Abstract
Background
Malaria is more common in pregnant than in non-pregnant Nigerian women, and is associated with small birth size and the attendant short- and long-term health risks. The influence of malaria on maternal metabolic status in pregnancy and in cord blood and how this relates to birth size has not been studied. The study objective was to define relationships between maternal and cord serum metabolic markers, maternal malaria status and birth size.
Methods
During pregnancy, anthropometric measurements, blood film for malaria parasites and assays for lipids, glucose, insulin and TNF were obtained from 467 mothers and these analytes and insulin-like growth factor-I (IGF-I) were obtained from cord blood of 187 babies.
Results
Overall prevalence of maternal malaria was 52%, associated with younger age, anaemia and smaller infant birth size. Mothers with malaria had significantly lower cholesterol (total, HDL and LDL) and higher TNF, but no difference in triglyceride. In contrast, there was no effect of maternal malaria on cord blood lipids, but the median (range) cord IGF-I was significantly lower in babies whose mothers had malaria: 60.4 (24,145)μg/L, versus no malaria: 76.5 (24, 150)μg/L, p = 0.03. On regression analysis, the key determinants of birth weight included maternal total cholesterol, malarial status and cord insulin and IGF-I.
Conclusions
Malaria in pregnancy was common and associated with reduced birth size, lower maternal lipids and higher TNF. In the setting of endemic malaria, maternal total cholesterol during pregnancy and cord blood insulin and IGF-I levels are potential biomarkers of foetal growth and birth size.
Similar content being viewed by others
Background
In Nigeria, increasing mortality from stroke and end-stage renal failure is associated with a high prevalence of hypertension, obesity, diabetes and high serum triglycerides especially in women [1]. Numerous studies have established associations between reduced birth weight and increased risk of coronary heart disease, diabetes, hypertension and stroke in adulthood [2, 3]. Malaria remains endemic in Nigeria and is more common among pregnant women, with prevalence ranging from 20% to 44%. It leads to significant consequences for maternal and infant health, such as maternal anemia, responsible for 11% of maternal deaths, and low birth weight (LBW), responsible for 5-12% of all LBW, 43% of preventable LBW babies and contributes to 75,000-200,000 infant deaths each year in Nigeria [4–9]. Therefore, malaria in pregnancy may contribute to later life morbidities in keeping with the 'developmental origins' hypothesis.
The biologic mechanisms underlying this hypothesis are poorly understood and mediators of the relationships between LBW, malaria in pregnancy and later cardiovascular and metabolic morbidity have not been clearly identified. Few studies have examined the relationships between maternal metabolic markers, foetal hormones and birth weight, nor has the effect of malaria in pregnancy on these relationships been explored. Maternal and cord blood levels of lipids, glucose and insulin and cord insulin-like growth factor-I (IGF-I) levels have been investigated as possible determinants of birth weight [10–12]. Maternal fasting triglyceride (TG) was independently associated with birth weight in non-diabetic women with maternal hyperglycaemia and with foetal growth and birth size in women with gestational diabetes [11, 13, 14]. Maternal plasma glucose was positively associated with birth weight in non-diabetic women [10, 15]. The relationship between birth weight and cord blood lipids was inconsistent [16, 17], but positive correlations between birth weight and cord blood glucose and insulin levels in both normal and low birth weight babies have been reported [18]. Cord blood IGF-I may also be involved in the control of foetal size during late gestation [19, 20]. Increased placental expression of cytokines such as tumour necrosis factor (TNF), interleukin 8, γ-interferon, IL-6 and IL-10 occurred in pregnancies affected by malaria, but only TNF has been linked to LBW [21, 22].
This study sought to explore the hypothesis that malaria parasitaemia in pregnancy would induce changes in maternal metabolic markers, which would be associated with reduced birth size. The relationships between maternal and cord blood metabolic profiles and birth size in the setting of endemic malaria was examined and potential pregnancy biomarkers of LBW were identified using an established cohort of mothers and infants born in Nigeria. [23]
Methods
Study site
The study was carried out in Yemetu-Adeoyo, a semi-urban community in Ibadan in south-west Nigeria where transmission of malaria is perennial. The hospital in this community, Adeoyo Maternity Hospital (AMH), is the oldest maternity hospital in Nigeria, dating from 1927. There are over 4,000 deliveries annually. Ethical approval for the study was obtained from the joint University of Ibadan/University College Hospital ethical committee and the University of Manchester Ethics committee.
Study procedures, follow-up, delivery and recruitment of babies
Healthy pregnant women aged 18-45 years presenting at AMH before 36 weeks' gestation and all babies born ≥ 37 weeks' gestation were eligible. Women who were HIV positive or had sexually transmitted infections at booking, those with pre-term deliveries, as well as those with multiple pregnancies or with chronic diseases, such as hypertension and diabetes were excluded. Babies with known syndromes, metabolic defects, major congenital abnormalities or severe birth trauma were also excluded.
Standard operating procedures (SOP) were developed and women were enrolled over one year to cover both wet and dry seasons. The study protocol and the rationale for the study were explained carefully in appropriate language, most commonly Yoruba or English, with questions answered as needed and written informed consent was obtained from all participants. After the delivery of their babies, another written informed consent was also obtained for the participation of the babies in the study.
Information on socio-demographic, obstetric, family, and health history including malarial frequency and use of anti-malarial drugs, was collected. All participants were issued prescriptions of sulphadoxine-pyrimethamine for intermittent preventive therapy (IPT) for malaria according to standard hospital practice.
Antenatal visits followed routine practice with frequency of attendance determined by gestational age. Standardized measures of anthropometry were carried out on all women at every visit until delivery. Maternal weight was measured to the nearest 0.1 kg on a SECA scale, and height on a stadiometer, both without shoes according to the SOP.
Visit schedule and blood measurements
At booking, 2 ml of blood was collected into an EDTA tube for full blood count and blood films for malaria parasites (MP) were prepared, stained with 3% Giemsa at pH 7.2. Repeat thick blood films for MP were obtained at every subsequent visit and at delivery. In addition, the placenta was weighed, turned to the maternal surface, cotyledons exposed and 1 ml of blood obtained from the inter-villous space for a placental malarial blood smear.
Blood films were examined for MP under light microscopy and recorded as negative only after 100 or more high-power microscope fields had been scanned. In those with malaria, parasite densities were determined by counting the number of parasites (np) among 200 leucocytes on the thick film using the following equation: Absolute parasite counts = (np x TLC)/200 where TLC = subject's total leucocyte count. For quality control, 30% of negative samples and 40% of positive samples were re-examined by two different, trained microscopists.
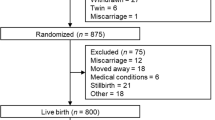
At the second antenatal visit, of 624 healthy pregnant women enrolled, 467 women gave their consent for fasting blood to be taken for biochemical markers while 396 of them had samples for malaria parasites. At delivery, 27 were excluded due to four maternal deaths (0.9%), 11 still births (2.4%), five miscarriages (1.1%) and seven neonatal deaths (1.5%), leaving 436 mother-baby pairs.
Cord blood was collected from the umbilical vein on the foetal surface of the placenta. Plasma was separated by centrifugation at 3,000 rpm and 4°C for 10 minutes and aliquoted into microtubes and stored at -80°C prior to lipid, glucose, insulin, TNF and IGF-I (cord blood only) assays. Cord glucose could not be measured immediately and so was not included in the assays.
Based on availability of complete maternal and cord blood samples, 187 mother-baby pairs were selected. There were no significant differences in the socio-demographic and clinical data of excluded women.
Definitions
Malaria was defined as the presence of asexual blood stages of Plasmodium falciparum in peripheral blood. There were two definitions of malaria:
-
a)
'Malaria at recruitment' = Malaria at second antenatal visit when blood for biochemical markers was also obtained. This was only used in analyses of effects on maternal biochemical markers at the same time-point. There were 72 blood smears positive for malaria parasites out of the 396 samples giving the prevalence of malaria as 18%.
-
b)
'Maternal malaria' = Malaria parasitaemia present at least once during pregnancy and/or at delivery (n = 211). These included (i) malaria parasitaemia in peripheral blood at least once during pregnancy (n = 138); and (ii) at delivery and/or in the placenta (n = 73). This was used in analyses of relationships with cord parameters and effects on birth indices.
Out of 187 mother-baby pairs with complete maternal and cord blood samples, 97 had malaria giving a prevalence of 52%.
Anaemia was defined as a packed cell volume (PCV) < 30%.
Biochemical assays
Total cholesterol (TC), High-density lipoprotein-cholesterol (HDL-C) and triglyceride concentrations were determined by using standard enzymatic procedures on an automatic analyser (COBAS MIRA/HITACHI 704 - Roche Diagnostics, Germany). The inter- and intra-assay coefficients of variation (CVs) for all parameters were < 5%. Low-density lipoprotein- cholesterol (LDL-C) was calculated using the Friedewald formula [24].
The normal values of lipids (mmol/L) in adult women are TC 3-5, HDL-C 1.2-2.2, LDL-C 2-3 and TG 0.6-1.68.
Maternal glucose was measured by the glucose oxidase method using a commercial kit (Randox, Crumlin, UK) on a YSI 2300 stat plus analyser (YSI, Farnborough, Hants, UK). The intra-assay CV was 1.5% at 4.1 mmol/L, and inter-assay CVs were 2.8% and 1.7% at 4.1 and 14.1 mmol/L respectively. Normal fasting glucose values are 3.9-6 mmol/L.
Insulin was measured by ELISA, using a commercial kit (Mercodia, Uppsala, Sweden). Assay sensitivity was 1 mU/L. Intra-assay CVs were 3.4% and 3.2% at 11 and 154 mU/L, and equivalent inter-assay CVs were 3.6% and 2.9%.
IGF-I and TNF were measured using Immulite 2000 assays (DPC, Lumigen Inc, Southfield, UK). Respective assay sensitivities were 25 μg/L and < 0.09 ng/L. Inter-assay CV values for IGF-1 at 48.9 and 158.5 μg/L were 7.6 and 9.2%. For TNF, intra-assay CVs were 6.7% and 5.3% at 6.3 and 19 ng/L, and the inter-assay CVs at 6.1 and 18.6 ng/L were 8.2 and 9.7% respectively.
Infant anthropometry
Babies were measured within 72 hours of birth. They were weighed naked to the nearest 0.1 kg and length measured on an infant stadiometer from crown to heel to the nearest 0.1 cm. Occipito-frontal circumference (OFC) was taken around the widest circumference of the head using a non-stretchable tape. Skinfold thicknesses (triceps, biceps, sub-scapular, and suprailiac) were measured using Holtain calipers on the left side to the nearest 0.1 mm. Measurements were obtained in duplicate or triplicate if disagreeing by > 15%.
Validity of anthropometric measurements
Based on the WHO Manual (1995), three nurses, already proficient in paediatric venipuncture, were trained in anthropometry methods. They carried out all measurements on the same equipment throughout the study. They also had three-monthly protocol-refresher training sessions and used training videos to minimize inter-observer and within-observer errors.
Statistical analysis
Data were analysed using SPSS version 14 (SPSS Inc, Chicago, IL). Results were expressed as mean (SD) or median (IQ range), using Student t/Mann Whitney and Chi square tests for associations between maternal and infant clinical characteristics and malaria. Levels of insulin, IGF-I and TNF were skewed and, therefore, tested non-parametrically, while lipids and glucose levels were normally distributed and tested parametrically. Correlations were assessed by Spearman's test. All tests were two-sided and p values < 0.05 were considered significant.
Simple linear regression was used to assess the determinants of infant size, indicating those with p < 0.001, p< 0.01 and < 0.05. Due to high co-linearity between biochemical parameters, a stepwise method of model selection was used to identify the key variables.
Results
The age and BMI of mothers were similar irrespective of trimester at recruitment. Mean (SD) gestational ages at booking and at delivery were 27.1 (5.1) and 39.3 (1.4) weeks respectively (Table 1).
Plasma levels of all lipids except HDL-C were significantly elevated in the third compared to the second trimester but fasting plasma glucose was reduced. Insulin and TNF levels were similar.
Effect of maternal malaria at recruitment on maternal characteristics and biochemical markers
The prevalence of malaria among the women at recruitment was 18%. Among all women recruited, 28% were primigravid, and this was associated with MP (28% vs 13% in the multigravid, p = 0.001). Primigravidity was associated with 2.5-fold increase in risk of having malaria [OR = 2.5, 95% CI, 1.5-4.2].
Most women were asymptomatic and only seven had fever. Malaria was also associated with younger maternal age (29.4 vs 27.7 years, p = 0.001) and lower packed cell volume [31.7 (4.7) vs 32.8 (3.3)%, p = 0.006].
Mothers with malaria had significantly lower TC, lower HDL-C, lower LDL-C, no change in TG but higher TNF in both the second and third trimesters (Table 2). There was no difference in glucose and insulin levels in the two trimesters with the presence of MP.
Effect of maternal malaria on newborn birth indices and cord blood biochemical markers
Prevalence of maternal malaria defined as malaria in pregnancy and/or at delivery overall was 52%. Mean birth weight, length, OFC, mid-upper arm circumference (MUAC) and biceps skinfold thicknesses of infants born to women with malaria were globally smaller than those of women without malaria. Mean birth weight was significantly lower by 160 gms (-5.3% compared to babies of mothers without malaria), biceps skinfold thickness by 1.5 mm (-4.1%) and MUAC by 1.8 mm (-1.8%), while birth length and OFC were lower by only 6.2 cm (-1.3%) and by 3.5 mm (-1%) respectively (Table 3). The placental weights of infants born to women with and without malaria were not significantly different but the placenta-foetal weight ratios for infants whose mothers have malaria were significantly lower than those whose mothers did not have malaria (p = 0.019) (Table 3).
Cord blood IGF-I levels were significantly lower in babies of mothers with malaria, with no differences in cord blood lipids, insulin and TNF (Table 4).
Associations of maternal and cord biochemical markers and maternal malaria with infant size
Maternal total cholesterol and LDL-C were significantly correlated with birth weight at p< 0.001 and insulin at p< 0.01 (Table 5), while maternal TG and glucose and cord blood insulin and IGF-I correlated with birth weight at p< 0.05 (Table 5).
To determine the overall independent effects on birth weight, three stepwise regression models were derived (Table 6):
1 In the first model, the influence of malaria at recruitment and all maternal biochemical markers on birth weight was evaluated. Maternal TC was a powerful, independent determinant of birth weight (p = 0.001), such that for every 0.15 mmol/L increase in TC, there was a 100 g increase in birth weight.
2 In the second model, the influence of maternal malaria through pregnancy and at delivery and all cord biochemical markers on birth weight was examined. Maternal malaria was the only significant determinant of birth weight (p = 0.007).
3 In the final model, maternal malaria, and maternal and cord biochemical markers that had shown significant correlations to birth weight were included. Maternal malaria with cord blood insulin and IGF-I were significantly related to birth weight.
Discussion
Effect of maternal malaria on maternal and cord blood biochemical markers
In agreement with other studies in various populations, including Nigerian pregnant women, this study showed an overall elevation in serum lipids, except HDL-C, during pregnancy. The elevation was highest in the third trimester [25–27]. Jimenez et al reported no significant changes in HDL-C during pregnancy while others report an elevation [28, 29]. These increases in TC and LDL-C and low to normal HDL-C are believed not to be atherogenic [25, 27] and are likely to be related to energy transfer to the foetus. The cord lipid profiles are similar to those in Manchester children from the hyperglycaemia and adverse pregnancy outcomes (HAPO) study [30] and to those in Ibadan about 30 years ago, but slightly lower than those reported in eastern Nigeria [17, 31, 32]. Cord HDL-C and TG levels are about half that of adults while TC and LDL-C are about one-third, hence HDL is the major lipid moiety [33, 34].
In these pregnant women, there is a marked cholesterol-lowering impact of malaria, including HDL and LDL, but triglyceride levels were unaffected. These findings corroborate older reports of lower TC and HDL associated with acute malaria but in that setting higher TG [35]. A study of lipid profiles associated with acute malaria in non-pregnant people has shown higher TG but no change in TC [36]. The mechanisms involved in changes in lipid profile associated with malaria are still unclear. In vitro experiments have shown a selective uptake of HDL-C by Plasmodium falciparum indicating that the HDL-C fraction appears to be a major lipid source for parasite growth [37] and increased intra-parasitic cholesterol and phospholipid levels [38]. P. falciparum is unable to synthesize lipids required during its erythrocytic cycle. Hence there is lipid transport through membrane flux into the parasite. Therefore, malaria in pregnancy results in demands for cholesterol from three sources including: the parasite for its growth and probably its attachment properties; the placenta for production of progesterone to maintain the pregnancy, and the fetus itself for growth [37, 38]. This combination may lead to low cholesterol levels resulting in prematurity and low birth weight babies.
Maternal plasma glucose and insulin were unaltered by malaria; the result contrasts with a previous study in pregnant women with acute malaria, who had hypoglycaemia associated with increased glucose turnover, attributed to enhanced pancreatic β-cell function [39]. Maternal TNF concentrations in the second trimester were nine-fold higher in those with malaria, but only doubled if malaria occurred in the third trimester. This higher TNF was found previously in Malawian and Kenyan pregnant women in peripheral and placental blood [21, 40]. However no associations between TNF and any of the other biochemical parameters or birth size were found.
In contrast this study indicated that malaria had a less pronounced effect on cord blood parameters: cord lipid profile, insulin and TNF levels were not altered, findings not previously described in an endemic malaria area. However cord IGF-I levels were lower.
Effect of maternal malarial on birth indices
In this study, malaria in pregnancy was associated with younger age, first pregnancy and maternal anaemia, as well as smaller, shorter, thinner babies with smaller head circumferences as previously reported [41, 42].
There was no significant difference between the placental weight in infants of mothers with malaria and those without as previously reported by Mcgregor et al [43]. The finding of lower placenta-foetal weight ratio in infants born to women with malaria parasitaemia confirms previous reports [44, 45]. Placental efficiency can be assessed using the placenta-foetal weight ratio and the lower ratio in babies of mothers with malaria suggests that their placentas have impaired function.
In babies of mothers with malaria, mean birth weight was lower by 5.3% compared to babies of mothers without malaria, biceps skinfold thickness by 4.1% and MUAC by 1.8%, while birth length and OFC were lower by only 1.3% and by 1% respectively (Table 3). This agrees with various studies on the impact of malaria in pregnancy on birth weight (more so than birth length) which showed that mean birth weights of babies born to mothers with malaria were lower by 105 g [46], 371 g [47], 382 g [48] and 461 g [49]. This reduced birth weight is probably due to placental insufficiency resulting from malaria, which leads to intrauterine growth restriction [45]. There are reports of higher positive rates of malaria parasite and/or malaria pigment in the placentae of LBW babies [45, 50, 51]. In pregnant women with malaria, it has been observed that there is accumulation of infected red blood cells and increased maternal phagocytic cells, especially monocytes in the maternal vascular area of the placenta (the intervillous space) to much higher densities than in the peripheral blood. In addition, there is placental sequestration of the trophozoite and schizont stages, which are absent from the circulation [52]. The inadequate uteroplacental and umbilical blood flow, and alterations in the transplacental transfer of glucose or production of foetal insulin result in LBW babies due to foetal growth restriction [53]. It has been suggested that disturbed placental folate metabolism, placental lactogen, IGF/somatomedin, and somatostatin-like substances, which influence foetal growth, may also play an important role [54].
Very few studies have assessed the impact of malaria in pregnancy on other growth parameters at birth apart from birth weight. In agreement with our findings, Kalanda et al documented significantly lower mean birth weight, length, head circumference and mid upper arm circumference in babies of mothers with malaria [53].
Determinants of birth size
Birth weight remains the single most important determinant of neonatal and infant survival and health. It is therefore important to understand factors that may influence birth weight. In this study, birth weight was strongly related to maternal total cholesterol, LDL-C, and less so to maternal triglyceride, glucose and insulin. This is in contrast to findings in Nigerian newborns about 15 years ago when maternal lipid samples were obtained at delivery but not during pregnancy [31, 33]. This study now indicates that variations in maternal cholesterol metabolism appear to have a significant effect on foetal growth during pregnancy and hence birth size. Maternal TG has a lesser impact, although in women with altered glucose tolerance, serum TG concentrations have been shown to predict birth weight [11, 13]. Additionally maternal glucose and insulin have a less important impact on foetal growth. In cord blood, insulin and IGF-I were related to birth weight. Both insulin and IGF-I are major growth factors in foetal life as shown by monogenic disorders that affect foetal insulin secretion [55] and IGF-I generation [56–58].
The regression models highlight the most significant factors that determine birth weight, including maternal total cholesterol, malaria status and cord insulin and IGF-I levels. These findings are corroborated by a recent report that showed that maternal and cord IGF-I were reduced in women with placental malaria compared to those without [59].
Conclusions
In the setting of endemic malaria, maternal total cholesterol during pregnancy and cord blood insulin and IGF-I levels are important markers of foetal growth restriction and reduced birth size likely mediated through placental insufficiency.
References
Opadijo OG, Akande AA, Jimoh AK: Prevalence of coronary heart disease risk factors in Nigerians with systemic hypertension. Afr J Med Med Sci. 2004, 33: 121-125.
Levy-Marchal C, Jaquet D: Long-term metabolic consequences of being born small for gestational age. Pediatr Diabetes. 2004, 5: 147-153. 10.1111/j.1399-543X.2004.00057.x.
Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D: Fetal and childhood growth and hypertension in adult life. Hypertension. 2000, 36: 790-794.
Akanbi OM, Odaibo AB, Ademowo OG: The burden of malaria infection on pregnant women and birth weight of infants in south western Nigeria. East Afr J Public Health. 2009, 6: 63-68.
Anorlu RI, Odum CU, Essien EE: Asymptomatic malaria parasitaemia in pregnant women at booking in a primary health care facility in a periurban community in Lagos, Nigeria. Afr J Med Med Sci. 2001, 30 (Suppl): 39-41.
Cot M, Deloron P: [Malaria during pregnancy: consequences and interventional perspectives](in French). Med Trop (Mars). 2003, 63: 369-380.
Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD: Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007, 7: 93-104. 10.1016/S1473-3099(07)70021-X.
FMOH: Federal Republic of Nigeria. Malaria situation analysis document. 2000, Federal Ministry of Health Nigeria
Guyatt HL, Snow RW: Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin Microbiol Rev. 2004, 17: 760-769. 10.1128/CMR.17.4.760-769.2004.
Breschi MC, Seghieri G, Bartolomei G, Gironi A, Baldi S, Ferrannini E: Relation of birthweight to maternal plasma glucose and insulin concentrations during normal pregnancy. Diabetologia. 1993, 36: 1315-1321. 10.1007/BF00400812.
Schaefer-Graf UM, Graf K, Kulbacka I, Kjos SL, Dudenhausen J, Vetter K, Herrera E: Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care. 2008, 31: 1858-1863. 10.2337/dc08-0039.
Jensen RB, Chellakooty M, Vielwerth S, Vaag A, Larsen T, Greisen G, Skakkebaek NE, Scheike T, Juul A: Intrauterine growth retardation and consequences for endocrine and cardiovascular diseases in adult life: does insulin-like growth factor-I play a role?. Horm Res. 2003, 60 (Suppl 3): 136-148.
Di Cianni G, Miccoli R, Volpe L, Lencioni C, Ghio A, Giovannitti MG, Cuccuru I, Pellegrini G, Chatzianagnostou K, Boldrini A, Del Prato S: Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med. 2005, 22: 21-25.
Kitajima M, Oka S, Yasuhi I, Fukuda M, Rii Y, Ishimaru T: Maternal serum triglyceride at 24-32 weeks' gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet Gynecol. 2001, 97: 776-780. 10.1016/S0029-7844(01)01328-X.
Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA: Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008, 358: 1991-2002.
Karon J, Patek J, Glowacki J, Tomala J: [Lipid-lipoprotein serum profile of the newborn and its birth weight](in Polish). Ginekol Pol. 1996, 67: 394-397.
Kelishadi R, Badiee Z, Adeli K: Cord blood lipid profile and associated factors: baseline data of a birth cohort study. Paediatr Perinat Epidemiol. 2007, 21: 518-524. 10.1111/j.1365-3016.2007.00870.x.
Setia S, Sridhar MG, Bhat V, Chaturvedula L, Vinayagamoorti R, John M: Insulin sensitivity and insulin secretion at birth in intrauterine growth retarded infants. Pathology. 2006, 38: 236-238. 10.1080/00313020600696256.
Ong K, Kratzsch J, Kiess W, Costello M, Scott C, Dunger D: Size at birth and cord blood levels of insulin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-1 (IGFBP-1), IGFBP-3, and the soluble IGF-II/mannose-6-phosphate receptor in term human infants. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J Clin Endocrinol Metab. 2000, 85: 4266-4269. 10.1210/jc.85.11.4266.
Lassarre C, Hardouin S, Daffos F, Forestier F, Frankenne F, Binoux M: Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr Res. 1991, 29: 219-225. 10.1203/00006450-199103000-00001.
Fried M, Muga RO, Misore AO, Duffy PE: Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol. 1998, 160: 2523-2530.
Moormann AM, Sullivan AD, Rochford RA, Chensue SW, Bock PJ, Nyirenda T, Meshnick SR: Malaria and pregnancy: placental cytokine expression and its relationship to intrauterine growth retardation. J Infect Dis. 1999, 180: 1987-1993. 10.1086/315135.
Ayoola OO, Gemmell I, Omotade OO, Adeyanju OA, Cruickshank JK, Clayton PE: Maternal malaria, birth size and blood pressure in Nigerian newborns: insights into the developmental origins of hypertension from the Ibadan growth cohort. PLoS One. 2011, 6 (9): e24548-10.1371/journal.pone.0024548.
Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972, 18: 499-502.
Ahaneku JE, Adinma JI, Nwosu OB, Ahaneku GI, Farotimi A, Analike R: Lipid and lipoprotein cardiovascular risk factor changes during normal pregnancy in Africans. Eur J Obstet Gynecol Reprod Biol. 1999, 82: 53-55. 10.1016/S0301-2115(98)00210-3.
Loke DF, Viegas OA, Kek LP, Rauff M, Thai AC, Ratnam SS: Lipid profiles during and after normal pregnancy. Gynecol Obstet Invest. 1991, 32: 144-147. 10.1159/000293016.
Piechota W, Staszewski A: Reference ranges of lipids and apolipoproteins in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1992, 45: 27-35. 10.1016/0028-2243(92)90190-A.
Jimenez DM, Pocovi M, Ramon-Cajal J, Romero MA, Martinez H, Grande F: Longitudinal study of plasma lipids and lipoprotein cholesterol in normal pregnancy and puerperium. Gynecol Obstet Invest. 1988, 25: 158-164. 10.1159/000293765.
Lippi G, Albiero A, Montagnana M, Salvagno GL, Scevarolli S, Franchi M, Guidi GC: Lipid and lipoprotein profile in physiological pregnancy. Clin Lab. 2007, 53: 173-177.
Bansal N, Charlton-Menys V, Pemberton P, McElduff P, Oldroyd J, Vyas A, Koudsi A, Clayton PE, Cruickshank JK, Durrington PN: Adiponectin in umbilical cord blood is inversely related to low-density lipoprotein cholesterol but not ethnicity. J Clin Endocrinol Metab. 2006, 91: 2244-2249. 10.1210/jc.2005-2714.
Okoro BA, Udeozo IO, Okeahialam TC: Influence of birthweight and social status on cordblood cholesterol in full term Nigerian neonates. Trop Geogr Med. 1985, 37: 356-358.
Taylor GO, Olufunwa SA, Agbedana EO, Akande EO: Maternal and cord plasma levels of high-density lipoprotein cholesterol and triglycerides in Nigeria. Br J Obstet Gynaecol. 1980, 87: 33-37. 10.1111/j.1471-0528.1980.tb04422.x.
Ibeziako PA, Jeyakuma LH, Ette SI: Cholesterol and phospholipid levels in Nigerian mothers and newborn. J Trop Pediatr. 1982, 28: 135-138.
Fujita H, Okada T, Inami I, Makimoto M, Hosono S, Minato M, Takahashi S, Mugishima H, Yamamoto T: Low-density lipoprotein profile changes during the neonatal period. J Perinatol. 2008, 28: 335-340. 10.1038/jp.2008.8.
Kittl EM, Diridl G, Lenhart V, Neuwald C, Tomasits J, Pichler H, Bauer K: HDL cholesterol as a sensitive diagnostic parameter in malaria. Wien Klin Wochenschr. 1992, 104: 21-24.
Onongbu IC, Onyeneke EC: Plasma lipid changes in human malaria. Tropenmed Parasitol. 1983, 34: 193-196.
Grellier P, Rigomier D, Clavey V, Fruchart JC, Schrevel J: Lipid traffic between high density lipoproteins and Plasmodium falciparum-infected red blood cells. J Cell Biol. 1991, 112: 267-277. 10.1083/jcb.112.2.267.
Sein KK, Aikawa M: The prime role of plasma membrane cholesterol in the pathogenesis of immune evasion and clinical manifestations of falciparum malaria. Med Hypotheses. 1998, 51: 105-110. 10.1016/S0306-9877(98)90102-5.
Davis TM, Suputtamongkol Y, Spencer JL, Wilson SG, Mekhton S, Croft KD, White NJ: Glucose turnover in pregnant women with acute malaria. Clin Sci (Lond). 1994, 86: 83-90.
Rogerson SJ, Brown HC, Pollina E, Abrams ET, Tadesse E, Lema VM, Molyneux ME: Placental tumor necrosis factor alpha but not gamma interferon is associated with placental malaria and low birth weight in Malawian women. Infect Immun. 2003, 71: 267-270. 10.1128/IAI.71.1.267-270.2003.
Aleyamma TK, Peedicayil A, Regi A: Falciparum malaria in pregnancy. Int J Gynaecol Obstet. 2007, 97: 48-49. 10.1016/j.ijgo.2006.12.012.
Ekejindu IM, Udigwe GO, Chijioke IR: Malaria and anaemia in pregnancy in Enugu, south east Nigeria. Afr J Med Med Sci. 2006, 35: 1-3.
McGregor IA, Wilson ME, Billewicz WZ: Malaria infection of the placenta in The Gambia, West Africa; its incidence and relationship to stillbirth, birthweight and placental weight. Trans R Soc Trop Med Hyg. 1983, 77: 232-244. 10.1016/0035-9203(83)90081-0.
Akum AE, Kuoh AJ, Minang JT, Achimbom BM, Ahmadou MJ, Troye-Blomberg M: The effect of maternal, umbilical cord and placental malaria parasitaemia on the birthweight of newborns from South-western Cameroon. Acta Paediatr. 2005, 94: 917-923. 10.1080/08035250510028605.
Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, Font F, Alonso PL: The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000, 181: 1740-1745. 10.1086/315449.
Cottrell G, Mary JY, Barro D, Cot M: The importance of the period of malarial infection during pregnancy on birth weight in tropical Africa. AmJTrop Med Hyg. 2007, 76: 849-854.
Matteelli A, Donato F, Shein A, Muchi JA, Abass AK, Mariani M, Leopardi O, Maxwell CA, Carosi G: Malarial infection and birthweight in urban Zanzibar, Tanzania. Ann Trop Med Parasitol. 1996, 90: 125-134.
Bouvier P, Breslow N, Doumbo O, Robert CF, Picquet M, Mauris A, Dolo A, Dembele HK, Delley V, Rougemont A: Seasonality, malaria, and impact of prophylaxis in a West African village. II. Effect on birthweight. AmJTrop Med Hyg. 1997, 56: 384-389.
Yakoob MY, Zakaria A, Waqar SN, Zafar S, Wahla AS, Zaidi SK, Sarwari AR, Qureshi RN, Siddiqui AR: Does malaria during pregnancy affect the newborn?. J Pak Med Assoc. 2005, 55: 543-546.
Walter PR, Garin Y, Blot P: Placental pathologic changes in malaria. A histologic and ultrastructural study. Am J Pathol. 1982, 109: 330-342.
Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW: Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007, 7: 105-117. 10.1016/S1473-3099(07)70022-1.
Beeson JG, Amin N, Kanjala M, Rogerson SJ: Selective accumulation of mature asexual stages of Plasmodium falciparum- infected erythrocytes in the placenta. Infect Immun. 2002, 70: 5412-5415. 10.1128/IAI.70.10.5412-5415.2002.
Kalanda BF, van BS, Verhoeff FH, Brabin BJ: Anthropometry of fetal growth in rural Malawi in relation to maternal malaria and HIV status. Arch Dis Child Fetal Neonatal Ed. 2005, 90: F161-F165. 10.1136/adc.2004.054650.
Brabin BJ, Romagosa C, Abdelgalil S, Menendez C, Verhoeff FH, McGready R, Fletcher KA, Owens S, D'Alessandro U, Nosten F, Fischer PR, Ordi J: The sick placenta-the role of malaria. Placenta. 2004, 25: 359-378. 10.1016/j.placenta.2003.10.019.
Hattersley AT, Beards F, Ballantyne E, Appleton M, Harvey R, Ellard S: Mutations in the glucokinase gene of the fetus result in reduced birth weight. Nat Genet. 1998, 19: 268-270. 10.1038/953.
Woods KA, Camacho-Hubner C, Savage MO, Clark AJ: Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996, 335: 1363-1367. 10.1056/NEJM199610313351805.
Ong K, Kratzsch J, Kiess W, Costello M, Scott C, Dunger D: Size at birth and cord blood levels of insulin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-1 (IGFBP-1), IGFBP-3, and the soluble IGF-II/mannose-6-phosphate receptor in term human infants. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J Clin Endocrinol Metab. 2000, 85: 4266-4269. 10.1210/jc.85.11.4266.
Ong K, Kratzsch J, Kiess W, Dunger D: Circulating IGF-I levels in childhood are related to both current body composition and early postnatal growth rate. J Clin Endocrinol Metab. 2002, 87: 1041-1044. 10.1210/jc.87.3.1041.
Umbers AJ, Boeuf P, Clapham C, Stanisic DI, Baiwog F, Mueller I, Siba P, King CL, Beeson JG, Glazier J, Rogerson SJ: Placental malaria-associated inflammation disturbs the insulin-like growth factor axis of fetal growth regulation. J Infect Dis. 2011, 203 (4): 561-569. 10.1093/infdis/jiq080.
Acknowledgements
We are grateful to all the pregnant women, their husbands and their babies for their participation in the study. We also thank the research study staff who carried out the field work and the Adeoyo Maternity Hospital staff for their support. We acknowledge the support of the National Institute of Health Research Manchester Biomedical Research Centre. This project was supported by a Wellcome Trust Research Training Fellowship to OOA (Reference: 080723/Z/06/Z). The Wellcome Trust, UK, played no role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
OA participated in overall study conception and design, data collection, analysis, interpretation and manuscript preparation. AW was involved in laboratory assay and data analysis. WB was involved in data collection. OJ participated in data collection. JC and PC both participated in overall study conception and design, data interpretation and manuscript preparation. All authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ayoola, O.O., Whatmore, A., Balogun, W.O. et al. Maternal malaria status and metabolic profiles in pregnancy and in cord blood: relationships with birth size in Nigerian infants. Malar J 11, 75 (2012). https://doi.org/10.1186/1475-2875-11-75
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-11-75