Abstract
Background
Acute malaria has been associated with a decreased antibody response to tetanus and diphtheria toxoids, meningococcal, salmonella, and Hib vaccines. Interest in giving malaria drug therapy and prevention at the time of childhood immunizations has increased greatly following recent trials of intermittent preventive therapy during infancy (IPTi), stimulating this re-analysis of unpublished data. The effect of malaria chemoprophylaxis on vaccine response was studied following administration of measles vaccines and diphtheria-tetanus-whole cell pertussis (DTP) vaccines.
Methods
In 1975, six villages divided into two groups of children ≤74 months of age from Burkina Faso, were assigned to receive amodiaquine hydrochloride chemoprophylaxis (CH+) every two weeks for seven months or no chemoprophylaxis (CH-). After five months, children in each group received either one dose of measles or two doses of DTP vaccines.
Results
For recipients of the measles vaccine, the seroconversion rates in CH+ and CH- children, respectively, were 93% and 96% (P > 0.05). The seroresponse rates in CH+ and CH- children respectively, were 73% and 86% for diphtheria (P > 0.05) and 77% and 91% for tetanus toxoid (P > 0.05). In a subset analysis, in which only children who strictly adhered to chemoprophylaxis criteria were included, there were, likewise, no significant differences in seroconversion or seroresponse for measles, diphtheria, or tetanus vaccines (P > 0.05). While analysis for pertussis showed a 43% (CH+) and 67% (CH-) response (P < 0.05), analyses using logistic regression to control for sex, age, chemoprophylaxis, weight-for-height Z-score, and pre-vaccination geometric mean titer (GMT), demonstrated that chemoprophylaxis was not associated with a significantly different conversion rate following DTP and measles vaccines. Seven months of chemoprophylaxis decreased significantly the malaria IFA and ELISA GMTs in the CH+ group.
Conclusion
Malaria chemoprophylaxis prior to vaccination in malaria endemic settings did not improve or impair immunogenicity of DTP and measles vaccines. This is the first human study to look at the association between malaria chemoprophylaxis and the serologic response to whole-cell pertussis vaccine.
Similar content being viewed by others
Background
Malaria accounts for an estimated 1 to 3 million deaths each year, with the majority occurring in children under five years of age in sub-Saharan Africa [1]. Vaccine-preventable diseases cause an estimated 1 to 2 million deaths in African children [2]. The WHO's Expanded Program on Immunization (EPI) is targeted at malarious areas, emphasizing the need to understand the effect of malaria and antimalaria drug use on vaccine immunogenicity and efficacy. Accordingly, a study that began in 1975 has been fully analysed following great increasing recent interest in the important topic of malaria chemoprophylaxis and, in particular, intermittent preventive (malaria) therapy of infants (IPTi) [3–7].
Acute malaria has been associated with a decreased response to tetanus toxoids, and meningococcal polysaccharide, Hib conjugate, and whole cell vaccines for typhoid fever [8–10]. Asymptomatic parasitaemia has been associated with a decreased response to the newer acellular pertussis and meningococcal vaccines, suggesting a benefit from malaria prophylaxis prior to vaccination [11–13]. Other studies have shown that asymptomatic parasitaemia or anti-malarial drug administration does not inhibit vaccine response to various live, attenuated, whole-cell killed, and toxoid vaccines [4, 5, 14–20]. No human studies have looked at the association between parasitaemia and the serologic response to whole-cell pertussis vaccine, a product still used in many vaccination programmes, particularly in developing countries. Antimalarials may also depress vaccine response as illustrated by the immunodepressive effect of 4-aminoquinolones[13, 21–24].
The study aimed to determine the effect of malaria chemoprophylaxis on vaccine seroconversion or seroresponse to live, attenuated measles vaccine, diphtheria and tetanus toxoids and whole-cell pertussis (DTP) vaccines.
Methods
Study area and population
The study was conducted from May through December in 1975 in six villages; all were located in the Guinean savanna and were hyper- and holo-endemic for malaria, depending on transmission season [25]. Before the study began (February-March, during the low transmission season), a 52% Plasmodium falciparum parasitaemia prevalence was found in 150 children (25 per site) <6 years of age, with no major differences between the sites; during this pre-study investigation, antibodies to P. falciparum were detected by indirect haemagglutination (IHA) in 100 percent of children tested from five of the six villages (25 children per village). Burkinabe clinicians in the nearest dispensaries and hospitals stated that the study area was endemic for measles (cases and deaths occurred during the study), diphtheria, tetanus, and pertussis, but the incidence was unknown; routine data had not been collected from the study villages because the EPI had not yet begun [26]. Hence, previous vaccination of children was extremely unlikely and was confirmed by interrogation of individual families. There was no malaria prophylaxis; treatment for fevers and other illness was obtained from traditional healers and in dispensaries within five km of the villages. P. falciparum resistance to 4-aminoquinolones was unknown in the area. Exclusion criteria for participation in the study included acute or chronic severe illness and the presence of detectable pre-vaccination measles antibodies. Children were weighed with a calibrated hanging scale. The length of very young children was measured with them lying down on a calibrated board and, for older children, standing, according to Centers for Disease Control and Prevention (CDC) nutrition programme guidelines.
Study design
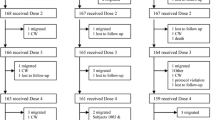
Children, aged 4 to 74 months (N = 996) living in the 6 malarious villages were assigned to 2 groups of 3 villages each, depending on whether they lived in villages east or west of Bobo-Dioulasso. One group received amodiaquine prophylaxis, CH+ [N = 488], and the other received no prophylaxis, CH- [N = 508]. Virtually all children within the same village received either measles [N = 482], or DTP vaccine [N = 514] (Figure 1). The study villages had differing populations of target-aged children. Hence, village and subject separation into measles versus DTP groups was based on the estimated number of children needed for each vaccine, and was dependent on the initial calculation of sample size (see Statistical Analysis). There was no blinding of study participants or researchers.
Malaria chemoprophylaxis and response to childhood vaccinations: flow chart of study design. Number of subjects excluded from analysis due to moving, death or lack of available sera: 1. 4 (37 excluded due to detectable pre-vaccination measles antibody titers) 2. 163 3. 177 4. 162 5. 70 6. 66 7. 36 8. 31 (68 excluded due to detectable pre-vaccination measles antibody titers, 18 excluded due to receipt of some prophylaxis)
The populations were of a related Mandinke ethnic group (Bobo in the eastern and Senufo in the western villages) and the communities had similar Anopheles gambiae ecology. Villages were chosen, based on the level of endemicity of malaria, the immunologic naivete of those receiving immunizations, and their proximity to one another; villages were spaced far enough apart so that families in CH- villages did not know that children in CH+ villages were receiving chemoprophylaxis, yet were close enough (within 75 km of Bob-Dioulasso) for travel convenience and management by the research team.
Chemoprophylaxis
CH+ children received a single prophylactic dose of amodiaquine hydrochloride suspension or tablets every 2 weeks for 7 months beginning in May and June, the start of the transmission season; those <12 months of age received 100 mg, those 12 to 47 months received 200 mg, and those 48 to 73 months old received 300 mg. Both amodiaquine and chloroquine are 4-aminoquinolines. Amodiaquine is the prodrug for the active ingredient desethylamodiaquine (DAQ). DAQ has a terminal half life of one to three weeks and is schizonticidal in very low concentrations [27, 28]. While amodiaquine lost favour because of its association with agranulocytosis, the drug is now being re-evaluated. Because amodiaquine has not been used for over 2 decades, it has somewhat greater efficacy than chloroquine for P. falciparum resistant to chloroquine [29, 30].
Seroconversion rates were measured in all CH+ and CH- children for whom paired sera were available. In addition, a CH+ group having strict compliance to drug ingestion was analyzed to examine more carefully the effect of malaria or drug use on serconversion and seroresponse. For this study, criteria for strict compliance included: receipt of ≥75% of the doses, no two consecutive doses missing, no doses missed in the month prior to vaccination(s), and no doses missed between the second vaccination and the last blood draw. At study completion, during the beginning of the low transmission season, families of children in the CH+ group were given a short-term supply of amodiaquine prophylaxis and instructions for home treatment in case of a rebound malaria attack. Follow-up visits occurred every one to two months in all villages for six months after the study.
Vaccination
All children were vaccinated with either measles vaccine or the first dose of DTP at month 5 (October or November, peak malaria transmission) and the second DTP dose one month later. Two doses of DTP were given during the study (rather than the standard initial series of three doses) to increase the likelihood that any effect from chemoprophylaxis would be discerned; a third dose was given at the end of the study but bloods were not drawn after this third vaccination. Vaccinations and amodiaquine were administered on the same day. The manufacturer and source of the licensed vaccines used were the Dow, Lirugen (Schwarz strain) measles vaccine, Lot No. 185806 AA and Merrill-National DTP, adsorbed, USP, vaccine, filling number 1036 DM, bulk Lot No. 1832. Measles and DTP vaccines were injected intramuscularly (0.5 ml) via a hydraulic (pressurized), injection device, the Ped-O-Jet® injector. Following study completion, all children in participating villages received the vaccine that they did not receive during the study. Prior to use, the measles and DTP vaccines were tested for potency and met standard requirements.
Vaccine serology
Venous blood samples were kept refrigerated and within 1 to 3 days serum was separated and kept at -20°C prior to shipment to the CDC, Atlanta, Georgia, USA, in dry ice. Serologic testing for the measles vaccine was performed in 1977 at the then Virology Division, Bureau of Laboratories, CDC. Antibody response to the DTP vaccine was performed in 1977 at the then Division of Bacterial Products, Bureau of Biologics, Food and Drug Administration, in Bethesda, MD.
Measles antibody was assessed by haemagglutination inhibition [31–33]. Seroconversion was defined by a rise in titer to ≥1:20 from an initial titer of <1:10 (the lowest detectable titer). Diphtheria and tetanus antibody titers were measured by passive microhemagglutination, using tanned sheep red blood cells [34, 35]. Individuals showing a >4-fold rise in antibodies to uncoated sheep red blood cells were not included in the analysis. Pertussis titers were measured by microagglutination using killed cells of Bordetella pertussis strains 134 and 165 [36]. The titer is the log2 of the reciprocal of the highest final serum dilution resulting in detectable agglutination. When sufficient serum was available, the lowest final serum dilution tested was 1:8; by convention, samples negative at a 1:8 dilution were assigned the titer log2 = 2. If the serum sample volume was low, higher initial dilutions were used. When such sera were negative at the lowest dilution tested, the titer was reported as <lowest dilution tested. Because the actual end-point was not known, titers for these sera were not included in the calculation of geometric mean titer (GMT) or geometric mean fold rise in titer (GMR). For some individuals, it was possible to verify a ≥4-fold rise even if the actual endpoint was not known for both sera. A ≥4-fold rise for DTP antigens was considered positive. For DTP, in cases where the titer decreased from pre- to post-vaccination, a ΔGMT = 0 was used in the calculation of GMR.
Malaria serology
P. falciparum IgG antibodies were measured by the Immunofluorescence Assay (IFA) [37] and by the Enzyme-Linked Immunosorbent Assay (ELISA) [38] following collection of blood 7 months after the children were CH+ or CH- status.
Statistical Analysis
Sample size was determined initially by a method comparing two proportions. For measles, 215 subjects were required for each group in order to have 90% assurance of significant results to detect this 10% difference in response rates. Similarly, for DTP vaccines, assuming 70% seroconversion for the test group and 60% for the control group, 387 subjects were needed for each group. SAS software, version 9.00 (SAS Institute, USA), was used. Weight-for-height Z-score was calculated using an anthro-system (version 1.02, WHO-CDC, Switzerland).
The primary outcome was rate of seroconversion or seroresponse in CH+ and CH- individuals. As secondary outcomes, geometric mean titers (GMT) and mean fold rise in titer (GMR) were measured for measles, DTP, and malaria antibodies for CH+ and CH- individuals for the strict compliance group.
Study population characteristics at vaccination were compared for the CH+ and CH- children using the Chi-squared test and Student's pooled t-test. The Chi-squared test was used for comparison of seroconversion to measles vaccine and seroresponse to DTP vaccinations; the Student's pooled t-test was used for pre- and post-vaccination GMT and GMR. A univariate logistic analysis was performed to assess effects of sex, age (> or < 24 months), prophylaxis, weight-for-height Z-score (> or < median Z-score), and pre-vaccination GMT (> or < median GMT) on seroconversion or seroresponse. Multivariate logistic regression was performed on those factors found to be independently associated with seroresponse. Analyses for seroconversion, GMTs, GMRs, and logistic regression were adjusted for village effect.
Consent
The study protocol was approved by the Burkina Faso (Upper Volta) Ministry of Health and the Institutional Review Board at the CDC. Verbal permission for the study was obtained from the village chiefs, their "council of elders," and each participating family, as was the custom for working in Burkinabe villages.
Results
The groups were similar at baseline with regard to age, sex, and nutritional status, except for a slight excess of males in the CH- group for DTP (Table 1). Twenty percent of the children were <12 months of age (N = 202). Figure 1 shows the distribution flow of children receiving or not receiving chemoprophylaxis by vaccine type. Although the vaccine was administered as combined DTP, the number of children with serological data that were evaluated differed for the three DTP assays as defined above. The final number of children analysed reflects the availability of paired sera, or loss due to moves or death (Figure 1). When comparing the compliant CH+ children to the non-compliant CH+ children, there were no significant differences in sex, age, or weight-for-height Z-score for those children receiving measles vaccine (P = 0.33, 0.56, 0.52 respectively) or in sex for children receiving DTP (P = 0.22). For the DTP group, age and weight-for-height Z-score was significantly less in the noncompliant CH+ group (P < 0.01, P = 0.02, respectively).
Table 2 shows that seroconversion rates to measles vaccine and seroresponse to diphtheria and tetanus vaccines were not significantly different in the CH+ and CH- groups, both when all children were included and when non-compliant CH+ children were excluded from the analysis (P > 0.05). When all children were included in this analysis, there was a lower rate of seroconversion to diphtheria and tetanus in the CH+ group, but this difference was not statistically significant. Percent seroresponse to pertussis was greater in the CH- group (P < 0.01). In this cluster analysis, there was adjustment for the random effect of village. When the analysis was done without adjusting for the random effect of village, there was a significantly greater rate of seroconversion to diphtheria and tetanus in the CH- group. When non-compliant children were excluded, the difference was no longer significant for diphtheria and tetanus. Percent seroresponse to pertussis remained greater in the CH- group (P < 0.01).
For measles, pre-vaccination measles titers for all children were <1:10 (lowest detectable titer) (Figure 2); GMR was not significantly different in the CH+ vs. CH- group (P = 0.44). For all three antigens GMR was higher in the CH- group, but this difference was statistically significant only for pertussis (P < 0.01).
Pre and post-vaccination Geometric Mean Titers (GMTs) and Geometric Mean Fold Rise (GMR) for prophylaxis (CH+) and no prophylaxis (CH-) groups. P values listed correspond to the difference in GMR between the two groups. GMTs expressed as log2. Children included in the CH+ group met criteria for compliance for chemoprophylaxis. (A) Measles vaccine: GMR for CH+ and CH- groups does not differ significantly. (B) Diphtheria vaccine: GMR for CH+ and CH- groups does not differ significantly. (C) Tetanus vaccine: GMR for CH+ and CH- groups does not differ significantly. (D) Pertussis vaccine: GMR is significantly > for CH- group.
Multivariate logistic regression analysis indicated that for tetanus, a lower pre-vaccination GMT was positively associated with seroresponse (P = 0.02); for pertussis, a lower pre-vaccination GMT (P < 0.01) and younger age (P = 0.04) were positively associated with seroresponse. There was no significant difference in pre-vaccination pertussis titres between the CH+ and CH- group (P 0.22) when looking at all children regardless of compliance; however, when excluding non-compliant children, pre-vaccination pertussis titres were higher in CH+ children (P <0.01). While pre-vaccination pertussis titres were higher in compliant CH+ children (log2 of GMT = 6.19, N = 37) compared to non-compliant CH+ children (log2 of GMT = 5.80, N = 84), this difference was not significantly different (P = 0.80). Chemoprophylaxis was not associated with seroresponse for any of the vaccines. Thus, especially for pertussis, the lower vaccine response rate observed in the CH+ group appears to be due, in part, to the greater proportion of subjects with high pre-immunization titers.
Malaria antibody titers were significantly lower in the CH+ group compared to the CH- group following seven months of prophylaxis. GMTs for children receiving measles vaccine were: CH+, 196 (N = 128) vs. CH-, 1089 (N = 219) (P < 0.01) using IFA and CH+, 285 (N = 60) vs CH-, 1990 (N = 64) using ELISA (P < 0.01) and for children receiving DTP vaccine: CH+, 109 (N = 132) vs CH-, 178 (N = 178) (P < 0.01) using IFA and CH+, 86 (N = 63) vs CH-,153 (N = 30) (P = 0.01) using ELISA. Only 13 percent of all CH+ children with detectable malaria titers prior to chemoprophylaxis had undetectable titers post-chemoprophylaxis (N = 159). This indicates that chemoprophylaxis given to young children for five to seven months after previous exposure to malaria was not adequate to eliminate malaria antibodies.
No adverse events were recorded after chemoprophylaxis, blood sample collection, or vaccination other than a few children with 1–3 mm nodules on their arms after receiving the vaccine by injector and one child who developed a cellulitis where the venopuncture occurred: this child recovered with systemic antibiotic treatment.
Discussion
Proposed mechanisms for malaria-associated immunodepression include impaired macrophage function [39–41], altered cytokine production [39, 42], a depletion of T or B cells [43], impaired dendritic cells[42, 44, 45], elevated nitric oxide production [46] and elevated prostaglandin E during febrile malaria[47]. Clinical evidence includes an association of malaria with increased susceptibility to bacterial infections [48], reactivation of viral infections [49, 50], a low prevalence of autoimmune disease in endemic areas[51, 52], and reports of decreased responses to vaccinations.
In contrast to asymptomatic parasitaemia, acute malaria impairs vaccine response[8–10, 12, 17, 18, 20]. In vitro challenge studies in individuals with acute malaria have demonstrated a depression in the cellular immune response involving alterations in lymphoproliferation and cytokine responses [42, 53, 54]. The pyrogenic cytokine TNF-alpha is elevated in febrile malaria and may depress humoral immunity by impairing antigen handling by dendritic cells. T-cell levels, CD4 cells in particular, are depressed [55]. IL-1, in addition to TNF-alpha, is elevated in acute illnesses [56, 57]. Both promote increased T-cell adhesion to endothelium, which may lead to T-cell margination and sequestration and, thus, a decrease of functional T-cells [55]. CD4 cells secrete cytokines that activate CD8 cells, B-cells and macrophages. In acute malaria, a depression of CD4 cells leads to depressed cellular and humoral immunity, impairing vaccine response.
The absence of association between malaria chemoprophylaxis and vaccine response in this study is consistent with findings from other chemoprophylaxis studies in malarious areas involving children with asymptomatic parasitaemia [4, 5, 14, 16–18, 58]. No prior studies have published data on the association between chemoprophylaxis and pertussis (killed, whole-cell) vaccine response in humans.
The agglutination test remains the test of choice for whole-cell pertussis vaccines. Although the antigen-specific ELISA tests can amplify the information provided by the agglutination test, the agglutination test is the only one that has been shown clinically to correlate with vaccine efficacy of whole-cell pertussis vaccines and has been used in relatively recent trials [59, 60]. Although ELISA or cell-culture based methods are more widely used today than the passive haemagglutination method for tetanus and diphtheria antitoxins, passive haemagglutination remains acceptable for evaluations of immunized populations [61]. Had they been available, the newer serological tests for measles, including neutralization testing, would have provided greater insight regarding clinical protection from disease.
Results of this study indicated that for the pertussis component, children <24 months of age had a better seroresponse. Vaccinating children <24 months of age will more effectively target the population in greatest need. Pertussis is most serious for very young infants and because complications leading to hospitalization, pneumonias, and death occur most often in those <24 months of age, the recommended age for initiation of pertussis immunization is generally two to three months.
Three doses of DTP vaccine comprise the usual primary series;thus, it would have been useful to assess seroconversion after a third DTP dose in addition to the response following the second dose. Technical and logistical considerations precluded this; additionally, there was concern regarding the possibility of decreased compliance with a longer study, as well as the potential to minimize any differences in the effect of chemoprophylaxis on seroconversion.
Malaria serologies demonstrated a significant difference in GMTs between the two treatment groups at the time of vaccination, adding some assurance that chemoprophylaxis decreased asymptomatic parasitaemia. Despite assumed effective chemoprophylaxis for five to seven months, virtually all compliant children had malaria antibodies; this probably reflected a durable antibody response to infections acquired before the study began. While not the primary study objective, fever prevalence data and blood smear records would have provided valuable insight on malaria prevention in the chemoprophylaxis group. Given efforts to administer intermittent preventive therapy of infants (IPTi) in conjunction with the vaccines included in the EPI, additional prospective studies are needed to establish more firmly the effect of antimalarials on response to childhood vaccinations [6].
Conclusion
Malaria chemoprophylaxis does not appear to enhance nor impair the antibody response to DTP and measles vaccines. There have been several changes over the 30 years since the study completion, including development of falciparum malaria resistance to 4-aminoquinolones throughout Africa, and establishment of the EPI (1977) and the Roll Back Malaria Partnership (1998). The continuing development and deployment of new antimalarial drugs and childhood vaccines mandates that the possible immunologic and protective interrelationships of these new products be investigated. Studies are in progress by the IPTi Consortium to address these issues http://www.ipti-malaria.org [7].
Financial Support
The study was supported partly by a grant from the Malaria Unit at the World Health Organization. Amodiaquine was contributed by Warner Lambert Pharmaceuticals.
References
Breman JG, Ailio MS, Mills A: Conquering the intolerable burden of malaria: what's new, what's needed: a summary. American Journal of Tropical Medicine and Hygiene. 2004, 71(Suppl 2): 1-15.
Bryce J, Boschi-Pinto C, Shibuya K, Black RE: WHO estimates of the causes of death in children. Lancet. 2005, 365: 1147-1152. 10.1016/S0140-6736(05)71877-8.
Geerligs PD, Brabin BJ, Eggelte TA: Analysis of the effects of malaria chemoprophylaxis in children on haematological responses, morbidity and mortality. Bull World Health Organ. 2003, 81: 205-216.
Massaga JJ, Kitua AY, Lemnge MM, Akida JA, Malle LN, Ronn AM, Theander TG, Bygbjerg IC: Effect of intermittent treatment with amodiaquine on anaemia and malarial fevers in infants in Tanzania: a randomised placebo-controlled trial. Lancet. 2003, 361: 1853-1860. 10.1016/S0140-6736(03)13504-0.
Schellenberg D, Menendez C, Kahigwa E, Aponte J, Vidal J, Tanner M, Mshinda H, Alonso P: Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: a randomised, placebo-controlled trial. Lancet. 2001, 357: 1471-1477. 10.1016/S0140-6736(00)04643-2.
Rosen JR, Breman JG: Malaria Intermittent Preventive Treatment in Infants (IPTi), Chemoprophylaxis, and Childhood Vaccinations. Lancet. 2004, 363: 1386-1388. 10.1016/S0140-6736(04)16052-2.
Schellenberg D, Menendez C, Aponte JJ, Kahigwa E, Tanner M, Mshinda H, alonso P: Intermittent preventive antimalarial treatment for Tanzanian infants: follow-up to age 2 years of a randomized placebo-controlled trial. Lancet. 2005, 365: 1481-1483. 10.1016/S0140-6736(05)66418-5.
Greenwood BM, Bradley-Moore AM, Bryceson AD, Palit A: Immunosuppression in children with malaria. Lancet. 1972, 1: 169-172. 10.1016/S0140-6736(72)90569-7.
Williamson WA, Greenwood BM: Impairment of the immune response to vaccination after acute malaria. Lancet. 1978, 1: 1328-1329. 10.1016/S0140-6736(78)92403-0.
Usen S, Milligan P, Ethevenaux C, Greenwood B, Mulholland K: Effect of fever on the serum antibody response of Gambian children to Haemophilus influenzae type b conjugate vaccine. Pediatr Infect Dis J. 2000, 19: 444-449. 10.1097/00006454-200005000-00010.
Tarzaali A, Viens P, Quevillon M: Inhibition of the immune response to whooping cough and tetanus vaccines by malaria infection, and the effect of pertussis adjuvant. Am J Trop Med Hyg. 1977, 26: 520-524.
Greenwood BM, Bradley AK, Blakebrough IS, Whittle HC, Marshall TF, Gilles HM: The immune response to a meningococcal polysaccharide vaccine in an African village. Trans R Soc Trop Med Hyg. 1980, 74: 340-346. 10.1016/0035-9203(80)90095-4.
Simondon F, Preziosi MP, Pinchinat S, Yam A, Chabirand L, Wassilak S, Pines E, Trape JF, Salomon H, Hoffenbach A: Randomised study of the possible adjuvant effect of BCG vaccine on the immunogenicity of diphtheria-tetanus-acellular pertussis vaccine in Senegalese infants. Eur J Clin Microbiol Infect Dis. 1999, 18: 23-29. 10.1007/s100960050221.
Monjour L, Bourdillon F, Schlumberger M, Fayet MT, Michon C, Ballet JJ, Gouba E, Gentilini M: [Humoral and cellular immunity following antitetanus vaccination in malnourished and malaria-induced African children. 1. Study of the antitetanus antibody response]. Bull World Health Organ. 1982, 60: 589-596.
Monjour L, Palminteri R, Froment A, Renault T, Alfred C, Gentilini M, Gouba E: Is cell-mediated immune response related to nutritional state, but unaffected by concomitant malarial infection?. Ann Trop Med Parasitol. 1982, 76: 575-577.
Cenac A, Develoux M, Djibo A: Chloroquine treatment of malaria does not increase antibody response to measles vaccination. A controlled study of 580 rural children living in an endemic malaria area. Trans R Soc Trop Med Hyg. 1988, 82: 405-10.1016/0035-9203(88)90135-6.
Bradley-Moore AM, Greenwood BM, Bradley AK, Bartlett A, Bidwell DE, Voller A, Craske J, Kirkwood BR, Gilles HM: Malaria chemoprophylaxis with chloroquine in young Nigerian children. II. Effect on the immune response to vaccination. Ann Trop Med Parasitol. 1985, 79: 563-573.
Greenwood AM, Greenwood BM, Bradley AK: Enhancement of the immune response to meningococcal polysaccharide vaccine in a malaria endemic area by administration of chloroquine. Ann Trop Med Parasitol. 1981, 75: 261-263.
Spindel R, Baruzzi RG, Souza VA, Ferreira AW, Avila SL: Measles vaccine coverage and immune response in children of Caiabi and Metuktire Indian tribes living in malarial endemic area: Parque indigena do Xingu, Central Brazil. Trop Doct. 2001, 31: 142-144.
McGregor I, Barr M: Antibody response to tetanus toxoid innoculation in malarious and non-malarious Gambian children. Trans R Soc Trop Med Hyg. 1962, 56: 364-367. 10.1016/0035-9203(62)90005-6.
Kollaritsch H, Que JU, Kunz C, Wiedermann G, Herzog C, Cryz SJJ: Safety and immunogenicity of live oral cholera and typhoid vaccines administered alone or in combination with antimalarial drugs, oral polio vaccine, or yellow fever vaccine. J Infect Dis. 1997, 175: 871-875.
Fryauff DJ, Church LW, Richards AL, Widjaja H, Mouzin E, Ratiwayanto S, Hadiputranto H, Sutamihardja MA, Richie TL, Subianto B, Tjitra E, Hoffman SL: Lymphocyte response to tetanus toxoid among Indonesian men immunized with tetanus-diphtheria during extended chloroquine or primaquine prophylaxis. J Infect Dis. 1997, 176: 1644-1648.
Taylor DN, Wasi C, Bernard K: Chloroquine prophylaxis associated with a poor antibody response to human diploid cell rabies vaccine. Lancet. 1984, 1: 1405-10.1016/S0140-6736(84)91893-2.
Pappaioanou M, Fishbein DB, Dreesen DW, Schwartz IK, Campbell GH, Sumner JW, Patchen LC, Brown WJ: Antibody response to preexposure human diploid-cell rabies vaccine given concurrently with chloroquine. N Engl J Med. 1986, 314: 280-284.
Escodie A, Hamon J: Le paludisme en Afrique occidentale d'expression francaise. Med Trop. 1961, 21: 661-687.
www.who.int/vaccines/globalsummary/immunization/countryprofileresult.cfm: .
Krishna S, White NJ: Pharmacokinetics of quinine,chloroquine and amodiaquine. Clinical implications. Clinical Pharmacokinetics. 1996, 30: 263-269.
Winstanley PA, Simooya O, Kofi-Ekue JM, Oalker O, Salako LA, Edwards G, Orme ML, Breckenridge AM: The disposition of amodiaquine in Zambians and Nigerians with malaria. British Journal of Pharmacology. 1990, 29: 695-701.
Watkins WM, Sixsmith DG, Spencer HC, Boriga DA, Kariuki DM, Kipingor T, Koech DK: Effectiveness of amodiaquine as treatment for chloroquine-resistant Plasmodium falciparum infections in Kenya. Lancet. 1984, 1: 357-359. 10.1016/S0140-6736(84)90410-0.
Mutabingwa TK, Anthony D, Heller A, Hallett R, Ahmed J, Drakeley C, Greenwood B, Whitty CJM: Amodiaquine alone, amodiaquine + sulfadoxine-pyrimethamine, amodiaquine + artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomized effectiveness trial. Lancet. 2005, 365: 1474-1480. 10.1016/S0140-6736(05)66417-3.
Hierholzer JC, Suggs MT, Hall EC: Standardized viral hemagglutination and hemagglutination-inhibition tests: II. Description and statistical evaluation. Applied Microbiology. 1969, 18: 824-833.
Hierholzer JC, Suggs MT: Standardized viral hemagglutination and hemagglutination-inhibition tests: I. Standardization of erythrocyte suspension. Applied Microbiology. 1969, 18: 816-823.
Wassilak SG, Bernier RH, Herrmann KL, Orenstein WA, Bart KJ, Amler R: Measles seroconfirmation using dried capillary blood specimens in filter paper. Pediatric Infectious Disease. 1984, 3: 117-121.
Boyden SV: The adsorption of proteins on erythrocytes treated with tannic acid and subsequent hemagglutination by antiprotein sera. J Exp Med. 1951, 93: 107-120. 10.1084/jem.93.2.107.
Stavitsky AB: Micromethods for the study of protein and antibodies. I. Procedure and general applications of hemagglutination and hemagglutination-inhibition reactions with tannic acid and protein-treated red blood cells. J Immunol. 1954, 72: 360-367.
Manclark CR: Serological Response to Bordetella pertussis. Manual of Clinical Immunology. 1976, Baltimore, The Williams and Wilkins Co., 312-314.
Sulzer AJ, Wilson M, Hall EC: Indirect fluorescent antibody test for parasitic diseases. V. An evaluation of a thick-smear antigen in the IFA test for malaria antibodies. Am J Trop Med Hyg. 1969, 18: 199-205.
Spencer HC, Collins WE, Chin W, Skinner JC: The Enzyme-linked immunosorbent assay (ELISA) for malaria I. The use of in vitro-cultured Plasmodium falciparum as antigen. Am J Trop Med Hyg. 1979, 28: 927-932.
Scorza T, Magez S, Brys L, De Baetselier P: Hemozoin is a key factor in the induction of malaria-associated immunosuppression. Parasite Immunol. 1999, 21: 545-554. 10.1046/j.1365-3024.1999.00254.x.
McBride JS, Micklem HS: Immunosuppression in murine malaria. II. The primary response to bovine serum albumin. Immunology. 1977, 33: 253-259.
Schwarzer E, Bellomo G, Giribaldi G, Ulliers D, Arese P: Phagocytosis of malarial pigment haemozoin by human monocytes: a confocal microscopy study. Parasitology. 2001, 123: 125-131. 10.1017/S0031182001008216.
Ocana-Morgner C, Mota MM, Rodriguez A: Malaria blood stage suppression of liver stage immunity by dendritic cells. J Exp Med. 2003, 197: 143-151. 10.1084/jem.20021072.
Ho M, Webster HK, Looareesuwan S, Supanaranond W, Phillips RE, Chanthavanich P, Warrell DA: Antigen-specific immunosuppression in human malaria due to Plasmodium falciparum. J Infect Dis. 1986, 153: 763-771.
Urban BC, Ferguson DJ, Pain A, Willcox N, Plebanski M, Austyn JM, Roberts DJ: Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999, 400: 73-77. 10.1038/21900.
Urban BC, Willcox N, Roberts DJ: A role for CD36 in the regulation of dendritic cell function. Proc Natl Acad Sci U S A. 2001, 98: 8750-8755. 10.1073/pnas.151028698.
Rockett KA, Awburn MM, Rockett EJ, Cowden WB, Clark IA: Possible role of nitric oxide in malarial immunosuppression. Parasite Immunol. 1994, 16: 243-249.
Snyder DS, Beller DI, Unanue ER: Prostaglandins modulate macrophage Ia expression. Nature. 1982, 299: 163-165. 10.1038/299163a0.
Mabey DC, Brown A, Greenwood BM: Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis. 1987, 155: 1319-1321.
Gunapala DE, Facer CA, Davidson R, Weir WR: In vitro analysis of Epstein-Barr virus: host balance in patients with acute Plasmodium falciparum malaria. I. Defective T-cell control. Parasitol Res. 1990, 76: 531-535. 10.1007/BF00931060.
Cook IF: Herpes zoster in children following malaria. J Trop Med Hyg. 1985, 88: 261-264.
Clark IA, al-Yaman FM, Cowden WB, Rockett KA: Does malarial tolerance, through nitric oxide, explain the low incidence of autoimmune disease in tropical Africa?. Lancet. 1996, 348: 1492-1494. 10.1016/S0140-6736(96)07342-4.
Adebajo AO: Low frequency of autoimmune disease in tropical Africa. Lancet. 1997, 349: 361-362. 10.1016/S0140-6736(05)62867-X.
Wangoo A, Ganguly NK, Mahajan RC: Immunosuppression in murine malaria: suppressor role of macrophages and their products during acute and chronic Plasmodium berghei infection. Apmis. 1990, 98: 407-414.
Plebanski M, Flanagan KL, Lee EA, Reece WH, Hart K, Gelder C, Gillespie G, Pinder M, Hill AV: Interleukin 10-mediated immunosuppression by a variant CD4 T cell epitope of Plasmodium falciparum. Immunity. 1999, 10: 651-660. 10.1016/S1074-7613(00)80064-3.
Feeney C, Bryzman S, Kong L, Brazil H, Deutsch R, Fritz LC: T-lymphocyte subsets in acute illness. Crit Care Med. 1995, 23: 1680-1685. 10.1097/00003246-199510000-00012.
Shimizu Y, Newman W, Gopal TV, Horgan KJ, Graber N, Beall LD, van Seventer GA, Shaw S: Four molecular pathways of T cell adhesion to endothelial cells: roles of LFA-1, VCAM-1, and ELAM-1 and changes in pathway hierarchy under different activation conditions. J Cell Biol. 1991, 113: 1203-1212. 10.1083/jcb.113.5.1203.
Tanaka Y, Adams DH, Hubscher S, Hirano H, Siebenlist U, Shaw S: T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993, 361: 79-82. 10.1038/361079a0.
Monjour L, Bourdillon F, Froment A, Daniel-Ribeiro C, Tirard S, Datry A, Chastang C, Tselentis Y, Gentilini M: [Measles vaccination in the Sudan-Sahel region of Africa. Absence of the immunodepressive effect of malaria]. Pathol Biol (Paris). 1985, 33: 232-235.
Meade BD, Lynn F, Reed GF, Mink CM, Romani TA, Deforest A, Deloria MA: Relationships between functional assays and enzyme immunoassays as measurements of responses to acellular and shole-cell pertussis vaccines. Pediatrics. 1995, 96: 595-600.
Vaccination against whooping cough: relation between protection in children and results for laboratory tests; a report to the Whooping-cough Immunization Committee of the Medical Research Council and to the medical officers of health for Cardiff, Leeds, Leyton, Manchester, Middlesex, Oxford, Poole, Tottenham, Walthamstow, and Wembley. British Medical Journal. 1956, 2: 454-651.
Durbaca S: Antidiphtheria and antitetanus immunity of recruits in Romania. Roumanian archives of microbiology and immunology. 1996, 55: 295-303.
Acknowledgements
We thank Kenneth Herrmann, Barbara Dove, John Robbins, James Nakano (deceased), Henry Mathews, Allen Hightower, Peggy Stanfil, Lois Norman (deceased), Cecile Viboud, Juan Arciniega, and Vicki Breman for their contributions and Cherice Holloway for manuscript assistance. We are deeply indebted to the children and the families who participated in the study.
Drs. Rosen and Breman are co-first authors. Dr. Breman was with the Centers for Disease Control and Prevention, and he and Dr. Saliou were assigned to the Centre Muraz, Organisation de Coordination et de Coopération pour la lutte contre les Grandes Endémies when this study was performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
Dr. Breman was responsible for writing the protocol, carrying out the study in the field, data analysis and writing; Dr. Rosen for synthesis, data analysis and writing; Dr. Manclark for coordinating DTP serologies and doing the pertussis antibody tests; Dr. Meade for editing, assisting with analysis and interpretation of serologic data; Dr. Collins for supervising the malaria antibody testing; Dr. Lobel for assisting with the protocol, field work and expedition of the serologic analyses; Dr. Saliou for participating in field work and manuscript analysis; Ms. Roberts for data registration and preliminary analysis; Dr. Campaoré for serving as the responsible health officer in Burkina Faso; and Dr. Miller for analysis and interpretation of results.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Rosen, J.B., Breman, J.G., Manclark, C.R. et al. Malaria chemoprophylaxis and the serologic response to measles and diphtheria-tetanus-whole-cell pertussis vaccines. Malar J 4, 53 (2005). https://doi.org/10.1186/1475-2875-4-53
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-4-53