Abstract
Background
Chloroquine (CQ) and primaquine (PQ) are still the drugs of choice to treat Plasmodium vivax malaria in many endemic areas, Brazil included. There is in vivo evidence for the P. vivax resistance to CQ in the Brazilian Amazon, where the increase in the proportion of P. vivax malaria parallels the increase of unusual clinical complications related to this species. In this study, in vitro CQ and mefloquine (MQ)-susceptibility of P. vivax isolates from the Western Brazilian Amazon was tested using the double-site enzyme-linked lactate dehydrogenase immunodetection (DELI) assay.
Methods
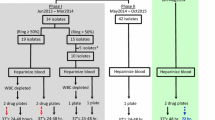
A total of 112 P. vivax isolates were tested in vitro for CQ-susceptibility and out of these 47 were also tested for MQ-susceptibility. The DELI assay was used to detect P. vivax growth at 48-hour short-term culture in isolates with ring stages ranging from 50 to %. Each isolate was tested in triplicate and geometric means of IC50’s was obtained. Nineteen isolates were genetically characterized for pvdhfr, pvmrp1, pvmdr1 and pvdhps candidate genes likely related to CQ resistance (10 with IC50<40 nM and 9 with IC50 >100 nM).
Results
Twelve out of 112 isolates were considered resistant to CQ, resulting in 10.7% (IC95% 5.0-16.4), while 3 out of 47 (6.4%; IC95% 0.0-12.8) were resistant to MQ. A discrete correlation was observed between IC50’s of CQ and MQ (Spearman=0.294; p=0.045). For pvdhps gene, a non-synonymous mutation was found at codon 382 (S→C) in 5/8 CQ-sensitive samples and 1/9 CQ-resistant samples (p=0.027). The other molecular markers were not associated to CQ-susceptibility.
Conclusions
In vitro CQ-resistance estimated in this study, estimated by the DELI test, was very similar to that observed in clinical trials, suggesting that in vitro procedures developed by capable local laboratories are useful in the surveillance of CQ-resistance in the Amazon; concurrent Amazon P. vivax strains with both CQ and MQ resistance may be common; and a non-synonymous mutation at pvdhps codon 382 (S→C) was associated to in vitro susceptibility to CQ, needing further studies to be confirmed.
Similar content being viewed by others
Background
Plasmodium vivax remains more widely distributed than Plasmodium falciparum and is a potential cause of morbidity and mortality amongst the 2.85 billion people living at risk of infection, the majority of whom are in the tropical belt of Central and Southeastern Asia and Latin America [1]. In Brazil, P. vivax is responsible for more than 80% of the cases in the last years [2]. In this country, the increase in the proportion of P. vivax malaria parallels the increase in the frequency of unusual clinical complications in P. vivax- infected patients [3]. Chloroquine (CQ) and primaquine (PQ) are still the drugs of choice to treat vivax malaria in many endemic areas, Brazil included. The simultaneous occurrence of severe vivax disease and CQ-resistance in some countries has raised the question of a possible association between severity and resistance [4]. CQ-resistance actually has been reported in Brazil almost at the same time as clinical severity [5].
There is in vivo evidence for the P. vivax CQ-resistance in the Brazilian Amazon. In this region, the resistance to CQ was firstly reported in Manaus in 1999 [5]. More recent data from studies conducted in the RAVREDA multicenter study (Amazonian Network for Surveillance for Resistance to Antimalarial Drugs) with a proper follow-up of patients exclusively using CQ and in whom drug plasma concentration was evaluated, seem to confirm such an observation [5]. However, since CQ plus PQ, the drug association used for the radical cure of P. vivax infection, has a synergistic action [6], further studies are needed to evaluate the contribution of CQ by itself for the effectiveness of the drug association, before any definitive conclusion can be proposed.
In vitro surveillance of anti-malarials resistance is largely used in P. falciparum with public health purposes, however P. vivax can grow only to a certain extent under in vitro conditions, with a very low reinvasion rate, what poses technical limitations to the routine use of this approach. The first in vitro P. vivax short-term culture was performed in 1974 [7], but more recent publications have presented reproducible results based on the WHO microtest using quantification of schizont maturation [8]. The method is cheap, however it is time-consuming since slides from the drug-free well have to be made every 6 hours trying to achieve the moment when there is schizonts maturation of 40%. Even demanding monoclonal antibodies, what makes it more expensive, the double-site Plasmodium lactate dehydrogenase (LDH) antigen capture enzyme-linked immunosorbent assay (DELI) [9] quantifies the total pLDH produced by maturing stages, and can be performed in 48 hours after the beginning of the culture, avoiding sequential slides reading. The aim of this study was to estimate the in vitro CQ-susceptibility of P. vivax isolates from the Western Brazilian Amazon using the DELI assay.
Methods
Site of study and patients
The study was performed from December 2007 to July 2008 in the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (FMT-HVD), which reports 30% of all the malaria cases in Manaus (03°06′S, 60°01′W). Patients living in the urban or peri-urban areas of this city with uncomplicated P. vivax malaria confirmed by a thick blood smear (TBS) were randomly selected in the outpatient clinics, from which epidemiological and clinical history was fully obtained. Parasite densities with differential counting of rings, trophozoites and schizonts were estimated by experienced microscopists, by counting the number of parasites in 200 leukocytes in high magnification fields, and the number of leukocytes/mm3. The study included patients of both sexes, aged 12–60 years, presenting blood parasite density from 250 to 100,000 parasites/mm3 and axillary temperature ≥ 37.5°C or history of fever in the last 48 hours. The major exclusion criterion was the use of anti-malarials in the previous 60 days. Due to the selective action of CQ upon young P. vivax trophozoites (rings) [10], only samples with ring stages ranging from 50-70% at the initial counting were analysed in this study.
In vitro determination of susceptibility to drugs
Blood samples were collected in on Vacutainer (Becton Dickinson®, Oxnard, CA) EDTA tubes prior to patient treatment. CQ sulfate and MQ hydrochloride were obtained from Sigma-Aldrich®. Isolates from the same outpatient clinics were randomly selected (47 were tested for CQ and MQ susceptibility and additional 65 samples were tested only for CQ). The blood samples were washed twice with a solution of RPMI-1640 Medium (Gibco®) and then one time with culture complete medium. The blood samples were then resuspended in the complete culture medium to obtain a 1.5% haematocrit. Finally, 200 μL of this suspension were distributed to each well in the anti-malarial pre-dosed plates. The plates were systematically incubated for 48h at 37°C (5% CO2, 5% O2 and 90% N2) and were immediately frozen at −20°C until the DELI test was performed with many samples at the same time. Only cultures with viable schizonts at 48 hours were considered valid. For the DELI test, plates were frozen and thawed three times to haemolyse the culture and release pLDH [9]. Each isolate was tested in triplicate and geometric means of IC50’s was obtained. IC50 threshold criterion for resistance to CQ or MQ was similar to that described elsewhere [9]. Dd2 and 3D7 P. falciparum clones were used as controls.
Molecular characterization
Nineteen isolates were genetically characterized for a few candidate genes linked to CQ resistance (10 with IC50<40 nM and 9 with IC50 >100 nM). Whole DNA extraction was carried out using a QIAamp® DNA Mini Kit (QIAGEN®, Germany) according to the manufacturer’s protocol. PCR primers and different reaction conditions used to amplify P. vivax dihydrofolate reductase (pvdhfr), multidrug resistance-associated protein 1 (pvmrp1), multidrug resistance 1 (pvmdr-1) locus, and dihydropteroate synthase (pvdhps) gene sequences were made as previously described [11–16] (Additional file 1). Briefly, after initial denaturing at 94°C for 2 minutes, the samples were submitted to 35 cycles (94°C for 1 minute more, 58°C for 30 seconds, and 72°C for 1 minute), with a final extension at 72°C for 10 minutes. For each fragment, PCR products were visualized by 1% agarose gel electrophoresis stained with ethidium bromide to confirm single band. DNA concentration was measured by NanoDrop® 2000 (Thermo Scientific®). Sequencing reactions were carried out using an ABI 3130xl genetic analyzer (Applied Biosystems®, USA) as specified by manufacturer’s protocol.
Data analysis
IC50 was calculated in the software Analysis of Malaria In Vitro Drug Sensitivity Data (HN-NonLin V 1.05 Beta®). Correlation between CQ and MQ IC50 was calculated through Pearson test. Fisher’s test was employed to evaluate the association between point mutations and CQ-resistance. Analysis was performed using SPSS software for Windows (version 16; SPSS, Inc., Chicago, IL®). P<0.05 was considered significant.
Ethical procedures
Collection of human samples and all study procedures were approved by the Ethical Review Committee of Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (protocol number 1850/2007). Written informed consent was obtained from all the participant patients. All malaria cases were treated according to the Brazilian Ministry of Health’s National guidelines, with CQ and PQ.
Results
Twelve out of 112 isolates were considered resistant to CQ, resulting in 10.7% (CI95% 5.0-16.4) of CQ-resistance, while three out of 47 (6.4%) (CI95% 0.0-12.8) were resistant to MQ (Figure 1). A discrete correlation was observed between IC50’s of CQ and MQ (Spearman=0.294; p=0.045) (Figure 2).
As seen in the Table 1, for pvdhps gene, a non-synonymous mutation at codon 382 (S→C) was found in 5/8 samples in vitro sensitive and 1/9 samples in vitro resistant to CQ (p=0.027). The other molecular markers were not associated to CQ susceptibility.
Discussion
Plasmodium vivax in vitro CQ-resistance levels in Latin America are unknown. In 2007, the proper 28 days follow-up of 109 patients with P. vivax prescribed only with CQ lead to the unexpected confirmation of 10.1% of resistance after plasmatic drug dosage [17], a rate very similar to that obtained from this study, suggesting that in vitro procedures developed by capable local laboratories are useful to the surveillance of CQ-resistance in the Amazon.
An interesting finding of this study was the concurrent resistance to both CQ and MQ within P. vivax strains from Amazon. In this region, a CQ-resistance rate of around 10% requires the evaluation of alternative drugs such MQ. Although MQ was thought to be a useful alternative treatment for P. vivax malaria in [18] and indirect evidence suggested that this drug may be efficacious against CQ-resistant P. vivax in Indonesian New Guinea [19], there is no previous information from Latin America supporting these data. In 1999, Alecrim et al.[5] reported a case of a patient with P. vivax malaria who showed resistance to chloroquine and mefloquine in the Brazilian Amazon region. CQ and MQ-resistance overlap have also had commonly reported from Thailand [20]. Nomura [21] pointed to different mechanisms of resistance between the two species, however some data including results presented here show that caution is required.
Among the isolates with IC50 defined, 19 have been genetically characterized (10 with IC50<40 nM and nine with IC50 >100 nM). For pvdhps gene, a non-synonymous mutation was found at codon 382 (S→C) in 5/8 samples in vitro sensitive and 1/9 samples in vitro resistant (p=0.027). It is known that the spread of sulphadoxine-pyrimethamine in P. falciparum exerted selective pressure for pvdhps mutations in P. vivax. Molecular studies on a population basis for P. falciparum have demonstrated that patients who were infected with parasites that carried this mutation may not respond to treatment [14], but when the parasite carries highly mutant versions of pvdhps and pvdhfr genes, clinical effectiveness is compromised [22]. Another study showed that resistance-conferring mutations were not found in pvdhps[23]. The other genetic markers were not associated with drug response. Suwanarusk et al.[24] studying the same gene, found that CQ IC50 was significantly higher in P. vivax isolates carrying the Y976F mutation than in isolates with the wild-type allele. Ranjitkar et al.[25] found that the low resistance to CQ was due to the low prevalence of mutation Y976F in pvmdr1 (5%). However, most studies did not find any association between mutation in the pvmdr-1 gene and CQ-resistance corroborating these results [16, 21, 26, 27].
Major limitations of this study were: (1) the small number of samples; (2) the lack a standard cut-off IC50 for the characterization of resistance in Latin American samples; (3) DELI test is a less time-consuming test due to the detection of pLDH in 48-hour culture plates, however it was never validated as compared to the microscopy test; (4) in vitro resistance was not individually compared to in vivo resistance. On the other hand, standard techniques are not easily reproducible among the different scenarios in endemic areas, due to personnel, reagents laboratory conditions. Data from the same laboratories over time is more reliable information and may be used as a baseline for future further studies using the same technique. This paper presents preliminary data on the in vitro P. vivax resistance in the Western Amazon. More information is needed regarding the usefulness of this approach in detecting in vivo resistance and the association of more severe cases.
Conclusion
In summary, this study has shown that: 1) in vitro CQ-resistance estimated in this study was very similar to that obtained from clinical trials in the same area [17], suggesting that in vitro procedures developed by capable local laboratories are useful in the surveillance of the CQ-resistance in the Amazon; 2) P. vivax strains simultaneous resistance to both CQ and MQ within in the Amazon needs to be clarified; and 3) a non-synonymous mutation at pvdhps gene codon 382 (S→C) was associated to in vitro susceptibility to CQ.
The high levels of anti-malarial drug resistance in this region call for reinforced surveillance of drug efficacy. Plasmodium vivax CQ-resistance may be rising in the Brazilian Amazon and probably this fact is contributing to spread vivax malaria and clinical severity of this disease. However, the public health urgency to detect and measure the progression of CQ-resistant vivax malaria has been neglected. To maintain an efficient malaria control programme, drug resistance surveillance assays must be conducted on a regular basis to assess anti-malarial efficacy and to ensure that the information is available to policy makers.
References
Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, Baird JK, Snow RW, Hay SI: The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010, 4: e774-10.1371/journal.pntd.0000774.
Oliveira-Ferreira J, Lacerda MV, Brasil P, Ladislau JL, Tauil PL, Daniel-Ribeiro CT: Malaria in Brazil: an overview. Malar J. 2010, 9: 115-10.1186/1475-2875-9-115.
Lacerda MV, Mourao MP, Alexandre MA, Siqueira AM, Magalhaes BM, Martinez-Espinosa FE, Filho FS, Brasil P, Ventura AM, Tada MS, Couto VS, Silva AR, Silva RS, Alecrim MG: Understanding the clinical spectrum of complicated Plasmodium vivax malaria: a systematic review on the contributions of the Brazilian literature. Malar J. 2012, 11: 12-10.1186/1475-2875-11-12.
Price RN, Douglas NM, Anstey NM: New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis. 2009, 22: 430-435. 10.1097/QCO.0b013e32832f14c1.
Alecrim MG, Alecrim W, Macedo V: Plasmodium vivax resistance to chloroquine (R2) and mefloquine (R3) in Brazilian Amazon region. Rev Soc Bras Med Trop. 1999, 32: 67-68.
Bray PG, Deed S, Fox E, Kalkanidis M, Mungthin M, Deady LW, Tilley L: Primaquine synergises the activity of chloroquine against chloroquine-resistant P. falciparum. Biochem Pharmacol. 2005, 70: 1158-1166. 10.1016/j.bcp.2005.07.021.
Powell RD, Berglund EM: Effects of chloroquine upon the maturation of asexual erythrocytic forms of Plasmodium vivax in vitro. Am J Trop Med Hyg. 1974, 23: 1007-1014.
Tasanor O, Noedl H, Na-Bangchang K, Congpuong K, Sirichaisinthop J, Wernsdorfer WH: An in vitro system for assessing the sensitivity of Plasmodium vivax to chloroquine. Acta Trop. 2002, 83: 49-61. 10.1016/S0001-706X(02)00056-6.
Druilhe P, Brasseur P, Blanc C, Makler M: Improved assessment of Plasmodium vivax response to antimalarial drugs by a colorimetric double-site plasmodium lactate dehydrogenase antigen capture enzyme-linked immunosorbent assay. Antimicrob Agents Chemother. 2007, 51: 2112-2116. 10.1128/AAC.01385-06.
Sharrock WW, Suwanarusk R, Lek-Uthai U, Edstein MD, Kosaisavee V, Travers T, Jaidee A, Sriprawat K, Price RN, Nosten F, Russell B: Plasmodium vivax trophozoites insensitive to chloroquine. Malar J. 2008, 7: 94-10.1186/1475-2875-7-94.
Barnadas C, Ratsimbasoa A, Tichit M, Bouchier C, Jahevitra M, Picot S, Menard D: Plasmodium vivax resistance to chloroquine in Madagascar: clinical efficacy and polymorphisms in pvmdr1 and pvcrt-o genes. Antimicrob Agents Chemother. 2008, 52: 4233-4240. 10.1128/AAC.00578-08.
Barnadas C, Musset L, Legrand E, Tichit M, Briolant S, Fusai T, Rogier C, Bouchier C, Picot S, Menard D: High prevalence and fixation of Plasmodium vivax dhfr/dhps mutations related to sulfadoxine/pyrimethamine resistance in French Guiana. Am J Trop Med Hyg. 2009, 81: 19-22.
de Pecoulas PE, Tahar R, Ouatas T, Mazabraud A, Basco LK: Sequence variations in the Plasmodium vivax dihydrofolate reductase-thymidylate synthase gene and their relationship with pyrimethamine resistance. Mol Biochem Parasitol. 1998, 92: 265-273. 10.1016/S0166-6851(97)00247-8.
Hawkins VN, Joshi H, Rungsihirunrat K, Na-Bangchang K, Sibley CH: Antifolates can have a role in the treatment of Plasmodium vivax. Trends Parasitol. 2007, 23: 213-222. 10.1016/j.pt.2007.03.002.
Raj DK, Mu J, Jiang H, Kabat J, Singh S, Sullivan M, Fay MP, McCutchan TF, Su XZ: Disruption of a Plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione. J Biol Chem. 2009, 284: 7687-7696. 10.1074/jbc.M806944200.
Orjuela-Sanchez P, de Santana Filho FS, Machado-Lima A, Chehuan YF, Costa MR, Alecrim MG, del Portillo HA: Analysis of single-nucleotide polymorphisms in the crt-o and mdr1 genes of Plasmodium vivax among chloroquine-resistant isolates from the Brazilian Amazon region. Antimicrob Agents Chemother. 2009, 53: 3561-3564. 10.1128/AAC.00004-09.
de Santana Filho FS, Arcanjo AR, Chehuan YM, Costa MR, Martinez-Espinosa FE, Vieira JL, Barbosa MG, Alecrim WD, Alecrim MG: Chloroquine-resistant Plasmodium vivax. Brazilian Amazon. Emerg Infect Dis. 2007, 13: 1125-1126. 10.3201/eid1307.061386.
Maguire JD, Krisin , Marwoto H, Richie TL, Fryauff DJ, Baird JK: Mefloquine is highly efficacious against chloroquine-resistant Plasmodium vivax malaria and Plasmodium falciparum malaria in Papua, Indonesia. Clin Infect Dis. 2006, 42: 1067-1072. 10.1086/501357.
Ohrt C, Richie TL, Widjaja H, Shanks GD, Fitriadi J, Fryauff DJ, Handschin J, Tang D, Sandjaja B, Tjitra E, Hadiarso L, Watt G, Wignall FS: Mefloquine compared with doxycycline for the prophylaxis of malaria in Indonesian soldiers. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1997, 126: 963-972. 10.7326/0003-4819-126-12-199706150-00006.
Karwacki JJ, Webster HK, Limsomwong N, Shanks GD: Two cases of mefloquine resistant malaria in Thailand. Trans R Soc Trop Med Hyg. 1989, 83: 152-153. 10.1016/0035-9203(89)90620-2.
Nomura T, Carlton JM, Baird JK, del Portillo HA, Fryauff DJ, Rathore D, Fidock DA, Su X, Collins WE, McCutchan TF, Wootton JC, Wellems TE: Evidence for different mechanisms of chloroquine resistance in 2 Plasmodium species that cause human malaria. J Infect Dis. 2001, 183: 1653-1661. 10.1086/320707.
Nzila AM, Mberu EK, Sulo J, Dayo H, Winstanley PA, Sibley CH, Watkins WM:Towards an understanding of the mechanism of pyrimethamine-sulfadoxine resistance inPlasmodium falciparum: genotyping of dihydrofolate reductase and dihydropteroate synthase of Kenyan parasites.Antimicrob Agents Chemother. 2000, 44: 991-996. 10.1128/AAC.44.4.991-996.2000.
Lu F, Wang B, Cao J, Sattabongkot J, Zhou H, Zhu G, Kim K, Gao Q, Han ET: Prevalence of drug resistance-associated gene mutations in Plasmodium vivax in Central China. Korean J Parasitol. 2012, 50: 379-384. 10.3347/kjp.2012.50.4.379.
Suwanarusk R, Russell B, Chavchich M, Chalfein F, Kenangalem E, Kosaisavee V, Prasetyorini B, Piera KA, Barends M, Brockman A, Lek-Uthai U, Anstey NM, Tjitra E, Nosten F, Cheng Q, Price RN: Chloroquine resistant Plasmodium vivax: in vitro characterisation and association with molecular polymorphisms. PLoS One. 2007, 2: e1089-10.1371/journal.pone.0001089.
Ranjitkar S, Schousboe ML, Thomsen TT, Adhikari M, Kapel CM, Bygbjerg IC, Alifrangis M: Prevalence of molecular markers of anti-malarial drug resistance in Plasmodium vivax and Plasmodium falciparum in two districts of Nepal. Malar J. 2011, 10: 75-10.1186/1475-2875-10-75.
Picot S, Brega S, Gerome P, Velut G, de Monbrison F, Cheminel V, Peyron F: Absence of nucleotide polymorphism in a Plasmodium vivax multidrug resistance gene after failure of mefloquine prophylaxis in French Guyana. Trans R Soc Trop Med Hyg. 2005, 99: 234-237. 10.1016/j.trstmh.2004.09.007.
Sa JM, Nomura T, Neves J, Baird JK, Wellems TE, del Portillo HA: Plasmodium vivax: allele variants of the mdr1 gene do not associate with chloroquine resistance among isolates from Brazil, Papua, and monkey-adapted strains. Exp Parasitol. 2005, 109: 256-259. 10.1016/j.exppara.2004.12.005.
Acknowledgements
We acknowledge the collaboration of the local laboratory workers Maria José Siqueira da Costa and Elizângela Celestino. This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant number 555666/2009-3) and by Fundação de Apoio à Pesquisa do Estado do Amazonas (FAPEAM) (grant number 1160/2010). MVGL has a Level 2 Productivity Fellowship from the National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YFCM, MRFC, JSC, FN, LWB and GCM participated in laboratory procedures. YFCM, MRFC, MGCA, HS, WMM and MVGL participated in overall study conception and design, data collection, analysis, interpretation and manuscript preparation. All authors read and approved the final manuscript.
Electronic supplementary material
12936_2013_2838_MOESM1_ESM.docx
Additional file 1: Oligonucleotide primers used for PCR amplification and DNA sequencing of Plasmodium vivax genes.(DOCX 34 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chehuan, Y.F., Costa, M.R., Costa, J.S. et al. In vitro chloroquine resistance for Plasmodium vivax isolates from the Western Brazilian Amazon. Malar J 12, 226 (2013). https://doi.org/10.1186/1475-2875-12-226
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-12-226