin-1 (IL-1) became available in the late 1980s, and assays based on them replaced earlier methods, the concept spread to other pro-inflammatory cytokines, and from malaria and sepsis to viral and certain autoimmune diseases. It is now also well-entrenched in the literature of the post-trauma syndrome. Clearly, different triggers for cytokine generation and release can be expected to generate different foci, profiles, concentrations and kinetics of these mediators – now numerous enough to form superfamilies – and thus clinical variation within the same general principle is to be expected. But an accepted fundamental pattern has emerged.
At a time of shifting perceptions on the interaction between sickness and host activity (reviewed by Dantzer [2]), Hart [3] argued that the distinctive behaviour of sick humans and animals (lethargy, anorexia, depressed motor activity etc.) was not simply another untoward aspect of being ill. Instead, it was reasoned to be an adaptive syndrome that had evolved as a protective mechanism to maximize chances of survival through encouraging the sick animal to seek out and remain in a safe resting place, and not search for food, until a survivable infectious episode had passed. Hart also proposed that sickness behaviour was caused by the inflammatory cytokines, TNF and IL-1. This was later confirmed by others [4], and investigated further through showing that IL-1 contributes significantly to the anorexia caused by both endotoxin and influenza infection [5, 6]. The literature on this field is now considerable. Indeed, Cavadini and co-workers [7] note that this link between TNF and IL-1 and sickness behaviour induced them to investigate if these inflammatory cytokines suppress expression of the clock genes that regulate circadian rhythm. It has recently [8] been realized that the circadian cycle controls many more biological functions than previously supposed.
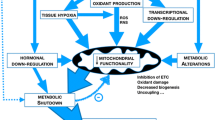
Circadian rhythm and hibernation are variations of a theme of genetic control of activity and metabolism, and the degree to which these two clocks are functionally linked is often studied [9]. The first example of a human disease being thought of in these terms was ischaemic heart disease, in which the concepts were adopted to explain the self-protective downregulation of metabolic function seen in cardiomyocytes after repeated bouts of ischaemia. Like the seasonal hibernation seen in Arctic mammals, it involves biochemical changes directed at preserving energy, so in 1997 was termed myocardial hibernation [10]. Functional hibernation as a survival strategy in severe sepsis was proposed in 2003 [11], and subsequently documented in myocytes in the mouse caecal ligation and puncture model of systemic inflammatory disease [12]. Levy's recent reasoning [13] that broadens the possible relevance of cytokine-induced cardiomyocyte hibernation to other organs is, as Singer suggested [11], essentially sickness behaviour [3], with altered circadian rhythm being considered in terms of cellular bioenergetics. The consequence is to reduce demand for energy, in the form of ATP, to a level commensurate with its supply.
Energy saving and the consequences of it being overdone
As outlined above, it seems reasonable that sickness behaviour, including lowering the metabolic rate to conserve energy and turning off appetite to avoid the urge to forage, will help tide the ill animal or human over an illness crisis. Recently, Cavadini and co-workers [7] reported that TNF and IL-1β suppress the expression of genes (Dbp, Tef, Hlf, Per1, Per2 and Per3) in vivo, and a nuclear receptor (Rev-erbα) involved in controlling circadian rhythm. The authors proposed that these cytokines thereby provide a link between the fatigue associated with autoimmune diseases, such as rheumatoid arthritis and Crohn's disease.
Nevertheless these cytokines, and others related to them, can cause a much wider spectrum of harmful changes than this [14]. Why these changes, so familiar from the literature and everyday observation, should occur as a group, forming a distinct syndrome, currently lacks a rationale. It was therefore considered how widely the implications of severe sickness behaviour, through suppressed circadian rhythm [7], might extend, and whether this approach might be able to explain the composition of the severe systemic inflammatory syndrome common to malaria, sepsis, viral infections, and also seen post-trauma. The literature to date has focussed on mechanisms for individual changes, but not questioned why the mosaic contains the components one is accustomed to observing.
In other words, are inflammatory cytokines simply innately harmful in excess, pathophysiology arising from excessive manifestation of the physiological roles they possess when in trace amounts? Or, as suggested here (Figure 1), do the wider functions of the nuclear receptor (Rev-erbα) and genes (Dbp, Tef, Hlf, Per1, Per2 and Per3) [7] lead to an understanding of the syndrome to be expected when a severe inflammatory illness occurs? In documenting this suppression Cavadini and co-authors administered, to mice, only a tenth of the dose of bacterial lipopolysaccharide (LPS) required for a severe response in this species. Accordingly, the literature was examined to see whether these cytokine-suppressed nuclear receptor and genes involved in circadian rhythm have other functions that help us understand the pattern of changes routinely observed in the systemic inflammatory syndrome.
Reversible encephalopathy
An active brain consumes much more energy than any other organ of similar size, largely through the demands of the Na+/K+ ATPase that runs the cell membrane Na+/K+ pump [15]. Hence, it is likely to have been a prime conservation site for any set of adaptive changes that have evolved to conserve energy in order for the organism to survive, whether at the level of sickness behaviour or hibernation, both of which can be conveniently approached in terms of altered circadian rhythm. The fatigue of chronic inflammatory diseases such as rheumatoid arthritis is consistent with the capacity of TNF, to which this fatigue is linked in the literature, to suppress circadian rhythm genes [7]. Sleep saves energy and pathways that lead to sleep are induced by TNF [16] or IL-1β [17]. Also, electroencephalogram (EEG) delta wave power is decreased for several days when short interfering RNA (siRNA) targeting TNF is microinjected into the primary somatosensory cortex [18]. This is consistent with TNF increasing cortical EEG delta wave power and, therefore, [19] being involved in sleep regulation.
The TNF and IL-1β-susceptible genes through which these changes are controlled have not been identified, but the above observations imply the same principles apply as the need to reduce ATP expenditure increases in urgency with more severe illness, progressing through the increased fatigue and sleep periods to the clinical signs of encephalopathy, including coma, in severe malaria, sepsis, or influenza. Clearly, this encephalopathy can be associated with a harmful outcome in these systemic inflammatory states, but its evolutionary function may simply be to save energy. Indeed, this strategy is likely to be successful in, for example, most adult cerebral malaria patients, who recover with negligible neurological deficit if other organ failure is not involved [20]. TNF [21] and IL-1 [22] are increased in proportion to the degree of coma in falciparum malaria. The attractiveness of impaired consciousness in this disease being based on awareness of the basic physiology and broad effects of these and functionally similar cytokines across acute disease in general – rather than on primary vascular blockage by sequestered parasitized red cells [23] – is enhanced by recent evidence, from Papua New Guinea [24] and West Papua [25], of the essentially non-sequestering Plasmodium vivax causing this encephalopathy to the same degree as does Plasmodium falciparum.
How might this coma come about? Biology's known gaseous signalling molecules offer possibilities, since they are increased in inflammatory states, and induce a hibernation-like reversible state of suspended animation that protects against low ATP levels in Drosophila melanogaster larvae (NO [26]), Caenorhabditis elegans (CO [27]) and mice (H2S [28, 29]). All three gases have the potential to operate through inhibiting mitochondrial cytochrome oxidase [30–32]. A striking contrast exists, however, in the roles of these three gases in mammalian inflammation – both NO [33] and CO [34] inhibit NF-κB, whereas H2S upregulates it [35]. This dichotomy may prove to explain why both NO and CO are anti-inflammatory in mice, including protecting against the mouse model of malaria encephalopathy [36, 37], whereas H2S is pro-inflammatory, increasing plasma TNF levels in mice [38], and its inhibition protects in sepsis models [38, 39]. These three gases warrant a detailed comparative investigation in this context.
LPS tolerance and inflammation-induced gene silencing
A major unaddressed question is the origin of the widespread gene silencing that develops after the initial illness crisis of severe systemic inflammation [40], including H5N1 influenza in mice [41]. Immunosuppression is common to haemoprotozoan diseases such as malaria [42] and trypanosomiasis [43], as well as in sepsis [44], influenza [45], and trauma [46]. Patients who survive the initial acute effects of excessive levels of inflammatory cytokines often succumb during a subsequent period of immunological and metabolic shutdown, in a state variously termed anergy, immune paralysis, or monocyte deactivation, with monocytes unable to generate HLA antigens or enough cytokines for normal immunological responses [47], let alone the excess that might be harmful to the host [48]. In short, a systemic hyperinflammatory response turns into a harmful hypoinflammatory state of unknown origin [49]. Explanations for these events in sepsis have included anti-inflammatory cytokines out-producing inflammatory cytokines [50] and severe lymphoid cell apoptosis [51]. However, both pro- and anti-inflammatory cytokines proved to switch off as the disease progresses [52], and the point of maximum T cell anergy correlates to a diminished apoptotic response [53].
LPS tolerance is a common model for gene silencing, and repressed gene expression, arising from disrupted transcription, is as widespread in LPS tolerance as in severe inflammatory disease. Indeed the two coincide, with patients recovering from malaria being tolerant to LPS [54] as well as to high loads of malaria parasites [55]. In landmark studies in 1975 Lewis Thomas widened our awareness of the broad relevance of the changes seen in LPS tolerance by studying it in parallel to tolerance to haemorrhagic shock and trauma shock [56]. Arguably, therefore, LPS tolerance is simply a particular way of detecting a process common to many disease states. For instance, the patterns of NF-κB expression in sepsis and LPS tolerance closely resemble each other [57], and monocyte inactivation is as generalized, with the same characteristics, in LPS tolerance as in chronic sepsis [58]. LPS tolerance, and therefore sepsis gene silencing, has a functional link to the theme of this review, in that it has a distinct circadian rhythm [59, 60], and can be created by depleting mice of Per2, one of the circadian genes that TNF suppresses [7].
More recently, LPS tolerance has been explained in terms of RelB induction generating transcriptionally inactive NF-κB p65/RelB heterodimers [61] and epigenetic silencing of TNF [62]. The latter seems a particularly cogent argument, with LPS tolerant, or silenced, cells exhibiting repressed production of TNF mRNA, retained binding of heterochromatin binding protein 1α, sustained methylation of histone H3 lysine 9, reduced phosphorylation of histone H3 serine 10, and diminished binding of NF-κB RelA/p65 to the TNF promoter. This group has combined these elements, which are consistent with basic studies on gene silencing [63], into a compelling case to explain this phenomenon in severe systemic inflammation [40]. It is, moreover, consistent with the hypoinflammatory state being a logical consequence of the earlier cytokine storm that caused the excessive sickness behaviour.
Changes less likely to have survival advantage
Dyserythropoiesis
A significant contribution is made to anaemia, in both acute and chronic inflammatory states, by defective generation of new red cells in bone marrow, or dyserythropoiesis. It can be reproduced by injecting TNF [64], and not surprisingly has been recorded in a range of infectious states in which pro-inflammatory cytokines are increased, including malaria [65], trypanosomiasis [66] and viral diseases [67–69]. As in many other tissues, the bone marrow's haemopoietic cells are governed by time-dependent variations in clock gene expression. This includes Per1, Per2, and the nuclear receptor Rev-erbα [70], all three of which are included in the nuclear elements shown to be suppressed by TNF [7]. This suppression gives a pathway whereby TNF could reduce haemopoietic activity in infectious diseases. It can be viewed as part of a generic attempt to save energy, in which any initial savings would soon become a liability as haematocrit, and therefore the capacity to carry oxygen in the circulation, falls.
Seizures
Febrile seizures are traditionally attributed to fever itself, rather than fever and seizures being visualized as having a common cause. Seizures are particularly common in malarial illness. In one large study [71] based in Kenya, 69% of patients experienced them, and other reports from West Africa record a considerably higher incidence. They are also frequently observed in paediatric sepsis [72], burn injury [73], and a range of acute severe viral diseases, including influenza [74] and Lassa fever [75].
An unexpected development in understanding the origin of certain types of seizures came from the realization, several years ago [76], that mice with deletions of the genes for three circadian PARbZip transcription factors, DBP, HLF and TEF, are highly susceptible to generalized spontaneous and audiogenic seizures. Moreover, fits were four times as likely to occur in the major sleep period of the circadian cycle than in its major active period. These authors identified Pdxk as a target gene of these transcription factors, noting the role of this gene in the generation of pyridoxal phosphate, a coenzyme in the synthesis of neurotransmitters such as γ-aminobutyric acid, serotonin and dopamine. Through the genes for these three transcription factors being among those recently reported to be suppressed by TNF and IL-1β [7], this mechanism for seizures is now linked to infectious disease and fever seizures, since these and similar pyrogenic cytokines dominate disease pathogenesis. These authors found an appreciable degree of down-regulation by a dose of cytokine a tenth of that required to make mice seriously ill, so these two cytokines warrant investigation as the cause of seizures, through Dbp, Tef, and Hlf downregulation, in circumstances in which levels of these cytokines are raised, such as severe haemoprotozoal, bacterial and viral diseases.
Coagulopathy
Coagulopathy is another characteristic component of the systemic inflammatory syndrome. As well as being detectable in human volunteers receiving parenteral TNF [77], it is, like seizures, common to malaria [78], sepsis [79], and viral diseases (eg dengue [80], Ebola [81] and influenza [82]). Plasminogen activator inhibitor type 1 (PAI-1), which retards the generation of plasmin, thereby adding to coagulopathy by slowing clot dissolution, is a major regulator of the fibrinolytic system. It is expressed with a circadian rhythmicity, peaking in the early morning. The nuclear receptor Rev-erbα, a core component of the circadian loop, causes this cyclic expression of human PAI-1 gene expression through two Rev-erbα binding sites in the PAI-1 promoter [83]. Hence, the capacity of TNF to suppress Rev-erbα, and thus circadian rhythm [7], may also explain why it enhances coagulopathy [77], and why this occurs in protozoal, bacterial, and viral diseases. Higher levels of PAI-1 predict a poor outcome in patients with sepsis [84]. Thus, it is argued here that the coagulopathy of these systemic disease states is a further example of sickness behaviour, i.e. circadian rhythm shutdown, taken to harmful extremes.
Hypoalbuminaemia
Plasma albumin has several important biological functions, including being an extracellular transition metal ion-binding and radical-scavenging antioxidant, and an important contributor to plasma osmolarity. Its level is therefore normally controlled within tight limits. Levels are characteristically low in systemic inflammatory states, and this decrease can also be accounted for by this proposal. Hypoalbuminaemia occurs routinely in malaria [85], sepsis [86, 87], various severe viral diseases (eg Korean haemorrhagic fever [88], dengue [89], Ebola [90], viral hepatitis [91], SARS [92]), visceral leishmaniasis [93] and trauma [94]. Serum albumin level is an independent indicator of outcome in severe sepsis [95], so much so that it was included in the criteria when the APACHE scoring system was upgraded from II to III [96]. The liver-specific albumin gene is positively regulated by Dbp [97], one of the circadian genes that TNF suppresses [7]. TNF has been demonstrated, in picomolar concentrations, to reduce albumin production by human hepatocytes [98].
Hypertriglyceridaemia
Usually, and for sound metabolic reasons, the concentration of triglycerides in plasma is closely regulated. Circulating levels can be increased by exogenous TNF [99], and this cytokine has been shown to bring this about in thermal injury [100]. Thus hypertriglyceridaemia can be expected in diseases in which TNF is acutely increased, as documented in malaria [101] and sepsis [86, 102]. However, no reference exists to circulating triglyceride levels having been examined in viral diseases. Since TNF suppresses the nuclear receptor Rev-erbα [7], and hypertriglyceridaemia occurs in Rev-erbα KO mice [103], the presence of this change in the systemic inflammatory syndrome is predictable.
Consistent with a primary cytokine origin of systemic disease
These arguments strengthen the concept that the pro-inflammatory cytokines are the primary driver of the pathophysiology of severe infectious, including malaria, and post-trauma illness. An alternative view, that each pathogen causes disease through a different primary mechanism (eg death of viral-infected host cells or vasculature blockage by parasitized red cells in malaria [23]), with cytokines providing only non-specific changes such as fever, still exists. This view is further weakened by the above literature and reasoning, since it is difficult to credit vascular obstruction (falciparum malaria) or cell death (viral infection) with the ability to generate the above changes, such as coagulopathy, hypoalbuminaemia or immunosuppression, in a single infectious disease, let alone a range of them, or post-trauma.
Conclusion
While sickness behaviour, comprising a series of changes caused through pro-inflammatory cytokines shutting down circadian rhythm, has survival advantages, its more intense expression in severe disease generates a range of more harmful alterations, recognizable as the severe systemic inflammatory response. These are controlled by more severe effects of the same cytokines (TNF and IL-1β) affecting the same genes and nuclear receptor(s) as suppress circadian rhythm. Accordingly, the coexistence of reversible encephalopathy, gene silencing, dyserythropoiesis, seizures, coagulopathy, hypoalbuminaemia and hypertriglyceridaemia in severe infectious disease, including malaria, can best be understood as an extreme form of sickness behaviour. It is further proposed that this pattern, familiar in such conditions, has persisted in evolution because it has a survival advantage when, most commonly, the syndrome is less severe. In terms of a basis for developing treatments for human disease, this implies that the observed hypoinflammatory changes seen in these conditions require no primary explanation other than they are logical consequences of excess earlier production of pro-inflammatory cytokines, although local variations in secondary, cytokine-induced mechanisms occur.
As knowledge of the range of genes controlled by the superfamilies of pro-inflammatory cytokines and the genetic control of pathological changes both expand, it seems likely that other components of the systemic inflammatory syndrome will be added to the list begun in this paper.
References
Clark IA: How TNF was recognized to be a key mechanism of disease. Cytok Gr Fact Rev. 2007, 18: 335-343. 10.1016/j.cytogfr.2007.04.002.
Dantzer R, Kelley KW: Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007, 21: 153-160. 10.1016/j.bbi.2006.09.006.
Hart BL: Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988, 12: 123-137. 10.1016/S0149-7634(88)80004-6.
Bluthe RM, Pawlowski M, Suarez S, Parnet P, Pittman Q, Kelley KW, Dantzer R: Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology. 1994, 19: 197-207. 10.1016/0306-4530(94)90009-4.
Swiergiel AH, Smagin GN, Dunn AJ: Influenza virus infection of mice induces anorexia: comparison with endotoxin and interleukin-1 and the effects of indomethacin. Pharmacol Biochem Behav. 1997, 57: 389-396. 10.1016/S0091-3057(96)00335-8.
Swiergiel AH, Smagin GN, Johnson LJ, Dunn AJ: The role of cytokines in the behavioral responses to endotoxin and influenza virus infection in mice: effects of acute and chronic administration of the interleukin-1-receptor antagonist (IL-1ra). Brain Res. 1997, 776: 96-104. 10.1016/S0006-8993(97)01009-3.
Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A: TNF-{alpha} suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci USA. 2007, 104: 12843-12848. 10.1073/pnas.0701466104.
Ptitsyn AA, Zvonic S, Conrad SA, Scott LK, Mynatt RL, Gimble JM: Circadian clocks are resounding in peripheral tissues. PLoS Comput Biol. 2006, 2: e16-10.1371/journal.pcbi.0020016.
Revel FG, Herwig A, Garidou ML, Dardente H, Menet JS, Masson Pevet M, Simonneaux V, Saboureau M, Pevet P: The circadian clock stops ticking during deep hibernation in the European hamster. Proc Natl Acad Sci USA. 2007, 104: 13816-13820. 10.1073/pnas.0704699104.
Camici PG, Wijns W, Borgers M, De Silva R, Ferrari R, Knuuti J, Lammertsma AA, Liedtke AJ, Paternostro G, Vatner SF: Pathophysiological mechanisms of chronic reversible left ventricular dysfunction due to coronary artery disease (hibernating myocardium). Circulation. 1997, 96: 3205-3214.
Brealey D, Singer M: Mitochondrial dysfunction in sepsis. Curr Infect Dis Rep. 2003, 5: 365-371. 10.1007/s11908-003-0015-9.
Levy RJ, Piel DA, Acton PD, Zhou R, Ferrari VA, Karp JS, Deutschman CS: Evidence of myocardial hibernation in the septic heart. Crit Care Med. 2005, 33: 2752-2576. 10.1097/01.CCM.0000189943.60945.77.
Levy RJ: Mitochondrial dysfunction, bioenergetic impairment, and metabolic down-regulation in sepsis. Shock. 2007, 28: 24-28. 10.1097/01.shk.0000235089.30550.2d.
Spriggs DR, Sherman ML, Michie H, Arthur KA, Imamura K, Wilmore D, Frei E, Kufe DW: Recombinant human tumor necrosis factor administered as a 24-hour intravenous infusion. A phase 1 and pharmacologic study. J Natl Cancer Inst. 1988, 80: 1039-1044. 10.1093/jnci/80.13.1039.
Erecinska M, Silver IA: Ions and energy in mammalian brain. Prog Neurobiol. 1994, 43: 37-71. 10.1016/0301-0082(94)90015-9.
Kapas L, Hong L, Cady AB, Opp MR, Postlethwaite AE, Seyer JM, Krueger JM: Somnogenic, pyrogenic, and anorectic activities of tumor necrosis factor-alpha and TNF-alpha fragments. Am J Physiol. 1992, 263: R708-R715.
Fang JD, Wang Y, Krueger JM: Effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am J Physiol. 1998, 274 (3 Pt 2): R655-R660.
Taishi P, Churchill L, Wang M, Kay D, Davis CJ, Guan X, De A, Yasuda T, Liao F, Krueger JM: TNFalpha siRNA reduces brain TNF and EEG delta wave activity in rats. Brain Res. 2007, 2: 125-132. 10.1016/j.brainres.2007.04.072.
Darko DF, Miller JC, Gallen C, White J, Koziol J, Brown SJ, Hayduk R, Atkinson JH, Assmus J, Munnell DT, Naitoh P, McCutchan JA, Mitler MM: Sleep electroencephalogram delta-frequency amplitude, night plasma levels of tumor necrosis factor alpha, and human immunodeficiency virus infection. Proc Natl Acad Sci USA. 1995, 92: 12080-12084. 10.1073/pnas.92.26.12080.
Marsh K, Snow RW: Host-parasite interaction and morbidity in malaria endemic areas. Philosoph Trans R Soc Lond. 1997, 352: 1385-1394. 10.1098/rstb.1997.0124.
Kern P, Hemmer CJ, Van Damme J, Gruss H-J, Dietrich M: Elevated tumour necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989, 87: 139-143.
Kwiatkowski D, Hill AVS, Sambou I, Twumasi P, Castracane J, Manogue KR, Cerami A, Brewster DR, Greenwood BM: TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990, 336: 1201-1204. 10.1016/0140-6736(90)92827-5.
Maier AG, Rug M, O'Neill MT, Brown M, Chakravorty S, Szestak T, Chesson J, Wu Y, Hughes K, Coppel RL, Newbold C, Beeson JG, Craig A, Crabb BS, Cowman AF: Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell. 2008, 134: 48-61. 10.1016/j.cell.2008.04.051.
Genton B, D'Acremont V, Rare L, Baea K, Reeder JC, Alpers MP, Muller I: Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med. 2008, 5: e127-10.1371/journal.pmed.0050127.
Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, Lampah DA, Price RN: Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 2008, 5: e128-10.1371/journal.pmed.0050128.
Teodoro RO, O'Farrell PH: Nitric oxide-induced suspended animation promotes survival during hypoxia. EMBO J. 2003, 22: 580-587. 10.1093/emboj/cdg070.
Nystul TG, Roth MB: Carbon monoxide-induced suspended animation protects against hypoxic damage in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2004, 101: 9133-9136. 10.1073/pnas.0403312101.
Blackstone E, Morrison M, Roth MB: H2S induces a suspended animation-like state in mice. Science. 2005, 308: 518-10.1126/science.1108581.
Blackstone E, Roth MB: Suspended animation-like state protects mice from lethal hypoxia. Shock. 2007, 27: 370-372. 10.1097/SHK.0b013e31802e27a0.
Brown GC: Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Letters. 1995, 369: 136-139. 10.1016/0014-5793(95)00763-Y.
Zuckerbraun BS, Chin BY, Bilban M, d'Avila JD, Rao J, Billiar TR, Otterbein LE: Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007, 21: 1099-1106. 10.1096/fj.06-6644com.
Dorman DC, Moulin FJ, McManus BE, Mahle KC, James RA, Struve MF: Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol Sci. 2002, 65: 18-25. 10.1093/toxsci/65.1.18.
Matthews JR, Botting CH, Panico M, Morris HR, Hay RT: Inhibition of NF-kappa b DNA binding by nitric oxide. Nucleic Acids Res. 1996, 24: 2236-2242. 10.1093/nar/24.12.2236.
Sarady JK, Otterbein SL, Liu F, Otterbein LE, Choi AM: Carbon monoxide modulates endotoxin-induced production of granulocyte macrophage colony-stimulating factor in macrophages. Am J Respir Cell Mol Biol. 2002, 27: 739-745.
Zhang H, Zhi L, Moochhala S, Moore PK, Bhatia M: Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2007, 292 (4): L960-L971. 10.1152/ajplung.00388.2006.
Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, Intaglietta M, Heyde van der HC: Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med. 2006, 12: 1417-2422. 10.1038/nm1499.
Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, Chora A, Rodrigues CD, Gregoire IP, Cunha Rodrigues M, Portugal S, Soares MP, Mota MM: Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med. 2007, 13: 703-710. 10.1038/nm1586.
Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto Tellez M, Moore PK: Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005, 19: 1196-1198.
Zhang H, Zhi L, Moore PK, Bhatia M: Role of hydrogen sulfide in cecal ligation and puncture-induced sepsis in the mouse. Am J Physiol Lung Cell Mol Physiol. 2006, 290: L1193-L1201. 10.1152/ajplung.00489.2005.
McCall CE, Yoza BK: Gene silencing in severe systemic inflammation. Am J Respir Crit Care Med. 2007, 175: 763-767. 10.1164/rccm.200610-1436CP.
Tumpey TM, Lu X, Morken T, Zaki SR, Katz JM: Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J Virol. 2000, 74: 6105-6116. 10.1128/JVI.74.13.6105-6116.2000.
Voller A, Gall D, Manawadu BR: Depression of the antibody response to tetanus toxoid in mice infected with malaria parasites. Z Tropenmed Parasitol. 1972, 23: 152-155.
Mabbott NA, Coulson PS, Smythies LE, Wilson RA, Sternberg JM: African trypanosome infections in mice that lack the interferon-gamma receptor gene: nitric oxide-dependent and -independent suppression of T-cell proliferative responses and the development of anaemia. Immunol. 1998, 94: 476-480. 10.1046/j.1365-2567.1998.00541.x.
Weighardt H, Heidecke CD, Emmanuilidis K, Maier S, Bartels H, Siewert JR, Holzmann B: Sepsis after major visceral surgery is associated with sustained and interferon-gamma-resistant defects of monocyte cytokine production. Surgery. 2000, 127: 309-315. 10.1067/msy.2000.104118.
Kantzler GB, Lauterier SF, Cusumano CL, Lee JD, Ganguly R, Waldman RH: Immunosuppression during influenza virus infection. Infect Immun. 1974, 10: 996-1002.
Zellweger R, Ayala A, Demaso CM, Chaudry IH: Trauma-hemorrhage causes prolonged depression in cellular immunity. Shock. 1995, 4: 149-153.
Pietsch JB, Meakins JL, MacLean LD: The delayed hypersensitivity response: application in clinical surgery. Surgery. 1977, 82: 349-355.
Volk HD, Reinke P, Krausch D, Zuckermann H, Asadullah K, Muller JM, Docke WD, Kox WJ: Monocyte deactivation – rationale for a new therapeutic strategy in sepsis. Intens Care Med. 1996, 22: S474-S481. 10.1007/BF01743727.
Robertson CM, Coopersmith CM: The systemic inflammatory response syndrome. Microbes Infect. 2006, 8: 1382-1389. 10.1016/j.micinf.2005.12.016.
Bone RC: Immunologic dissonance – a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS). Ann Intern Med. 1996, 125: 680-687.
Hotchkiss RS, Karl IE: Medical progress: The pathophysiology and treatment of sepsis. N Engl J Med. 2003, 348: 138-150. 10.1056/NEJMra021333.
Cabioglu N, Bilgic S, Deniz G, Aktas E, Seyhun Y, Turna A, Gunay K, Esen F: Decreased cytokine expression in peripheral blood leukocytes of patients with severe sepsis. Arch Surg. 2002, 137: 1037-1043. 10.1001/archsurg.137.9.1037.
Pellegrini JD, De AK, Kodys K, Puyana JC, Furse RK, Miller-Graziano C: Relationships between T lymphocyte apoptosis and anergy following trauma. J Surg Res. 2000, 88: 200-206. 10.1006/jsre.1999.5797.
Rubenstein M, Mulholland JH, Jeffery GM, Wolff SM: Malaria-induced endotoxin tolerance. Proc Soc Exp Biol Med. 1965, 118: 283-287.
Sinton JA: Immunity or tolerance in malarial infections. Proc R Soc Med. 1938, 31: 1298-1302.
Zweifach BW, Thomas L: The relationship between the vascular manifestations of shock produced by endotoxin, trauma, and hemorrhage. I. Certain similarities between the reactions in normal and endotoxin-tolerant rats. J Exp Med. 1957, 106: 385-401. 10.1084/jem.106.3.385.
Adib-Conquy M, Adrie C, Moine P, Asehnoune K, Fitting C, Pinsky MR, Dhainaut JF, Cavaillon JM: NF-kappaB expression in mononuclear cells of patients with sepsis resembles that observed in lipopolysaccharide tolerance. Am J Respir Crit Care Med. 2000, 162: 1877-1883.
Astiz M, Saha D, Lustbader D, Lin R, Rackow E: Monocyte response to bacterial toxins, expression of cell surface receptors, and release of anti-inflammatory cytokines during sepsis. J Lab Clin Med. 1996, 128: 594-600. 10.1016/S0022-2143(96)90132-8.
Liu J, Malkani G, Shi X, Meyer M, Cunningham Runddles S, Ma X, Sun ZS: The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immun. 2006, 74: 4750-4756. 10.1128/IAI.00287-06.
Franklin AE, Engeland CG, Kavaliers M, Ossenkopp KP: The rate of behavioral tolerance development to repeated lipopolysaccharide treatments depends upon the time of injection during the light-dark cycle: a multivariable examination of locomotor activity. Behav Brain Res. 2007, 180: 161-173. 10.1016/j.bbr.2007.03.021.
Yoza BK, Hu JY, Cousart SL, Forrest LM, McCall CE: Induction of RelB participates in endotoxin tolerance. J Immunol. 2006, 177: 4080-4085.
Gazzar ME, Yoza BK, Hu JY, Cousart SL, McCall CE: Epigenetic silencing of tumor necrosis factor during endotoxin tolerance. J Biol Chem. 2007, 282: 26857-26864. 10.1074/jbc.M704584200.
Lachner M, Jenuwein T: The many faces of histone lysine methylation. Curr Opin Cell Biol. 2002, 14: 286-298. 10.1016/S0955-0674(02)00335-6.
Clark IA, Chaudhri G: Tumour necrosis factor may contribute to the anaemia of malaria by causing dyserythropoiesis and erythrophagocytosis. Br J Haematol. 1988, 70: 99-103. 10.1111/j.1365-2141.1988.tb02440.x.
Helleberg M, Goka BQ, Akanmori BD, Obeng Adjei G, Rodriques O, Kurtzhals JA: Bone marrow suppression and severe anaemia associated with persistent Plasmodium falciparum infection in African children with microscopically undetectable parasitaemia. Malaria J. 2005, 4: 56-10.1186/1475-2875-4-56.
Igbokwe IO: Dyserythropoiesis in animal trypanosomosis. Rev Elev Med Vet Pays Trop. 1989, 42: 423-429.
Kovelenov A, Lobzin Iu V, Mikhal'tsov AN, Malkov AN: Clinical and laboratory features of severe forms of acute viral hepatitis B. Ter Arkh. 2003, 75: 17-23.
Carpenter SL, Zimmerman SA, Ware RE: Acute parvovirus B19 infection mimicking congenital dyserythropoietic anemia. J Pediatr Hematol Oncol. 2004, 26: 133-135. 10.1097/00043426-200402000-00017.
Lu PL, Hsiao HH, Tsai JJ, Chen TC, Feng MC, Chen TP, Lin SF: Dengue virus-associated hemophagocytic syndrome and dyserythropoiesis. Kaohsiung J Med Sci. 2005, 21: 34-39.
Tsinkalovsky O, Smaaland R, Rosenlund B, Sothern RB, Hirt A, Steine S, Badiee A, Abrahamsen JF, Eiken HG, Laerum OD: Circadian variations in clock gene expression of human bone marrow CD34+ cells. J Biol Rhythms. 2007, 22: 140-150. 10.1177/0748730406299078.
Waruiru CM, Newton CRJC, Forster D, New L, Winstanley P, Mwangi I, Marsh V, Winstanley M, Snow RW, Marsh K: Epileptic seizures and malaria in Kenyan children. Trans R Soc Trop Med Hyg. 1996, 90: 152-155. 10.1016/S0035-9203(96)90120-0.
Teach SJ, Geil PA: Incidence of bacteremia, urinary tract infections, and unsuspected bacterial meningitis in children with febrile seizures. Pediatr Emerg Care. 1999, 15: 9-12. 10.1097/00006565-199902000-00003.
Mukhdomi GJ, Desai MH, Herndon DN: Seizure disorders in burned children: a retrospective review. Burns. 1996, 22: 316-319. 10.1016/0305-4179(95)00013-5.
Chung B, Wong V: Relationship between five common viruses and febrile seizure in children. Arch Dis Child. 2007, 92: 589-593. 10.1136/adc.2006.110221.
Cummins D, Bennett D, Fisher Hoch SP, Farrar B, Machin SJ, McCormick JB: Lassa fever encephalopathy: clinical and laboratory findings. J Trop Med Hyg. 1992, 95: 197-201.
Gachon F, Fonjallaz P, Damiola F, Gos P, Kodama T, Zakany J, Duboule D, Petit B, Tafti M, Schibler U: The loss of circadian PAR bZip transcription factors results in epilepsy. Genes Dev. 2004, 18: 1397-1412. 10.1101/gad.301404.
Poll van der T, Buller HR, Gate H, Wortel CH, Bauer KA, van Deventer SJH, Hack E, Sauerwein HP, Rosenberg RD, Gate JW: Activation of coagulation after administration of tumor necrosis factor to normal subjects. N Engl J Med. 1990, 322: 1622-1627.
Dennis LH, Eichelberger JW, Inman MM, Conrad ME: Depletion of coagulation factors in drug-resistant Plasmodium falciparum malaria. Blood. 1967, 29: 713-721.
Levi M, Vanderpoll T, Tencate H, Vandeventer SJH: The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Invest. 1997, 27: 3-9. 10.1046/j.1365-2362.1997.570614.x.
Suharti C, van Gorp ECM, Setiati TE, Dolmans WMV, Djokomoeljanto RJ, Hack CE, ten Cate H, Meer van der JWM: The role of cytokines in activation of coagulation and fibrinolysis in Dengue Shock Syndrome. Thromb Haemost. 2002, 87 (1): 42-46.
Mahanty S, Bray M: Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis. 2004, 4: 487-498. 10.1016/S1473-3099(04)01103-X.
Keller TT, Sluijs van der KF, de Kruif MD, Gerdes VE, Meijers JC, Florquin S, Poll van der T, van Gorp EC, Brandjes DP, Buller HR, Levi M: Effects on coagulation and fibrinolysis induced by influenza in mice with a reduced capacity to generate activated protein C and a deficiency in plasminogen activator inhibitor type 1. Circ Res. 2006, 99: 1261-1269. 10.1161/01.RES.0000250834.29108.1a.
Wang J, Yin L, Lazar MA: The orphan nuclear receptor Rev-erb alpha regulates circadian expression of plasminogen activator inhibitor type 1. J Biol Chem. 2006, 281: 33842-33848. 10.1074/jbc.M607873200.
Mesters RM, Florke N, Ostermann H, Kienast J: Increase of plasminogen activator inhibitor levels predicts outcome of leukocytopenic patients with sepsis. Thromb Haemost. 1996, 75: 902-907.
Graninger W, Thalhammer F, Hollenstein U, Zotter GM, Kremsner PG: Serum protein concentrations in Plasmodium falciparum malaria. Acta Trop. 1992, 52: 121-128. 10.1016/0001-706X(92)90027-U.
Alvarez C, Ramos A: Lipids, lipoproteins, and apoproteins in serum during infection. Clin Chem. 1986, 32: 142-145.
Khan M, Coovadia Y, Connolly C, Sturm AW: Risk factors predicting complications in blood culture-proven typhoid fever in adults. Scand J Infec Dis. 2000, 32: 201-205. 10.1080/003655400750045349.
Kim YO, Yoon SA, Ku YM, Yang CW, Kim YS, Kim SY, Choi EJ, Chang YS, Bang BK: Serum albumin level correlates with disease severity in patients with Hemorrhagic Fever with Renal Syndrome. J Korean Med Sci. 2003, 18: 696-700.
Rigau Perez JG: Clinical manifestations of dengue hemorrhagic fever in Puerto Rico, 1990–1991. Rev Panam Salud Publica. 1997, 1: 381-388.
Fisher-Hoch SP, Platt GS, Lloyd G, Simpson DI, Neild GH, Barrett AJ: Haematological and biochemical monitoring of Ebola infection in rhesus monkeys: implications for patient management. Lancet. 1983, 2: 1055-1058. 10.1016/S0140-6736(83)91041-3.
Acharya SK, Panda SK, Duphare H, Dasarathy S, Ramesh R, Jameel S, Nijhawan S, Irshad M, Tandon BN: Chronic hepatitis in a large Indian hospital. Natl Med J India. 1993, 6: 202-206.
Leong HN, Earnest A, Lim HH, Chin CF, Tan CS, Puhaindran ME, Tan AC, Chen MI, Leo YS: SARS in Singapore – predictors of disease severity. Ann Acad Med Singapore. 2006, 35: 326-331.
Tanir G, Taylan Ozkan A, Daglar E: Pediatric visceral leishmaniasis in Turkey. Pediatr Int. 2006, 48: 66-69. 10.1111/j.1442-200X.2006.02153.x.
Yukl RL, Bar Or D, Harris L, Shapiro H, Winkler JV: Low albumin level in the emergency department: a potential independent predictor of delayed mortality in blunt trauma. J Emerg Med. 2003, 25: 1-6. 10.1016/S0736-4679(03)00105-7.
Goldwasser P, Feldman J: Association of serum albumin and mortality risk. J Clin Epidemiol. 1997, 50: 693-703. 10.1016/S0895-4356(97)00015-2.
Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A, Harrell FE: The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991, 100: 1619-1636. 10.1378/chest.100.6.1619.
Cereghini S: Liver-enriched transcription factors and hepatocyte differentiation. FASEB J. 1996, 10: 267-282.
Perlmutter DH, Dinarello CA, Punsal PI, Colten HR: Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986, 78: 1349-1354. 10.1172/JCI112721.
Memon RA, Grunfeld C, Moser AH, Feingold KR: Tumor necrosis factor mediates the effects of endotoxin on cholesterol and triglyceride metabolism in mice. Endocrinol. 1993, 132: 2246-2253. 10.1210/en.132.5.2246.
Vega GL, Baxter CR: Tumor necrosis factor mediates hypertriglyceridemia during thermal injury in mice genetically susceptible to lipopolysaccharides. J Burn Care Rehabil. 1991, 12: 463-467. 10.1097/00004630-199109000-00013.
Parola P, Gazin P, Patella F, Badiaga S, Delmont J, Brouqui P: Hypertriglyceridemia as an indicator of the severity of falciparum malaria in returned travelers: a clinical retrospective study. Parasitol Res. 2004, 92: 464-466. 10.1007/s00436-003-1012-5.
Scholl RA, Lang CH, Bagby GJ: Hypertriglyceridemia and its relation to tissue lipoprotein lipase activity in endotoxemic, Escherichia coli bacteremic, and polymicrobial septic rats. J Surg Res. 1984, 37: 394-401. 10.1016/0022-4804(84)90205-1.
Raspe E, Duez H, Mansen A, Fontaine C, Fievet C, Fruchart JC, Vennstrom B, Staels B: Identification of Rev-erbalpha as a physiological repressor of apoC-III gene transcription. J Lipid Res. 2002, 43: 2172-2179. 10.1194/jlr.M200386-JLR200.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors declare that they have no competing interests.
Authors' contributions
All three authors contributed to wide-ranging discussions on the ideas contained in this manuscript. IC wrote the manuscript, to which LA and AB made invaluable suggestions.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Clark, I.A., Budd, A.C. & Alleva, L.M. Sickness behaviour pushed too far – the basis of the syndrome seen in severe protozoal, bacterial and viral diseases and post-trauma. Malar J 7, 208 (2008). https://doi.org/10.1186/1475-2875-7-208
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-7-208