Abstract
Background
Plasmodium has a complex cell biology and it is essential to dissect the cell-signalling pathways underlying its survival within the host.
Methods
Using the fluorescence resonance energy transfer (FRET) peptide substrate Abz-AIKFFARQ-EDDnp and Fluo4/AM, the effects of extracellular ATP on triggering proteolysis and Ca2+ signalling in Plasmodium berghei and Plasmodium yoelii malaria parasites were investigated.
Results
The protease activity was blocked in the presence of the purinergic receptor blockers suramin (50 μM) and PPADS (50 μM) or the extracellular and intracellular calcium chelators EGTA (5 mM) and BAPTA/AM (25, 100, 200 and 500 μM), respectively for P. yoelii and P. berghei. Addition of ATP (50, 70, 200 and 250 μM) to isolated parasites previously loaded with Fluo4/AM in a Ca2+-containing medium led to an increase in cytosolic calcium. This rise was blocked by pre-incubating the parasites with either purinergic antagonists PPADS (50 μM), TNP-ATP (50 μM) or the purinergic blockers KN-62 (10 μM) and Ip5I (10 μM). Incubating P. berghei infected cells with KN-62 (200 μM) resulted in a changed profile of merozoite surface protein 1 (MSP1) processing as revealed by western blot assays. Moreover incubating P. berghei for 17 h with KN-62 (10 μM) led to an increase in rings forms (82% ± 4, n = 11) and a decrease in trophozoite forms (18% ± 4, n = 11).
Conclusions
The data clearly show that purinergic signalling modulates P. berghei protease(s) activity and that MSP1 is one target in this pathway.
Similar content being viewed by others
Background
Malaria is one of the most important infectious diseases in the world, responsible for an estimated 655,000 deaths each year [1]. While Plasmodium grows and develops inside red blood cells (RBCs), concomitant structural [2] and biochemical changes occurs at the host cell culminating in cell rupture and release of free merozoites[3, 4]. It is now well established that Plasmodium activates proteases during the blood stages, including during the entry into and exit from its host RBC and the intracellular feeding on haemoglobin [5–9].
As an adaptive evolutionary mechanism, the malaria parasite subverts its host's signalling system to survive and replicate[10–12]. The role of Ca2+ signalling underlying modulation of the Plasmodium cell cycle has been extensively investigated including an effect on protease activity[13, 14]. For example, some proteases are modulated by intracellular Ca2+ in rodent Plasmodium species[15]. Such signalling depends on the maintenance of low cytosolic Ca2+ during its RBC stages[16–26]. However, it is still unknown how a calcium signal is triggered and how particular metabolites derived from the host are central in providing signalling molecules to facilitate parasite growth. The host hormone, melatonin [27] and its derivatives that elicit a rise in cytosolic calcium in Plasmodium[17, 28–30] also induce proteolysis in Plasmodium falciparum and Plasmodium chabaudi[31].
Here we have investigated whether other metabolites derived from the host are able to induce proteolysis in Plasmodium berghei and Plasmodium yoelii. Purines such as adenosine, ADP, ATP and UDP mediate several biological processes[32], being essential in various metabolic cycles and extracellular signalling [33]. The role of ATP in cell signalling is well studied in many eukaryotic cells and includes a wide variety of processes such as secretion, immune responses, mechano-sensory transduction, inflammation, platelet aggregation, cell proliferation, differentiation, and cell migration[34].
ATP is released from RBCs when they are deformed, and this process could also be relevant to malaria parasite invasion since RBCs undergo extensive deformation after the initial merozoite attachment. In addition, it has been reported that following Plasmodium infection, the ATP content of RBCs increases [35] and blocking purinergic signalling decreases RBCs invasion by P. falciparum[36]. Interestingly, suramin, which inhibits purinergic signalling, has been shown to inhibit merozoite surface protein-1 (MSP1) processing and erythrocyte invasion[37]. In this manner, a role of ATP in Ca2+ signalling and proteolysis to modulate the Plasmodium RBC cell cycle is hypothesized.
By using fluorescence resonance energy transfer (FRET) peptides, it was previously shown that Ca2+ modulates protease activation in P. berghei and P. yoelii parasites[15]. Here, the importance of ATP in modulating proteolysis through Ca2+ pathways in these parasites has been investigated. In addition, the role of ATP in activating proteases or modulating the P. berghei cell cycle was studied in the presence of purinergic blocker KN-62. It is shown here that this compound blocks parasite maturation and affects processing of the merozoite surface protein MSP1[38]. Taken together, the present work contributes to the understanding of P. berghei biology.
Materials and methods
Reagents
Thapsigargin, (phenylmethylsulphonyl fluoride), saponin, probenecid, MOPS (3-(N-morpholino) propanesulfonic acid), EGTA (ethylene glycol-bis (2-aminoethylether)-N,N,N',N tetraacetic acid), adenosine, ATP (adenosine-5'-triphosphate), GTP (guanosine-5'-triphosphate), suramin, PPADS (pyridoxalphosphate-6-azophenyl-2',4'-disulphonic acid), IP5I (diinosine pentaphosphate), TNP-ATP (3'-O-(2,4,6-Trinitrophenyl)adenosine-5'-triphosphate tetra(triethylammonium) salt), KN-62 (4-[(2S)-2-[(5-isoquinolinylsulfonyl) methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl] phenyl isoquinolinesulfonic acid ester), Triton X-100 and dihydroethidium were purchased from Sigma- Aldrich (St. Louis, MO). BAPTA/acetoxymethyl ester (AM) and Fluo4/AM were bought from Molecular Probes Inc. (Eugene, OR). The peptide Abz-AIKFFARQ-EDDnp was analytical grade and synthesized according to Hirata, 1994[39–41].
Plasmodium berghei (strain NK65) and P. yoelii (strain 17X) parasites
Plasmodium berghei and P. yoelii were maintained as an asynchronous parasitaemia in mice (Balb/C strain) by transfer every four days. For parasite preparation, filtration of the infected blood through a cellulose column (Whatman CF11) removed leukocytes and platelets. The erythrocytes were then washed twice in PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM NaH2PO4,) by centrifugation at 1,500 g for 5 min and lysed in PBS containing 60 μg ml-1 saponin. The membranes were removed by centrifugation (10,000 × g for 10 min at 4°C) and further washing of the parasites (1,500 g for 5 min) in MOPS buffer (116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 5.5 mM D-glucose, 50 mM MOPS, and 1 mM CaCl2, pH 7.2) containing saponin. After erythrocyte lysis, the parasites were maintained in MOPS buffer during the whole experiment.
Ethical approval
All animal procedures were approved by the São Paulo University Ethics Committee for Animal Experiments (CEEA) according to the Colégio Brasileiro de Experimentação Animal guidelines (COBEA).
Cell culture of Plasmodium berghei (strain NK65) parasites
Plasmodium berghei parasites in mice (Balb/C) were transferred to culture at a parasitaemia of 6-10%. The infected RBCs were filtered through a cellulose column (Whatman CF11) as described above and washed twice with RPMI 1640 medium (GIBCO BRL) supplemented with 10% foetal calf serum (FCS). Infected RBCs were then transferred to a culture chamber and kept in suspension by a magnetic stirrer, under an atmosphere of 5% O2, 7% CO2 and 88% N2. The parasites were maintained in culture for 17 h. The stages of intraerythrocytic development were determined by morphology on Giemsa-stained smears.
Peptide and calcium indicator Fluo4/AM loading
The FRET peptide Abz-AIKFFARQ-EDDnp has a fluorescent group, Abz (ortho-aminobenzoic acid), and a quencher group, EDDnp (ethylene diamine-2-4-dinitrophenyl). The peptide is able to access free malaria parasites when the erythrocyte membrane was removed by saponin treatment in MOPS buffer after 1 min incubation. Stock solutions were prepared in DMSO/water (1:1) and concentrations were measured spectrophotometrically using a molar absorption coefficient of 17,300 M-1 cm-1 at 365 nm. For Ca2+ measurements isolated parasites were incubated for 30 min at room temperature with the fluorescent calcium indicator Fluo-4/AM (5 μM) in MOPS buffer containing 1.8 mM probenecid, an inhibitor of organic transport, to minimize indicator extrusion. The cell suspension was then washed three times in MOPS buffer to remove the extracellular dye[17].
Spectrofluorimetric determinations
Spectrofluorimeric measurements were performed in a Shimadzu RF-5301 PC at 37°C with isolated parasites (108 cells ml-1) incubated with MOPS buffer in a 1 ml cuvette. The fluorescence was measured continuously (acquisition rate: every 0.5 seconds) 1 min after addition of the FRET peptide (10 μM) for 400 seconds. Excitation/emission wavelengths were adjusted to 320/420 nm for Abz and 505/530 nm for Fluo-4 AM. For experiments with purinergic inhibitors parasites were pre-incubated with KN-62, IP5I, TNP-ATP, PPADS or suramin, for 30 min at room temperature. For experiments with the extra or intracellular calcium chelator EGTA (5 mM) or Bapta/AM (25, 100, 200 or 500 μM) parasites were pre-incubated for 5 or 40 min at room temperature, respectively. All incubations were performed before the addition of the FRET peptide.
Confocal imaging of FRET peptide
Isolated P. berghei parasites were resuspended in MOPS buffer and plated onto poly-lysine coated plates (200 μL). After 1 min incubation with the FRET peptide (10 μM), plated cells were taken to a confocal microscope (LSM 510, Zeiss) and observed under a 63× objective (water immersion). Cells were excited with UV laser at 351 nm and 364 nm. To select fluorescence, a 375 nm main dichroic mirror was used. Fluorescence was collected with a 385 long-pass dichroic mirror. Images were taken at 3 seconds intervals and ATP (200 μM) was added to the cells after baseline acquisition. Fluorescence was analysed using LSM 510 Image Examiner (Zeiss).
Flow cytometry analysis
Using Flow cytometry analysis viability was assessed in P. yoelii and P. berghei by dihydroethidine (1:200) staining for 20 min at 37°C and analysed by dot plots (side scatter versus fluorescence) of 105 cells. Dihydroethidine (DHT) was excited with a 488 nm Argon laser and fluorescence emission was collected at 518-605 nm. Parameters subject to adjustment of the FACSCalibur flow cytometer were forward scatter (FSC) (log scale, E-1), SSC (log scale, 269), FL-2 (log scale, 505). For all flow cytometry experiments initial gating was carried out with unstained erythrocytes to account for erythrocyte autofluorescence.
Immunoblotting
Plasmodium berghei infected erythrocytes were incubated in MOPS buffer with Ca2+ (1 mM) and KN-62 (200 μM) or DMSO (0.05%) for 2 h at 37°C. After incubation cells were kept for 2 h at -80°C and subsequently disrupted with lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% (v/v) NP-40 and 0.5% (w/v) sodium deoxycholate) including the phosphatase and protease inhibitors (10 μM NaF, 100 μM orthovanadate, 1 μg ml-1 leupeptin, 1 μg ml-1 pepstatin A, 1 μg ml-1 quimostatin, 100 μg ml-1 benzamidine and 1 mM PMSF) for 30 min at 4°C. Samples were quantified by spectrometry and 10 μg of protein was electrophoresed on an 8% polyacrylamide gel and transferred to nitrocellulose. Mouse monoclonal antibody MAb25.1 [42, 43] (1:1,000) that specifically binds MSP1[44] was added and incubated overnight at 4°C [45] After washing, blots were incubated for 2 h with secondary HRP-conjugated anti-mouse IgG antibody (1:10,000, GE Healthcare) and binding was detected using enhanced chemiluminescence.
Statistical analysis
Results are expressed as mean ± SEM of at least three individual experiments. Student's t-test was used for comparisons between two groups, whereas for repeated measures ANOVA was used for comparisons among larger groups. A P value less than 0.05 was considered indicative of a statistically significant difference. GraphPad Prism software (San Diego, CA, USA) was used for all statistical tests.
Results
Previous studies had shown that extracellular ATP induced a rise of intracellular Ca2+ concentration in P. falciparum parasites[36]. Differences in the modulation of proteolysis by Ca2+ among Plasmodium species indicated the necessity for new comparative studies to clarify the situation[15, 31].
In the present work, a dose dependent modulation of proteolysis of the FRET peptide substrate Abz-AIKFFARQ-EDDnp induced by ATP (50, 200 and 250 μM) in the presence of extracellular calcium was verified as shown in Figure 1 (panels A, B, C and D). Moreover, when observed under the confocal microscope, the fluorescence of the cleaved substrate Abz-AIKFFARQ-EDDnp clearly colocalizes with P. berghei isolated parasites (Figure 1, Panel B). Under these conditions ATP (200 μM) is able to induce proteolysis (Figure 1, Panels C and D). Additional results also showed that adenosine (10 μM) but not GTP (50 μM) induce proteolysis in isolated P. yoelii and P. berghei parasites (Additional file 1). In these experiments proteolysis was detected by the rate of change in fluorescence caused by hydrolysis of the FRET peptide.
Extracellular ATP triggers intracellular protease activity in P. yoelii and P. berghei . (A) Graphical representation of resonance energy transfer (FRET) peptide hydrolysis (peptide Abz-AIKFFARQ-EDDnp) in the presence of ATP (200 μM) and the control in isolated (saponin treated) free mixed blood stages of P. berghei parasites. (B) Fluorescence trace and confocal imaging of Abz-AIKFFARQ-EDDnp hydrolysis. ATP (200 μM) addition is indicated by the arrow. Fluorescence (fl) and phase contrast (ph) before and after ATP addition are below the trace. (C and D) Bar graph analyses of peptide hydrolysis at different concentrations of ATP (25, 50, 200 and 250 μM) relative to the control (1.2 ± 0.03, n = 12, P = 0.0332; 1.5 ± 0.07, n = 9, P = 0.0007; 1.7 ± 0.075, n = 17, P < 0.0001 and 1.33 ± 0.04, n = 10; P = 0.0048) and (1.17 ± 0.07, n = 5, P = 0.425; 1.4 ± 0.06, n = 12, P = 0.0039; 1.7 ± 0.15, n = 16, P = 0.0267 and 1.7 ± 0.2, n = 8; P = 0.0228) for P. berghei and P.yoelii parasites, respectively. P values were calculated by comparison with the control (ctr) (1.05 ± 0.08, n = 6) and (1.22 ± 0.03, n = 6), respectively. Isolated parasites (108 cells ml-1) were incubated in MOPS buffer with 1 mM calcium in a 1 ml cuvette. The fluorescence was measured continuously (acquisition rate: every 0.5 seconds)1 min after addition of the peptide Abz-AIKFFARQ-EDDnp (10 μM) for 400 seconds.
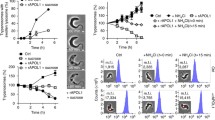
To verify whether intracellular and extracellular Ca2+ modulates the proteolytic activity induced by ATP (50 μM), experiments were performed in the presence of BAPTA/AM (25 μM, 100 μM, 200 μM or 500 μM) or EGTA (5 mM), respectively. Figure (2A and 2B) clearly shows that proteolysis was inhibited in the presence of intra- and extracellular Ca2+ chelator indicating the need of the cation in the pathway. Interestingly, it was observed that proteolysis is impaired in the presence of the purinergic inhibitors PPADS (100 or 50 μM) and suramin (50 μM) in P. berghei and P. yoelii parasites (Figure 3A and 3B, respectively). The P2X (a family of ligand-gated ion channel receptors) inhibitor KN-62 (at 10 μM) also inhibited the proteolytic activity in P. berghei parasites (Figure 3A).
Calcium is required for FRET activation triggered by ATP in P. berghei and P. yoelii isolated (saponin treated) free mixed blood stages parasites. (A and B) The rate of peptide hydrolysis induced by ATP (50 μM) as measured by fluorescence was inhibited after incubation with the extracellular and intracellular calcium chelators: EGTA (5 mM) for 5 min (1.0 ± 0.07, n = 6, P = 0.0004) and BAPTA/AM (100, 200 or 500 μM) for 40 min (1.2 ± 0.03, n = 11, P = 0.0002; 1.18 ± 0.01, n = 13, P < 0.0001; 1.2 ± 0.03, n = 5, P = 0.005, respectively) P values were calculated by comparison with the ATP (50 μM) data (1.5 ± 0.07, n = 9) in P. berghei parasites. P. yoelii parasites were also incubated with EGTA (5 mM) (1.02 ± 0.08, n = 7, P = 0.0009) and BAPTA/AM (25, 100, 200 or 500 μM) for 40 min (1.4 ± 0.07, n = 7, P = 0.823; 1.2 ± 0.03, n = 10, P = 0.002; 1.2 ± 0.03, n = 10, P = 0.017; 1.2 ± 0.03, n = 10, P = 0.022, respectively). P values were calculated by comparison with the ATP (50 μM) data (1.4 ± 0.06, n = 12). Isolated parasites (108 cells ml-1) were incubated in MOPS buffer without CaCl2. The fluorescence was measured continuously (acquisition rate - every 0.5 seconds) for 400 seconds.
Inhibitors suggest the presence of purinergic receptor(s) in P. berghei (A) and P. yoelii (B). Rate of peptide hydrolysis induced by ATP (50 μM) as measured by fluorescence after incubation with suramin (50 μM) (1.1 ± 0.04, n = 7, P = 0.0002 and 1.0 ± 0.07, n = 7, P = 0.0006, respectively), PPADS (50 μM) (1.1 ± 0.08, n = 12, P = 0.0008 and 1.4 ± 0.063, n = 6, P = 0.791, respectively) or KN-62 (10 μM) (1.3 ± 0.025, n = 13, P = 0.001). P values were calculated by comparison with the ATP (50 μM) data (1.5 ± 0.067, n = 9 and 1.4 ± 0.062, n = 12, respectively). Effects of ATP on FRET peptide Abz-AIKFFARQ-EDDnp (10 μM) hydrolysis were observed after 30 min incubation of isolated parasites (108 cells ml-1) with the pharmacological agents in MOPS buffer with 1 mM calcium in a 1 ml cuvette.. The fluorescence was measured continuously (acquisition rate: every 0.5 seconds) for 400 seconds.
Next, whether or not ATP can induce a calcium rise in P. berghei and P. yoelii using the fluorescent calcium probe Fluo4/AM was investigated. Figure (4A to 4D) shows the typical rise in cytosolic calcium in isolated P. berghei and P. yoelii after the addition of ATP (50 μM, 70 μM, 200 μM and 250 μM). Addition of the detergent digitonin induced the maximum rise in calcium that was subsequently abolished by addition of the calcium chelator EGTA. Supplementary experiments showed that adenosine (10 μM or 15 μM) is also able to increase cytosolic calcium in P. berghei and P. yoelii (Additional file 2).
Dose response effects of ATP on [Ca2+] c are inhibited by purinergic blockers in malarial parasites. (A and B) Representative traces of Fluo4/AM (green fluorescence calcium indicator) changes over time by addition of ATP (200 μM) in P. berghei and P. yoelii, respectively. (C and D) Bar graph analyses of Ca2+ concentration in P. berghei and P. yoelii Fluo4/AM labelled isolated parasites (108 cells ml-1) after addition of ATP (25, 50, 70, 200 and 250 μM) (1.15 a.u. ± 0.04, n = 8; 1.4 a.u. ± 0.06, n = 14; 1.5 a.u. ± 0.07, n = 11; 1.9 a.u. ± 0.1, n = 15; 1.6 a.u. ± 0.1, n = 11, respectively) in P. berghei or ATP (50, 70 and 200 μM) (2.3 a.u. ± 0.02, n = 3; 1.9 a.u. ± 0.1, n = 6; 2.1 a.u. ± 0.14, n = 12), respectively in P. yoelii. Mobilization of Ca2+ after treatment with ATP (200 μM) was blocked in the presence of purinoreceptor inhibitors PPADS (50 μM) (0.9 a.u. ± 0.03, n = 9, P < 0.0001), TNP-ATP (50 μM) (1.0 a.u. ± 0.01, n = 7, P < 0.0001), Ip5I (10 μM) (1.1 a.u. ± 0.02, n = 12, P < 0.0001) or KN-62 (10 μM) (1.0 a.u. ± 0.02, n = 9, P < 0.0001, respectively) in P. berghei and TNP-ATP (50 μM) (1.5 a.u. ± 0.09, n = 9, P = 0.001) in P. yoelii. P values were calculated by comparison with the ATP (200 or 50 μM) data (2.0 a.u. ± 0.1, n = 12 and 2.3 a.u. ± 0.02, n = 3) in P. berghei and P. yoelii, respectively. Bar graphs represent means with SEM. The fluorescence was measured continuously (acquisition rate: every 0.5 seconds) for 400 seconds.
These results indicate that activation of proteolysis by ATP could be triggered by an increase in Ca2+ through purinergic signalling in the rodent malarial parasites. This is better demonstrated by the data in Figures 4C and 4D, where isolated P. berghei and P. yoelii parasites that have been loaded with Fluo4/AM were submitted to different pharmacological treatments; the rise in cytosolic calcium was impaired in the presence of P2X blockers TNP-ATP (50 μM), KN-62 (10 μM), Ip5I (10 μM) and PPADS (50 μM).
The rodent malaria parasites P. berghei and P yoelii grow as relatively asynchronous populations, therefore, the distribution of intracellular stages during the above experiments (Figures 1, 2, 3, 4) was assessed by morphology using Giemsa-stained smears; the results indicated that the majority of parasites were at the trophozoite stage (Figure 5).
Distribution of Plasmodium stages and parasitemia during experiments. P. berghei (A) and P. yoelii (B) proportion of rings (25.2 ± 3, n = 6 and 27.3 ± 2, n = 6, respectively), trophozoites (60 ± 1.8, n = 6 and 68.8 ± 3, n = 6, respectively) and schizonts (14.7 ± 2.7, n = 6 and 39 ± 0.8, n = 6, respectively). To assess parasitaemia (C) (38.4 ± 1.5, n = 6 and 40.1 ± 4.3, n = 6; P = 0.718, respectively) in Balb/C mice no less than 1000 erythrocytes were counted on Giemsa-stained smears. Bar graphs represent means with SEM.
Parasite viability was verified by flow cytometry analyses using DHT staining. Figure (6A and 6B) shows that both P. berghei and P. yoelii parasites were viable at the beginning and end (3 h later) of the spectrofluorimetric assays (99.8% ± 0.04 and 99.5% ± 0.15, n = 3, P = 0.144; 99.8% ± 0.1 and 99.7% ± 0.09, n = 3, P = 0.822, respectively) indicating ideal experimental conditions for the parasites.
Flow Cytometry using dihydroethidine staining in P. berghei and P. yoelii to assess viability. (A and B) Histogram distribution of fluorescence in non labelled parasites (control), parasites labeled at the beginning (dashed line) and 3 hours later (solid line) in the same buffer. Dihydroethidine is a vital stain taken up by viable parasites, and which then stains nucleic acid. It is the chemically reduced form of DNA intercalating dye ethidium bromide (B-ring reduction). (C and D) Bar graph analyses of viability in P. berghei and P. yoelii. The data (mean of three independent experiments) show no statistical difference in DHT fluorescence from the beginning and the end of the experiment (99.8 ± 0.09, n = 3 and 99.8 ± 0.1, n = 3; P = 0.823, respectively) and (99.5 ± 0.2, n = 3 and 99.8 ± 0.04, n = 3; P = 0.145, respectively).
The ability of KN-62 to interfere with the processing of MSP1 in P. berghei-infected cells was investigated next. Western blot analyses showed that there is an increased amount of MSP1 and its proteolytic fragments in parasites treated with KN-62 (200 μM) for 2 h (Figure 7A and 7B). MAb25.1 reacts with an epitope in the N-terminal region of MSP1, therefore, band A is the full length precursor (~230 kDa), band C is the N-terminal fragment corresponding to the 83 kDa fragment of P. falciparum MSP1, Bands D and E are subfragments of this N-terminal fragment and B is an intermediate fragment containing all of the ~95 kDa fragment C together with other downstream sequences [42–44].
MSP1 protein expression is increased in KN-62 treated P. berghei iRBC. (A) Representative western blot comparing MSP1 protein and its fragments in control and KN-62 (200 μM) treated P. berghei iRBC showing increased expression levels after treatment. Each lane is loaded with 10 μg total cell lysate prepared from freshly harvested parasites. Blots were probed with monoclonal antibody mAb25.1 (1:1000); Anti-actin antibody (1:5000) was used as a control for protein loading. (B) Densitometric analysis of blot showed that MSP expression is increased, particularly in bands C and E.
The effects of purinoreceptor inhibitor KN-62 on the P. berghei cell cycle were verified by maintaining parasites with a similar distribution of development stages (Figure 8A) for 17 h in the presence of KN-62 (10 μM). The data showed a considerable drop in the number of trophozoites (18.01% ± 4.08, n = 11) compared with the number of ring stages (81.99% ± 4.07, n = 11) thus highlighting the relevance of purinoceptors in the parasite developmental cycle (Figure 8C).
Effects of purinergic inhibitor KN-62 on P. berghei erythrocyte invasion (A). Plasmodium berghei intraerythrocytic stages (ring, trophozoite or schizont) were assessed during an in vitro assay at time zero (beginning) (22.53 ± 2.93, n = 4; 73.21 ± 3.3, n = 4 and 3.26 ± 0.69, n = 4, respectively). (B) Culture of asynchronous P. berghei parasites were incubated for 17 h in the presence of DMEM (control), ATP (50 μM), ATP (50 μM) with DMSO (0.05%) or ATP (50 μM) with KN-62 (10 μM) and the parasitaemia calculated as a percentage of control (100, n = 4; 93.8 ± 9.3, n = 4; 84 ± 6.1, n = 7 and 80.2 ± 10.4, n = 9, respectively). Note no difference in erythrocyte invasion among the treatments (P > 0.05). Distribution of P. berghei intraerythrocytic stages in the presence of KN-62 (C). P. berghei intraerytrocytic stages: ring (41.9 ± 3.7, n = 9; 43.8 ± 3.6, n = 8; 45.2 ± 2.3, n = 9; 82 ± 4, n = 11; respectively), trophozoite (55.5 ± 3.8, n = 9; 53.7 ± 3.8, n = 8; 52.2 ± 2.5, n = 9; 18 ± 4, n = 11, respectively) or schizont (2.6 ± 0.4, n = 9; 2.5 ± 1, n = 8; 2.6 ± 1.1, n = 9; 0.00 ± 0.00, n = 11; respectively) were assessed after 17 h in the in vitro assay. Bars represents the number of rings, trophozoites and schizonts (average), expressed as a percentage of control ± S.E.M. Data were compared by one-way ANOVA and by the Newman-Keuls test. *Statistical significance with respect to control values P < 0.001.
Discussion
ATP is a ubiquitous signalling molecule that recognizes purinergic membrane receptors and modulates several processes studied in a myriad of organisms including slime mould, yeast lizards and mammals (for reviews see [28, 46–51]). Purinergic receptors are divided into two classes namely P1 and P2 (adenosine and ATP/ADP, respectively). The P2 receptor includes two types: P2X (family of ligand-gated ion channel receptors) and P2Y (a family of GPCR) [52]. Despite the presence of purinergic signalling mechanisms in invertebrates and lower eukaryotes the evolutionary distance from parasites to human prevents as molecular identification of the receptor from the genome [53–55]. It is noteworthy that in the parasitic helminth Schistosoma mansoni, a P2X receptor has been identified in the genome database[56], and using bioinformatic tools Madeira and colleagues identified genes for GPCR-like candidates in the genomes of P. falciparum, P. berghei, P. yoelii and P. chabaudi[57]. As RBCs do not synthesize their own purines de novo, Plasmodium must obtain purine compounds from the extracellular milieu[58]. Endocytosis from the host cell cytosol is thought to be involved in the uptake of nutrients, such as nucleosides, nucleobases and amino acids.
The P. falciparum plasma membrane nucleoside transporter, PfENT1 (P. falciparum equilibrative nucleoside transporter), has been the subject of extensive study [59, 60]. Localized in the plasma membrane [61] its kinetic parameters and substrate specificity have been determined [59, 60, 62]. The knockout of the PfENT1 gene leads to a reduction in hypoxanthine uptake, and in addition adenosine and inosine transport is affected [63]. The molecular-cellular mechanism by which the parasite obtains extracellular compounds might be related to lipid traffic in the infected RBC [64] and it is well known that the parasite has the ability to create membranous structures in the infected-RBC and new functions such as the anion channel (new permeation pathway)[65–67].
In the present contribution, it was shown that addition of ATP in a dose dependent manner (Figure 1) to P. berghei and P. yoelii activates intracellular proteolysis. Interestingly, extracellular and intracellular Ca2+ is needed to activate the proteolysis triggered by ATP (Figure 2). It was also found that the purinergic receptor is involved in the ATP signalling pathway to activate the proteases of these rodent malarial parasites since the presence of purinergic antagonists (suramin, PPADS or KN-62) blocked proteolytic activation triggered by ATP in P. berghei and P. yoelii (Figure 3). Differences in the PPADS concentration needed to block protease activity in P. berghei (50 μM) and P. yoelii (100 μM) may be due to differences in the total intracellular Ca2+ concentration mobilized by ATP in both species (Figure 4). This result is in agreement with the presence of a purinergic antagonist (PPADS, TNP-ATP, Ip5I or KN-62) able to inhibit the ability of ATP to induce a rise in calcium in these rodent malarial species (Figure 4). Of interest, the differences in calcium activation of proteolysis between P. berghei and P. yoelii were reported previously[15].
For the human malaria parasite P. falciparum, merozoite invasion and secondary processing of MSP1 is inhibited by suramin[37]. Here it was shown that MSP1 protein expression and processing in P. berghei parasites is increased by KN-62 (200 μM) treatment (Figure 7) indicating that MSP1 processing may be a downstream effect of the purinergic signalling pathway of P. berghei.
Proteolysis is central to several steps of the Plasmodium life cycle including merozoite invasion and egress from RBC, and haemoglobin digestion. In P. falciparum, blocking purinergic receptors with either KN-62 or Ip51 prevents parasite invasion of RBCs[36]; interestingly the present work showed that KN-62 modulates the P. berghei intracellular cell cycle (Figure 8).
Conclusion
The data presented here support the concept that Plasmodium subverts the host-endocrine system by using extracellular ATP to activate proteolysis to invade or escape from RBCs. The molecular identification of the purinergic receptor in Plasmodium as well as the protease (s) involved in these processes represent the basis of new strategies for development of anti-malarial drugs.
References
WHO Global Malaria Programme: World Malaria Report 2011. 2011, World Health Organization
Garcia CR, Takeuschi M, Yoshioka K, Miyamoto H: Imaging Plasmodium falciparum-infected ghost and parasite by atomic force microscopy. J Struct Biol. 1997, 119: 92-98. 10.1006/jsbi.1997.3886.
Garcia CR, de Azevedo MF, Wunderlich G, Budu A, Young JA, Bannister L: Plasmodium in the postgenomic era: new insights into the molecular cell biology of malaria parasites. Int Rev Cell Mol Biol. 2008, 266: 85-156.
Goldberg DE, Cowman AF: Moving in and renovating: exporting proteins from Plasmodium into host erythrocytes. Nat Rev Microbiol. 2010, 8: 617-621. 10.1038/nrmicro2420.
Banerjee R, Liu J, Beatty W, Pelosof L, Klemba M, Goldberg DE: Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine. Proc Natl Acad Sci USA. 2002, 99: 990-995. 10.1073/pnas.022630099.
Coppi A, Tewari R, Bishop JR, Bennett BL, Lawrence R, Esko JD, Billker O, Sinnis P: Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host Microbe. 2007, 2: 316-327. 10.1016/j.chom.2007.10.002.
Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, Berriman M, Florens L, Janssen CS, Pain A, Christophides GK: A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005, 307: 82-86. 10.1126/science.1103717.
Harris PK, Yeoh S, Dluzewski AR, O'Donnell RA, Withers-Martinez C, Hackett F, Bannister LH, Mitchell GH, Blackman MJ: Molecular identification of a malaria merozoite surface sheddase. PLoS Pathog. 2005, 1: 241-251.
Rosenthal PJ: Cysteine proteases of malaria parasites. Int J Parasitol. 2004, 34: 1489-1499. 10.1016/j.ijpara.2004.10.003.
Doerig C, Abdi A, Bland N, Eschenlauer S, Dorin-Semblat D, Fennell C, Halbert J, Holland Z, Nivez MP, Semblat JP: Malaria: targeting parasite and host cell kinomes. Biochim Biophys Acta. 2010, 1804: 604-612.
Koyama FC, Chakrabarti D, Garcia CR: Molecular machinery of signal transduction and cell cycle regulation in Plasmodium. Mol Biochem Parasitol. 2009, 165: 1-7. 10.1016/j.molbiopara.2009.01.003.
Kumar KA, Garcia CR, Chandran VR, Van Rooijen N, Zhou Y, Winzeler E, Nussenzweig V: Exposure of Plasmodium sporozoites to the intracellular concentration of potassium enhances infectivity and reduces cell passage activity. Mol Biochem Parasitol. 2007, 156: 32-40. 10.1016/j.molbiopara.2007.07.004.
Barale JC, Blisnick T, Fujioka H, Alzari PM, Aikawa M, Braun-Breton C, Langsley G: Plasmodium falciparum subtilisin-like protease 2, a merozoite candidate for the merozoite surface protein 1-42 maturase. Proc Natl Acad Sci USA. 1999, 96: 6445-6450. 10.1073/pnas.96.11.6445.
Perrin BJ, Huttenlocher A: Calpain. Int J Biochem Cell Biol. 2002, 34: 722-725. 10.1016/S1357-2725(02)00009-2.
Cruz LN, Alves E, Leal MT, Juliano MA, Rosenthal PJ, Juliano L, Garcia CR: FRET peptides reveal differential proteolytic activation in intraerythrocytic stages of the malaria parasites Plasmodium berghei and Plasmodium yoelii. Int J Parasitol. 2011, 41: 363-372. 10.1016/j.ijpara.2010.10.009.
Alves E, Bartlett PJ, Garcia CR, Thomas AP: Melatonin and IP3-induced Ca2+ release from intracellular stores in the malaria parasite Plasmodium falciparum within infected red blood cells. J Biol Chem. 2011, 286: 5905-5912. 10.1074/jbc.M110.188474.
Beraldo FH, Almeida FM, da Silva AM, Garcia CR: Cyclic AMP and calcium interplay as second messengers in melatonin-dependent regulation of Plasmodium falciparum cell cycle. J Cell Biol. 2005, 170: 551-557. 10.1083/jcb.200505117.
Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DV, Kumar V, Hasan S, Brown JR, Peishoff CE, Cardon LR, Garcia-Bustos JF: Thousands of chemical starting points for antimalarial lead identification. Nature. 2010, 465: 305-310. 10.1038/nature09107.
Krishna S, Pulcini S, Fatih F, Staines H: Artemisinins and the biological basis for the PfATP6/SERCA hypothesis. Trends Parasitol. 2010, 26: 517-523. 10.1016/j.pt.2010.06.014.
Lew VL, Tiffert T: Is invasion efficiency in malaria controlled by pre-invasion events?. Trends Parasitol. 2007, 23: 481-484. 10.1016/j.pt.2007.08.001.
Marchesini N, Luo S, Rodrigues CO, Moreno SN, Docampo R: Acidocalcisomes and a vacuolar H + -pyrophosphatase in malaria parasites. Biochem J. 2000, 347: 243-253. 10.1042/0264-6021:3470243.
Passos AP, Garcia CR: Characterization of Ca2+ transport activity associated with a non-mitochondrial calcium pool in the rodent malaria parasite. Chabaudi Biochem Mol Biol Int. 1997, 42: 919-925.
Sibley LD: Intracellular parasite invasion strategies. Science. 2004, 304: 248-253. 10.1126/science.1094717.
Vaid A, Sharma P: PfPKB, a protein kinase B-like enzyme from Plasmodium falciparum: II. Identification of calcium/calmodulin as its upstream activator and dissection of a novel signaling pathway. J Biol Chem. 2006, 281: 27126-27133. 10.1074/jbc.M601914200.
Valderramos SG, Scanfeld D, Uhlemann AC, Fidock DA, Krishna S: Investigations into the role of the Plasmodium falciparum SERCA (PfATP6) L263E mutation in artemisinin action and resistance. Antimicrob Agents Chemother. 2010, 54: 3842-3852. 10.1128/AAC.00121-10.
Varotti FP, Beraldo FH, Gazarini ML, Garcia CR: Plasmodium falciparum malaria parasites display a THG-sensitive Ca2+ pool. Cell Calcium. 2003, 33: 137-144. 10.1016/S0143-4160(02)00224-5.
Srinivasan V, Spence DW, Moscovitch A, Pandi-Perumal SR, Trakht I, Brown GM, Cardinali DP: Malaria: therapeutic implications of melatonin. J Pineal Res. 2010, 48: 1-8. 10.1111/j.1600-079X.2009.00728.x.
Beraldo FH, Sartorello R, Lanari RD, Garcia CR: Signal transduction in red blood cells of the lizards Ameiva ameiva and Tupinambis merianae (Squamata, Teiidae). Cell Calcium. 2001, 29: 439-445. 10.1054/ceca.2001.0207.
Budu A, Peres R, Bueno VB, Catalani LH, Garcia CR: N1-acetyl-N2-formyl-5-methoxykynuramine modulates the cell cycle of malaria parasites. J Pineal Res. 2007, 42: 261-266. 10.1111/j.1600-079X.2006.00414.x.
Gazarini ML, Thomas AP, Pozzan T, Garcia CR: Calcium signaling in a low calcium environment: how the intracellular malaria parasite solves the problem. J Cell Biol. 2003, 161: 103-110. 10.1083/jcb.200212130.
Farias SL, Gazarini ML, Melo RL, Hirata IY, Juliano MA, Juliano L, Garcia CR: Cysteine-protease activity elicited by Ca2+ stimulus in Plasmodium. Mol Biochem Parasitol. 2005, 141: 71-79. 10.1016/j.molbiopara.2005.01.015.
Ralevic V, Burnstock G: Postjunctional synergism of noradrenaline and adenosine 5'-triphosphate in the mesenteric arterial bed of the rat. Eur J Pharmacol. 1990, 175: 291-299. 10.1016/0014-2999(90)90567-P.
Burnstock G, Wood JN: Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 1996, 6: 526-532. 10.1016/S0959-4388(96)80060-2.
Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA: International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006, 58: 281-341. 10.1124/pr.58.3.3.
Akkaya C, Shumilina E, Bobballa D, Brand VB, Mahmud H, Lang F, Huber SM: The Plasmodium falciparum-induced anion channel of human erythrocytes is an ATP-release pathway. Pflugers Arch. 2009, 457: 1035-1047. 10.1007/s00424-008-0572-8.
Levano-Garcia J, Dluzewski AR, Markus RP, Garcia CR: Purinergic signalling is involved in the malaria parasite Plasmodium falciparum invasion to red blood cells. Purinergic Signal. 2010, 6: 365-372. 10.1007/s11302-010-9202-y.
Fleck SL, Birdsall B, Babon J, Dluzewski AR, Martin SR, Morgan WD, Angov E, Kettleborough CA, Feeney J, Blackman MJ, Holder AA: Suramin and suramin analogues inhibit merozoite surface protein-1 secondary processing and erythrocyte invasion by the malaria parasite Plasmodium falciparum. J Biol Chem. 2003, 278: 47670-47677. 10.1074/jbc.M306603200.
Child MA, Epp C, Bujard H, Blackman MJ: Regulated maturation of malaria merozoite surface protein-1 is essential for parasite growth. Mol Microbiol. 2010, 78: 187-202.
Hirata IY: CMCaCN: Internally quenched fluorogenic protease substrates: solid-phase synthesis and fluorescence spectroscopy of peptides containing ortho-aminobenzoyl/dinitrophenyl groups as donor-acceptor pairs. Lett Pept Sci. 1994, 1: 299-308.
Carmona AK, Juliano MA, Juliano L: The use of Fluorescence Resonance Energy Transfer (FRET) peptides for measurement of clinically important proteolytic enzymes. An Acad Bras Cienc. 2009, 81: 381-392. 10.1590/S0001-37652009000300005.
Hirata IY, Cezari MH, Nakaie CR, Boschcov P, Ito AS, Juliano MA, Juliano L: Internally quenched fluorogenic protease substrates: Solid-phase synthesis and fluorescence spectroscopy of peptides containing ortho-aminobenzoyl/dinitrophenyl groups as donor-acceptor pairs. Letters in Peptide Science. 1994, 1: 299-308.
Freeman RR, Trejdosiewicz AJ, Cross GA: Protective monoclonal antibodies recognising stage-specific merozoite antigens of a rodent malaria parasite. Nature. 1980, 284: 366-368. 10.1038/284366a0.
Holder AA, Freeman RR: Characterization of a high molecular weight protective antigen of Plasmodium yoelii. Parasitology. 1984, 88 (Pt 2): 211-219.
Holder AA, Freeman RR: Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981, 294: 361-364. 10.1038/294361a0.
Holder AA, Freeman RR: The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J Exp Med. 1984, 160: 624-629. 10.1084/jem.160.2.624.
Bagnaresi P, Rodrigues MT, Garcia CR: Calcium signaling in lizard red blood cells. Comp Biochem Physiol A Mol Integr Physiol. 2007, 147: 779-787. 10.1016/j.cbpa.2006.09.015.
Beraldo FH, Garcia CR: Divergent calcium signaling in RBCs from Tropidurus torquatus (Squamata-Tropiduridae) strengthen classification in lizard evolution. BMC Physiol. 2007, 7: 7-10.1186/1472-6793-7-7.
Beraldo FH, Sartorello R, Gazarini ML, Caldeira W, Garcia CR: Red blood cells of the lizards Ameiva ameiva (Squamata, Teiidae) display multiple mechanisms to control cytosolic calcium. Cell Calcium. 2002, 31: 79-87. 10.1054/ceca.2002.0258.
Burnstock G, Verkhratsky A: Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf). 2009, 195: 415-447. 10.1111/j.1748-1716.2009.01957.x.
Allan CY, Fisher PR: In vivo measurements of cytosolic calcium in Dictyostelium discoideum. Methods Mol Biol. 2009, 571: 291-308. 10.1007/978-1-60761-198-1_20.
Cunningham KW: Acidic calcium stores of Saccharomyces cerevisiae. Cell Calcium. 2011, 50: 129-138. 10.1016/j.ceca.2011.01.010.
Burnstock G, Kennedy C: Is there a basis for distinguishing two types of P2-purinoceptor?. Gen Pharmacol. 1985, 16: 433-440. 10.1016/0306-3623(85)90001-1.
Carlton JM, Angiuoli SV, Suh BB, Kooij TW, Pertea M, Silva JC, Ermolaeva MD, Allen JE, Selengut JD, Koo HL: Genome sequence and comparative analysis of the model rodent malaria parasite Plasmodium yoelii yoelii. Nature. 2002, 419: 512-519. 10.1038/nature01099.
Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S: Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002, 419: 498-511. 10.1038/nature01097.
Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, De La Vega P, Holder AA, Batalov S, Carucci DJ, Winzeler EA: Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003, 301: 1503-1508. 10.1126/science.1087025.
Raouf R, Blais D, Seguela P: High zinc sensitivity and pore formation in an invertebrate P2X receptor. Biochim Biophys Acta. 2005, 1669: 135-141. 10.1016/j.bbamem.2005.01.009.
Madeira L, Galante PA, Budu A, Azevedo MF, Malnic B, Garcia CR: Genome-wide detection of serpentine receptor-like proteins in malaria parasites. PLoS One. 2008, 3: e1889-10.1371/journal.pone.0001889.
Gherardi A, Sarciron ME: Molecules targeting the purine salvage pathway in Apicomplexan parasites. Trends Parasitol. 2007, 23: 384-389. 10.1016/j.pt.2007.06.003.
Carter NS, Ben Mamoun C, Liu W, Silva EO, Landfear SM, Goldberg DE, Ullman B: Isolation and functional characterization of the PfNT1 nucleoside transporter gene from Plasmodium falciparu. J Biol Chem. 2000, 275: 10683-10691. 10.1074/jbc.275.14.10683.
Parker MD, Hyde RJ, Yao SY, McRobert L, Cass CE, Young JD, McConkey GA, Baldwin SA: Identification of a nucleoside/nucleobase transporter from Plasmodium falciparum, a novel target for anti-malarial chemotherapy. Biochem J. 2000, 349: 67-75. 10.1042/0264-6021:3490067.
Rager N, Mamoun CB, Carter NS, Goldberg DE, Ullman B: Localization of the Plasmodium falciparum PfNT1 nucleoside transporter to the parasite plasma membrane. J Biol Chem. 2001, 276: 41095-41099. 10.1074/jbc.M107037200.
Downie MJ, Saliba KJ, Howitt SM, Broer S, Kirk K: Transport of nucleosides across the Plasmodium falciparum parasite plasma membrane has characteristics of PfENT1. Mol Microbiol. 2006, 60: 738-748. 10.1111/j.1365-2958.2006.05125.x.
El Bissati K, Zufferey R, Witola WH, Carter NS, Ullman B, Ben Mamoun C: The plasma membrane permease PfNT1 is essential for purine salvage in the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 2006, 103: 9286-9291. 10.1073/pnas.0602590103.
Murphy SC, Fernandez-Pol S, Chung PH, Prasanna Murthy SN, Milne SB, Salomao M, Brown HA, Lomasney JW, Mohandas N, Haldar K: Cytoplasmic remodeling of erythrocyte raft lipids during infection by the human malaria parasite Plasmodium falciparum. Blood. 2007, 110: 2132-2139. 10.1182/blood-2007-04-083873.
Ginsburg H, Stein WD: How many functional transport pathways does Plasmodium falciparum induce in the membrane of its host erythrocyte?. Trends Parasitol. 2005, 21: 118-121. 10.1016/j.pt.2005.01.004.
Kelly JX, Winter RW, Braun TP, Osei-Agyemang M, Hinrichs DJ, Riscoe MK: Selective killing of the human malaria parasite Plasmodium falciparum by a benzylthiazolium dye. Exp Parasitol. 2007, 116: 103-110. 10.1016/j.exppara.2006.12.001.
Kirk K, Saliba KJ: Targeting nutrient uptake mechanisms in Plasmodium. Curr Drug Targets. 2007, 8: 75-88. 10.2174/138945007779315560.
Acknowledgements
This work was partially supported by grants from Brazilian Agencies FAPESP and CNPq - InBqmed (to C.R.S.G), the UK Medical Research Council (File references: U117532067 and U117532063) and the EU through the Network of Excellence EviMalaR (Health-2009-2.3.2-1-242095). LNC and AB received a FAPESP Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LNC designed the study, collected the data and prepared the first draft. MAJ and LJ synthesized the FRET peptides. AB performed confocal experiment (1B) and wrote the draft related to it. AAH and MJB provided the monoclonal antibody MAb25.1 and analysed the data. CRSG designed the study and prepared the first draft. All authors put forward different ideas, contributed to the interpretation of the data, helped with early drafts and contributed to and agreed the final draft. All authors read and approved the final manuscript
Electronic supplementary material
12936_2011_2077_MOESM1_ESM.PDF
Additional file 1: Extracellular adenosine but not GTP triggers intracellular protease activity in P. berghei and P. yoelii (A and B). Bar graph analyses of peptide hydrolysis after GTP (50 μM) (0.99 ± 0.09, n = 5, P = 0.63 and 1.06 ± 0.05, n = 9, P = 0.365) or adenosine (10 μM) (1.51 ± 0.11, n = 7, P = 0.006 and 2.15 ± 0.11, n = 3, P < 0.0001) in P. berghei and P. yoelii, respectively. P values were calculated by comparison with control data (1.05 ± 0.08, n = 6 and 1.12 ± 0.03, n = 6, respectively). Isolated parasites (108 cells ml-1) were incubated in MOPS buffer with 1 mM calcium in a 1 ml cuvette. The fluorescence was measured continuously 1 min after addition of the peptide Abz-AIKFFARQ-EDDnp (10 μM). (PDF 65 KB)
12936_2011_2077_MOESM2_ESM.PDF
Additional file 2: Dose response effects of adenosine on [Ca2+] c rise in P. yoelii and P. berghei . (A and B). Analyses of Ca2+ concentration in P. berghei and P.yoelii Fluo4/AM labelled isolated parasites (108 cells ml-1) after addition of adenosine (10 and 15 μM) (1.59 a.u. ± 0.07, n = 18, P = 0.007 and 1.5 a.u. ± 0.062, n = 8, P = 0.002, respectively) in P. berghei or adenosine (10 μM) (1.78 a.u. ± 0.16, n = 8, P = 0.004) in P. yoelii. P values were calculated by comparison with control data (1.05 a.u. ± 0.01, n = 3 and 0.99 a.u. ± 0.064, n = 5; respectively). Bar graphs represent means with SEM. (PDF 63 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cruz, L.N., Juliano, M.A., Budu, A. et al. Extracellular ATP triggers proteolysis and cytosolic Ca2+ rise in Plasmodium berghei and Plasmodium yoelii malaria parasites. Malar J 11, 69 (2012). https://doi.org/10.1186/1475-2875-11-69
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-11-69