Abstract
Background
In 2005, the Ethiopian government launched a massive expansion of the malaria prevention and control programme. The programme was aimed mainly at the reduction of malaria in populations living below 2,000 m above sea level. Global warming has been implicated in the increase in the prevalence of malaria in the highlands. However, there is still a paucity of information on the occurrence of malaria at higher altitudes. The objective of this study was to estimate malaria prevalence in highland areas of south-central Ethiopia, designated as the Butajira area.
Methods
Using a multi-stage sampling technique, 750 households were selected. All consenting family members were examined for malaria parasites in thick and thin blood smears. The assessment was repeated six times for two years (October 2008 to June 2010).
Results
In total, 19,207 persons were examined in the six surveys. From those tested, 178 slides were positive for malaria, of which 154 (86.5%) were positive for Plasmodium vivax and 22 (12.4%) for Plasmodium falciparum; the remaining two (1.1%) showed mixed infections of Plasmodium falciparum and Plasmodium vivax. The incidence of malaria was higher after the main rainy season, both in lower lying and in highland areas. The incidence in the highlands was low and similar for all age groups, whereas in the lowlands, malaria occurred mostly in those of one to nine years of age.
Conclusion
This study documented a low prevalence of malaria that varied with season and altitudinal zone in a highland-fringe area of Ethiopia. Most of the malaria infections were attributable to Plasmodium vivax.
Similar content being viewed by others
Background
In most parts of Ethiopia, malaria is unstable and seasonal because of the altitude and climatic factors [1]. Areas at altitudes between 1,600 and 2,000 m above sea level (masl) are epidemic-prone hypoendemic zones of malaria [2]. Malaria epidemics are thought to occur among populations settling as high as 2,500 masl [3]. Thus, people in the Ethiopian highlands, which were considered previously to be malaria free, are at an increasing risk of malaria [4]. The prevalence of malaria has been found to vary among locations [1, 2], possibly because of the country's heterogeneous topography, and weather variables [5].
In Ethiopia, Plasmodium falciparum and Plasmodium vivax account for 60-70% and 30-40% of malaria cases, respectively [6]. Plasmodium falciparum has been the major cause of epidemics, and of most malaria deaths [7]. Anopheles arabiensis is the main vector of malaria, and it has a wide geographical distribution. Anopheles pharoensis, Anopheles funestus and Anopheles nili are secondary vectors in some areas [6]. Ethiopia's Ministry of Health has been successful in reducing malaria prevalence, and the number of villages affected by malaria epidemics decreased from 681 in 2004 to three in 2008 [8]. Unfortunately, malaria remains among the leading causes of outpatient visits and hospital admissions in the country [9].
There is a paucity of information on the prevalence of malaria in the Ethiopian highlands, but such information is considered vital to focus and improve malaria interventions. In addition, estimates of malaria prevalence could be used in the development of early-warning mechanisms for malaria. Previous malaria prevalence studies have been performed either at lower altitudes in areas of high transmission [10, 11], or in highland urban settings [12, 13]. Furthermore, all of them were conducted during seasons of peak malaria transmission. This study was designed to estimate the prevalence of malaria by taking temporal and spatial variation into account. Temporal variation of malaria was addressed by performing repeated cross-sectional surveys of malaria in three distinct seasons of each year for two consecutive years. To address the spatial variation, villages at different altitudes lying between 1,800 and 2,300 masl were selected randomly and investigated. This study provides information on the community-based prevalence of malaria in epidemic-prone highland areas in south-central Ethiopia using active survey data.
Methods
This study was part of the Ethiopian Malaria Prediction System, a multi-disciplinary research project designed to improve early warning systems for malaria in Ethiopia [14]. This study involved repeated population-based surveys to estimate the seasonal prevalence of Plasmodium vivax and Plasmodium falciparum.
Population-based surveys
The study area and population
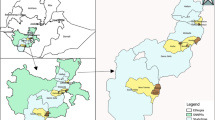
This study was conducted in six kebeles (the smallest administrative units) located about 130 km south of Addis Ababa in the Meskan and Mareko districts of the Gurage Zone in Ethiopia (Figure 1), which is designated as the Butajira area. These six kebeles are the location of the Demographic Surveillance Site (DSS) of the Butajira Rural Health Program (BRHP) [15].
There were 58,335 people living in the BRHP DSS in 2008. Half (50.1%, n = 29,243) of the population were female. Of the total population, 46% lived in the study area. The study area is part of an altitudinal transect between 1,800 and 2,300 masl. The mean annual rainfall in the study area during the last nine years was 945 mm (yearly range 510 mm to 1,329 mm). However, annual rainfall was below average in 2009 and 2010. The main rainy season usually occurs from June to September. The mean temperature was 18.2°C, with average annual minimum and maximum temperatures of 10.0°C and 26.3°C, respectively.
Most people in the area practise subsistence farming. The main cash crops are pepper at low altitude and khat (Catha edulis) in high altitude areas. The main staple food in the highlands is Enset (Ensete ventricosum), and in the lowlands maize, wheat, barley and Teff (Eragrostis tef) are consumed. Animal husbandry is also performed on the farms. The Odamo, Kelakel and Asass rivers flow through the area. The population usually seeks health services at the health posts found in each kebele, three health centres (two at low and one at high altitude) and two hospitals in the area.
Sample size calculation
Estimation of the sample size for the assessment of malaria prevalence was based on a 4.1% prevalence reported from three Ethiopian regions [16]. Employing assumptions of expected prevalence = 4%, margin of error = 1%, α = 5% (95% confidence level), design effect = 2 and 15% non-response rate, a sample size of 3,393 persons was calculated. Thus, with the assumption of an average family size of 4.5, a total of 3,393 people were recruited from 750 households. The sample size was calculated as n = (1.96)2 (0.04) (0.96)/0.01)2 = 1,475; total n = (1,472 × 2) + (1,475 × 2 × 0.15) = 2,950 + 443 = 3,393; and 3,393/4.5 = 750.
Survey design and sampling
The study used a community-based prospective design based on repeated cross-sectional surveys. The surveys used a multi-stage random sample design with kebeles as the first-stage unit, villages as the second, and households as the third stage [17].
Six rural kebeles were selected (Hobe, Bati Lejano, Dirama, Shershera Bido, Yeteker and Wurib), with two areas each representing low altitude (1,800-1,899 masl), mid-level (1,900- 1,999 masl) and high altitude (2,000-2,300 masl). The targeted households residing in those kebeles numbered 4,816. Sixteen villages with 750 households were selected randomly from the six kebeles, using probability proportion to size (PPS) sampling. Given that a lower prevalence of malaria was expected in the highlands, more villages and households were selected proportionally. Altitude readings for the households were recorded using a hand-held Global Positioning System (Garmin eTrex®).
Prospective data
Six cross-sectional surveys were performed on the same households for two consecutive years. The surveys were conducted in October-November 2008 (a month after the main rainy season), January-February 2009 (dry season), June-July 2009 (main rainy season), October- November 2009, January-February 2010, and June 2010. To ensure maximum participation, households with absentees were re-visited once.
Thin and thick blood smears were collected from all family members who consented to the study. Blood specimen processing, examination, and reporting of results were performed using standard guidelines [18]. In brief, the smears were air dried, placed in slide boxes, and examined by a trained microscopist at the field laboratory in Butajira. Thin films were fixed with methanol, and both thin and thick films were stained with 3% Giemsa stain for 30 min. Microscopic examination was done at 1,000× magnification. During the microscopic examination, a slide was regarded as negative after 100 fields had been examined without finding any parasites. Gametocytes were counted against 1,000 leukocytes.
To assure quality of the microscopic examinations, all positive and 10% of the negative slides were re-examined by a second microscopist. In the six surveys, seven of 2,094 slides (0.3%) showed discordant results. The agreement between the first two readers was good (kappa = 0.88). A third reader, blinded to the previous results, re-examined the seven discrepant slides (six vivax malaria and one negative).
Data management and statistical analysis
A training manual was prepared for the data collectors, who were trained in data collection. Data collection was performed using a pre-tested structured questionnaire and format prepared for this purpose. All data collectors were fluent in the local spoken languages. Data collection in the house-to-house survey was guided by DSS enumerators or the supervisors of each study kebele.
The questionnaire was translated from English to the local language (Amharic) and back to English to check for consistency. All family members who consented to participate in the study were included. The principal investigator and a supervisor from Butajira DSS performed the daily field supervision and cross-checking of the completed questionnaires.
Data entry and cleaning were performed using Epi Info version 6 (Centers for Disease Control and Prevention (CDC), Atlanta, Georgia (USA)). The data were analysed using SPSS version 18.0 (SPSS, Inc., 2009, Chicago). Descriptive statistics were applied to examine the characteristics of the sample; 95% confidence intervals were also calculated. Malaria prevalence was computed by dividing the number of people who showed infection with Plasmodia species by the total number of people examined from the study population. Households with missing values were not considered for analysis.
Ethical considerations
The study received ethical clearance from the Institutional Review Board of the College of Health Sciences of the Addis Ababa University, and from the Ministry of Science and Technology of Ethiopia. The local and regional health authorities were informed. Individual informed consent was obtained from adults, and from the parents or guardians of children aged less than 18 years. Blood specimens were collected aseptically using an alcohol swab and disposable blood lancets by trained staff. All people found to be malaria positive during the survey were treated directly according to the national guideline [19]. Febrile patients were screened for malaria parasites on the spot using malaria RDTs (CareStart®), and blood slides were also collected for the survey to investigate the nature of the malaria parasite using light microscopy. Malaria positive cases detected using malaria RDTs were treated immediately. Moreover, those found to be malaria positive on Giemsa-stained light microscopy were also treated. Plasmodium vivax positives were treated with chloroquine, 25 mg/kg for three days (10 mg base per kg on days 1 and 2, and 5 mg base per kg on day 3). Artemether-lumefantrine (20 mg artemether plus 120 mg lumefantrine in a fixed dose combination) was administered, based on body weight, two times a day for three days to Plasmodium falciparum-positive patients. Moreover, Coartem® and chloroquine stocks were kept at the health posts for treatment of cases that occurred between our repeated surveys.
Results
Characteristics of the study participants
In the six surveys, 19,207 blood slides were collected during house-to-house visits to 738 households in six kebeles, with an average of 3,780 persons included in each survey. This gave a response rate of 94.4% (n = 19,207/20,339). The remaining 5.6% (1,132 of 20,339) had missing data on different household variables and were not included in the analysis. The households for which data were excluded were not different from other households in any aspect. The average family size was 5.1 (range 1-12). The mean age was 20.5 years, and the median age was 15 years. Table 1 shows the age distribution and other study characteristics.
Community-based prevalence of malaria parasites
The overall prevalence of malaria was 0.93% [95% CI (0.79-1.07)] (178 malaria cases among 19,207 people). However, the prevalence varied among the villages, with the highest prevalence of 2.8% in Dadesso and Horosso villages (both below 1,850 masl), and the lowest prevalence (0.0%) at Sunke Wenz and Akababi village (2,100-2,180 masl) (data not presented). Malaria varied with altitude, with a prevalence of 1.91% [95% CI (1.55-2.27)] in low, 1.37% [95% CI (0.87-1.87)] in mid-level and 0.36% [95% CI (0.25-0.47)] in high altitude zones. The highest prevalence was found at low altitude between October and November 2009 (Table 2).
Two persons with P. vivax-positive slides, one from the lowlands and one from the highlands, reported that they had travelled outside their villages. Malaria infection varied among age groups, and in a different way at varying altitudes. At mid-level altitudes, malaria infection reached its peak in children aged one to four years, and at low altitude in children aged one to nine years. However, malaria prevalence at higher altitude was low and was similar across all age groups (Figure 2). Table 3 shows that Plasmodium falciparum malaria occurred rarely throughout the survey periods, with relatively more cases during the survey performed in October-November 2009 in the low altitude zone. Plasmodium vivax was found in all survey periods; however, the prevalence of Plasmodium vivax differed with respect to survey period and altitude (Table 4).
Discussion
This study shows that malaria occurs sporadically at high altitudes, and that the prevalence of malaria increases towards the lowlands. Malaria occurs throughout the year, but predominantly after the main rainy season. However, the prevalence varied greatly among villages at all altitudes. More children had malaria in the lowlands than in the highlands, which suggest that the highland population has lower immunity to malaria, as a result of limited prior exposure to the disease.
The strengths of the present study lay in the use of representative samples, the people investigated being residents of the study area, and the assessment of malaria in different seasons repeatedly for two consecutive years. Thus, this study provides an unbiased estimate of malaria prevalence in a localized area with increased epidemic risk.
The finding of malaria cases at high altitudes is consistent with a study performed in a nearby area [20]. However, that study may have overestimated the prevalence because it was performed only in the peak season for malaria transmission. Another study reported malaria infection at altitudes as high as 2,500 masl, and even extending above 3,000 masl [21]. However, the geographic origin of the cases was not well documented.
The low estimate for malaria prevalence obtained in this study is in agreement with another study carried out at a similar altitude in Ethiopia [22], and a previous estimate obtained in another region with unstable malaria transmission [23]. Areas of such low endemicity may be categorized as hypoendemic [24, 25], and they are considered to be vulnerable to epidemics.
The variability in the prevalence of malaria among villages is in agreement with previous studies in highland areas of Ethiopia [26], and studies performed in other regions with similar unstable malaria transmission [23, 27, 28]. This can be explained by the clustering of mosquitoes in villages near to mosquito breeding sites. Although this study lacks mosquito-sampling data, the influence of the clustering of mosquitoes in space on similar malaria case distributions is well documented elsewhere [29]. One study found a higher density of An. arabiensis in parts of a village with higher malaria prevalence near to Lake Ziway in Ethiopia [10]. Therefore, identification of areas in which malaria cases are clustered in space could be helpful in the design of focused interventions against malaria.
The present results showed the seasonality of malaria, and this is in agreement with other studies [20, 26]. The occurrence of malaria depends on adequate rainfall and temperature. In areas with a temperate climate, transmission of malaria is commonly limited to months in which the average temperature is above the minimum required for sporogony [24]. This explains the inverse relationship of decreasing malaria prevalence with increasing altitude. Temperature is a limiting factor for sporogony of the malaria parasite. A previous study demonstrated that an increase in altitude played a protective role against malaria infection [21]. The finding of malaria at high altitude should alert local health authorities to target malaria case detection and improve surveillance in highland areas, including those above 2,000 masl. The present finding of endemic malaria at altitudes as high as 2,200 masl during a non-epidemic year might be interpreted as the emergence of endemic malaria in the highlands. An increase in the daily minimum temperature of 0.4°C per decade has been recorded in the highlands of Ethiopia [30]. Presumably, this temperature rise has favoured the occurrence of malaria by keeping minimum temperatures in the highlands above the threshold for development of the malaria parasite in mosquitoes [24].
The finding of an increased proportion of vivax malaria is consistent with a study at high altitude in the Butajira area [20], and a study in the Akaki area [13]. Recent studies also show a shift from falciparum to vivax malaria [26, 31]. However, other studies report the consistent dominance of Plasmodium falciparum [32–34].
The increase in vivax malaria in highland-fringe areas could be explained by the high transmissibility of P. vivax, which is related to its typical biological features, including the immediate appearance of gametocytes, the presence of hypnozoites, and shorter sporogony [24, 25, 35]. A more than two-fold increase in the rate of drug resistance has been reported in south-central Ethiopia, close to the study area [36]. Thus chloroquine-resistant vivax malaria may have accounted for the dominance of vivax observed in this study. Some authors also speculate that the intensive malaria control efforts in Ethiopia, which have been implemented since 2005 [37], could explain the dominance of Plasmodium vivax [31].
References
Adhanom T, Deressa W, Witten KH, Getachew A, Seboxa T: Malaria. Epidemiology and ecology of health and disease in Ethiopia. Edited by: Berhane Y, Haile-Mariam D, Kloos H. 2006, Ethiopia, Addis Ababa: Shama Books, 556-576.
Abeku T, van Oortmarssen GJ, Borsboom G, de Vlas SJ, Habbema JDF: Spatial and temporal variations of malaria epidemic risk in Ethiopia: factors involved and implications. Acta Trop. 2003, 87: 331-340. 10.1016/S0001-706X(03)00123-2.
Negash K, Kebede A, Medhin A, Argaw D, Babaniyi O, Guintran JO, Delacollette C: Malaria epidemics in the highlands of Ethiopia. E Afr Med J. 2005, 82: 186-192.
Lindsay S, Martens WJM: Malaria in the African highlands: past, present and future. Bull World Health Organ. 1998, 76: 33-45.
Cheung W, Senay GB, Singh A: Trends and spatial distribution of annual and seasonal rainfall in Ethiopia. Int J Climatol. 2008, 10: 1002-
Tulu A: Malaria. The Ecology of Health and Disease in Ethiopia. Edited by: Ahmed Z, Kloos H. 1993, Colarado: Westview Press, 341-352.
Kiszewski A, Teklehaimanot A: A review of the clinical and epidemiologic burdens of epidemic malaria. AmJTrop Med Hyg. 2004, 71: 128-135.
Jima D, Getachew A, Bilak H, Steketee RW, Emerson PM, Graves PM, Gebre T, Reithinger R, Hwang J: Malaria indicator survey 2007, Ethiopia: coverage and use of major malaria prevention and control interventions. Malar J. 2010, 9: 58-10.1186/1475-2875-9-S2-P58.
Ministry of Health (MOH): Health and health-related indicators, 2008/2009. 2010, Addis Ababa, Ethiopia: Ministry of Health of Ethiopia
Abose T, Yeebiyo Y, Olana D, Alamirew D, Beyene Y, Regassa L, Mengesha A: Re-orientation and definition of the role of malaria vector control in Ethiopia Geneva. 1998, Switzerland: The World Health Organization
Yohannes M, Petros B: Urban malaria in Nazareth, Ethiopia: parasitological studies. Ethiop J Health Dev. 1996, 34: 83-91.
Tilaye T, Deressa W: Prevalence of urban malaria and associated factors in Gondar Town, Northwest Ethiopia. Ethiop Med J. 2007, 45: 151-158.
Woyessa A, Gebre-Michael T, Ali A: An indigenous malaria transmission in the outskirts of Addis Ababa, Akaki Town and its environs. Ethiop J Health Dev. 2004, 18: 2-7.
Ethiopian Malaria Prediction System (EMaPS). [http://emaps.uib.no].
Berhane Y, Wall S, Kebede D, Emmelin A, Enquselassie F, Byass P, Muhe L, Andersson T, Deyessa N, Gossaye Y, Högberg U, Alem A, Dahlblom K: Establishing an epidemiological field laboratory in rural areas: potentials for public health research and interventions. The Butajira rural health programme 1987-1999. Ethiop J Health Dev. 1999, 13: 1-47.
Shargie E, Gebre T, Ngondi J, Graves PM, Mosher AW, Emerson PM, Ejigsemahu Y, Endeshaw T, Olana D, WeldeMeskel A, Teferra A, Tadesse Z, Tilahun A, Yohannes G, Richard FO: Malaria prevalence and mosquito net coverage in Oromia and SNNPR regions of Ethiopia. BMC Publ Health. 2008, 8: 321-10.1186/1471-2458-8-321.
Kirkwood B, Sterne JA: Essential Medical Statistics. 2003, Massachusetts: Blackwell Science Ltd., 2
WHO: Basic Malaria Microscopy: Part I. 1991, Geneva, Switzerland: The World Heath Organization
Ministry of Health (MOH): Malaria diagnosis and treatment guidelines for health workers in Ethiopia. 2004, Addis Ababa: Federal Ministry of Health of Ethiopia
Tesfaye S, Belyhun Y, Teklu T, Mengesha T, Petros B: Malaria prevalence pattern observed in the highland fringe of Butajira, Southern Ethiopia: a longitudinal study from parasitological and entomological survey. Malar J. 2011, 10: 153-10.1186/1475-2875-10-153.
Graves P, Richards FO, Ngondi J, Emerson PM, Shargie EB, Endeshaw T, Ceccato P, Ejigsemahu Y, Mosher AW, Hailemariam A, Zerihun M, Teferi T, Ayele B, Mesele A, Yohannes G, Tilahun A, Gebre T: Individual, household and environmental risk factors for malaria infection in Amhara, Oromia and SNNP regions of Ethiopia. Trans R Soc Trop Med Hyg. 2009, 103: 1211-1220. 10.1016/j.trstmh.2008.11.016.
Ashton R, Kefyalew T, Tesfaye G, Pullan RL, Yadeta D, Reithinger R: School-based survey of malaria in Oromia Regional state, Ethiopia: a rapid survey method for malaria in low transmission settings. Malar J. 2011, 10: 25-10.1186/1475-2875-10-25.
Sintasath D, Ghebremeskel T, Lynch M, Kleinau E, Bretas G, Shililu J, Brantly E, Graves PM, Beier J: Malaria prevalence and associated risk factors in Eritrea. AmJTrop Med Hyg. 2005, 72: 682-687.
Molineaux L: The epidemiology of malaria as an explanation of its distribution, including some implications for its control. Malaria Principles and practice of malariology. Edited by: Wernsdorfer W. 1988, Great Britain: Churchill Livingstone Sir McGregor Eds, 913-998.
Snow R, Gilles HM: The epidemiology of malaria. Essential malariology. Edited by: Warrell D, Gilles H. 2002, London: Arnold, Fourth: 82-106.
Chala B, Petros B: Malaria in Finchaa sugar factory area in western Ethiopia: assessment of malaria as public health problem in Finchaa sugar factory based on clinical records and parasitological surveys, western Ethiopia. J Parasitol Vector Biology. 2011, 3: 52-58.
Brooker S, Clarke S, Njagi JK, Polack S, Mugo B, Estambale B, Muchiri E, Magnussen P, Cox J: Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Trop Med Int Health. 2004, 9: 757-766. 10.1111/j.1365-3156.2004.01272.x.
Munyekenye O, Githeko AK, Zhou G, Mushinzimana E, Minakawa N, Yan G: Plasmodium falciparum spatial analysis, Western Kenya Highlands. Emerg Infect Dis. 2005, 11: 1571-1577. 10.3201/eid1110.050106.
Ribeiro J, Seulu F, Abose T, Kidane G, Teklehaimanot A: Temporal and spatial distribution of anopheline mosquitos in an Ethiopian village: implications for malaria control strategies. Bull World Health Organ. 1996, 74: 299-305.
Conway D, Mould C, Bewket W: Over one century of rainfall and temperature observations in Addis Ababa, Ethiopia. Int J Climatol. 2004, 24: 77-91. 10.1002/joc.989.
Alemu A, Abebe G, Tsegaye W, Golassa L: Climatic variables and malaria transmission dynamics in Jimma town, South West Ethiopia. Parasit Vectors. 2011, 4: 30-10.1186/1756-3305-4-30.
Ghebreyesus T, Haile M, Witten KH, Getachew A, Yohannes M, Lindsay SW, Byass P: Household risk factors for malaria among children in the Ethiopian highlands. Trans R Soc Trop Med Hyg. 2000, 94: 17-21. 10.1016/S0035-9203(00)90424-3.
Karunamoorthi K, Bekele M: Prevalence of malaria from peripheral blood smears examination: A 1-year retorspective study from the Serbo Health Center, Kersa Woreda, Ethiopia. J Infect Public Health. 2009, 2: 171-176. 10.1016/j.jiph.2009.08.005.
Ramos J, Reyes F, Tesfamariam A: Change in epidemiology of malaria infections in a rural area in Ethiopia. J Travel Med. 2005, 12: 155-156.
Nájera JA, Kouznetzsov RL, Delacollette C: Malaria epidemics: detection and control, forecasting and prevention. 1998, Geneva, Switzerland: The World Health Organization
Ketema T, Getahun K, Bacha K: Therapeutic efficacy of chloroquine for treatment of Plasmodium vivax malaria cases in Halaba district, South Ethiopia. Parasit Vectors. 2011, 4: 46-10.1186/1756-3305-4-46.
Ministry of Health (MOH): National five year strategic plan for malaria prevention and control in Ethiopia: 2006-2010. 2006, Addis Ababa, Ethiopia: Ministry of Health of Ethiopia
Acknowledgements
The authors thank the study participants for their willingness to deliver the required information. Field staffs of the BRHP and local health personnel involved in the data collection are thanked for their strong commitment during the field activities. The authors are grateful for malaria microscopists who did the parasite examination. Mr Mulugeta Tadesse should be acknowledged for his supervisory role and facilitation of all field activities aspects. The Norwegian Programme for Development, Research and Education (NUFU) and University of Bergen, Norway funded this study as part of the Ethiopian Malaria Prediction System Project. The authors are grateful for funding the study. The authors also thank the National Malaria Control Unit of the Federal Ministry of Health of Ethiopia for giving us the Coartem® used for the treatment of malaria cases throughout the study period.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AW contributed to conception and design, acquisition of data, analysis and interpretation of data, and drafting the manuscript. WD contributed to conception and design of the study and reviewed the manuscript. AA contributed to conception and design of the study and reviewed the manuscript. BL contributed to conception and design, analysis and interpretation of data, and reviewed the manuscript. BL, AA, WD and AW reviewed the paper and all authors approved the final version.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Woyessa, A., Deressa, W., Ali, A. et al. Prevalence of malaria infection in Butajira area, south-central Ethiopia. Malar J 11, 84 (2012). https://doi.org/10.1186/1475-2875-11-84
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-11-84