Abstract
Background
The development and propagation of malaria parasites in their vertebrate host is a complex process in which various host and parasite factors are involved. Sometimes the evolution of parasitaemia seems to be quelled by parasite load. In order to understand the typical dynamics of evolution of parasitaemia, various mathematical models have been developed. The basic premise ingrained in most models is that the availability of uninfected red blood cells (RBC) in which the parasite develops is a limiting factor in the propagation of the parasite population.
Presentation of the hypothesis
We would like to propose that except in extreme cases of severe malaria, there is no limitation in the supply of uninfected RBC for the increase of parasite population.
Testing the hypothesis
In this analysis we examine the biological attributes of the parasite-infected RBC such as cytoadherence and rosette formation, and the rheological properties of infected RBC, and evaluate their effects on blood flow and clogging of capillaries. We argue that there should be no restriction in the availability of uninfected RBC in patients.
Implication of the hypothesis
There is no justification for the insertion of RBC supply as a factor in mathematical models that describe the evolution of parasitaemia in the infected host. Indeed, more recent models, that have not inserted this factor, successfully describe the evolution of parasitaemia in the infected host.
Similar content being viewed by others
Introduction
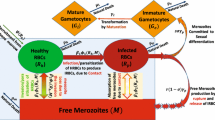
Parasites often have a mechanism which regulates parasite load according to parasite density. Although this auto-regulation is not fully understood, it maintains an equilibrium between activated specific and non-specific host defense processes, the sensitivity of red blood cells (RBC) to invasion and the virulence of the parasite [1]. Understanding of this auto-regulation would be facilitated by the development of a suitable mathematical model. Several attempts have been made in the past to generate mathematical models of the process of malaria infection in non-immune individuals [2–7]. The formulation of a model is essential since at some stages of their development in the host the malaria parasites cannot be seen, either because they are sequestered in the deep blood vasculature or else because detection limits are too high. The usefulness of such models is obvious as they could disclose the evolution of antimalarial immunity, anaemia that may be life threatening, the significance of antigenic variation to in-host and population evolution of the disease. Eventually, such models could be used for the assessment of drug response and the effects of vaccine to the point that they could advise the selection of vaccine target and the timing of drug treatment for optimization of both ways of medical intervention.
All models are derived from a paper by Anderson et al (1989) [8] in which the following basic assumptions have been made: 1) Uninfected cells are released from the bone marrow at a constant rate and have a natural life expectancy; 2) Red blood cells (RBC) are infected by a rate that is proportional to the density of uninfected RBC. 3) The death of infected cells due to maturation of schizonts is rapid compared to the above-mentioned rates. 4) The released merozoites either die or successfully infect new RBC.
Assumption 1) has recently been shown to be inadequate as the RBC survival time is only 1/3 that of healthy controls [9] and this is a major contributor to anaemia [10], in addition to impaired erythropoiesis [11]. These effects, however, have no bearing on the present discussion. Assumptions 3) and 4) are correct, but the dependence of the formation of infected cells on the concentration of uninfected cells (assumption 2) seems to be questionable. Its introduction into the model implies that in extreme cases of anaemia, the availability of uninfected RBC may rate-limit (by self-limiting) the evolution of infection. We would like to test this consideration in the broader context of the rheological effects of cytoadherence and invasion of RBC by merozoites in vivo.
Cytoadherence and rosetting
Cytoadherence is defined as the ability of parasitized red blood cells (PRBC) to attach to specific receptors on the endothelial cells of the microcapillaries, and rosetting is defined as the ability of PRBC to bind to uninfected RBC. Both processes could influence the invasion of RBC by merozoites emerging from the mature rupturing schizont. Although there are numerous works on cytoadherence, rosetting and invasion in cultures, very little is known about the details of invasion in vivo.
Let us examine the case of Plasmodium falciparum infection. Here, most if not all infected RBC harbouring mature parasite stages are sequestered in the post-capillary venules of the host due to their ability to cytoadhere to the endothelial cells of the venules [12–16]. This sequestration, on one hand, prevents the passage of the rigidified PRBC through the spleen and their ensuing removal by resident macrophages, and on the other hand, these cell-cell interactions could increase the probability of invasion.
Reports on the pathology of malaria show post-capillary venules clogged with uninfected RBC and PRBC [17–21]. Most of these are based on post-mortem histology and are, hence, probably relevant for severe cases of malaria. Here, we shall address only non-severe infection since, the situation in severe disease may be much more complex [22]. The first generation of post capillary venules range in size from 10 μm to 30 μm and the second generation from 40 μm to 200 μm, whereas the diameter of the spherical PRBC is not larger than 4.4 μm. There is, therefore, plenty of room for circulation to continue, especially with vasodilatation, for which there is some evidence of occurrence in malaria infections [23–25]. PRBC are more rigid and less deformable than uninfected RBC (although these are also less deformable than normal RBC) [26, 27] and thus increase blood viscosity [28–30]. However, we have to consider the phenomenon of rosetting as well, that is, the adherence of uninfected RBC to PRBC [31, 32]. Studies show that the cell-cell attachments within rosettes are strong and suggest that rosettes might survive both the arterial circulation and passage through microvessels and thus could contribute to the ischaemic complications of falciparum malaria [33, 34]. However, it has been recently suggested that rosettes cannot withstand arteriolar shear stress but do endure venular shear stress [35]. Hence, rosettes can be formed in the venules and reduce cytoadherence. This could be due to the masking of attachment ligands by uninfected RBCs from their receptors on the endothelial cells, or to competition for ligands between receptors on RBC and on endothelial cells. Yet, even if rosettes are only partially being formed during the passage through the venules, they would still slow down blood flow and thereby enhance cytoadherence, in as much as reduced flow is known to increase it [36]. However, if cytoadherence occurred before rosetting, then adherent cells should not efficiently form rosettes [37].
Effects of sequestration of PRBC on blood flow
Obviously then, cytoadherence with or without rosetting should increase resistance to blood flow through the venules. This was indeed shown to be the case under flow conditions in various experimental systems [36, 38–42]. Histology, however, indicates that even in severe malaria not all venules are obstructed. Vasodilatation, which has been observed by some investigators in malaria patients [23, 43], is known not to affect venules directly, but feeding arteries and arterioles. Flow in capillaries in the healthy host is typically intermittent and not all capillaries necessarily allow blood flow all the time. Vasodilatation of arterioles tends to reduce intermittency and recruit more capillaries into flow. Flow in the venules does not usually stop altogether, but in the smallest first generation venules there will be fluctuations depending on the capillaries supplying them. These fluctuations, along with other factors such as the tissue-specific distribution of cytoadherence receptors, could explain the differential sequestration of PRBC in some venules but not in others. It is also possible to infer on these phenomena from the behavior of white cells during inflammatory response: these cells adhere to the wall of venules as they migrate through [44]. White cells are much larger and more resistant to deformation than parasitized red cells and yet they do not stop flow. Nonetheless, it has been shown that microvascular resistance does increase when white cells adhere to the wall, most probably because the lumen is effectively narrowed.
Impaired blood flow in malaria patients
Investigations have shown that in malaria patients' blood flow is restricted through the kidney [23, 45, 46], the liver [47, 48], the brain [49, 50], the placenta [51] and that in Plasmodium berghei infection there is a general failure of capillary flow and disruption of venous outflow tracts by aggregates of infected and uninfected cells [52]. Another report could not find evidence for hypoperfusion in cerebral malaria [53]. This could be due to the autoregulation of local blood flow by anoxic blood that can accelerate flow by up to 200-fold or to neural regulation that can displace blood from a vasoconstricted area to a region of vasodilatation. In fact, in children with cerebral malaria cerebral blood flow is increased considerably [50]. It must be underscored that all these perturbations in blood flow were observed only in severely ill patients, and in extreme cases that are often fatal, could even lead to hypoglycemia and lactic acidosis [54–57].
Complete clogging of venules is incompatible with the evolution of parasitaemia
It is very likely therefore that in non-severe and even in acute malaria patients, the post-capillary venules are never fully clogged. In all the aforementioned pathological studies, histological inspection of post-mortem tissue sections reveals venules filled with PRBC. This filling could occur only if cytoadhering PRBC do not obstruct flow completely [58], allowing for bypassing cells to submit the PRBC to attach to endothelial receptors thus filling all potential cytoadhering sites. Reduced flow velocity due to partial clogging could assist cytoadherence whose efficiency is inversely related to the speed of flow [36]. Complete clogging is probably inconsistent with the nutritional requirements of the growing parasite (supply of metabolic substrates and removal of waste products), and most importantly, with effective invasion of RBC by released merozoites. Let us consider what happens in a fully clogged vessel when segmenters burst to release merozoites. It is tempting to suggest that the rupture of PRBC reinstates blood flow in the venules, and merozoites are entrained by the renewed blood flow into larger capillaries where they meet uninfected RBC supplied from parallel, unclogged venules, and invade them. This process would require that all segmenters rupture simultaneously. Otherwise, the merozoites that emerge from one segmenter will encounter only PRBC that are refractory to invasion. However, even in synchronous infection, rupture of segmenters and reinvasion takes well over one hour, suggesting that not all segmenters rupture at the same time. These considerations indicate that complete clogging of the post capillary venules by cytoadhering PRBC is disadvantageous for the optimal propagation of the parasites. Thus, total clogging probably never occurs. In fact, the rate of increase in parasitaemia in naive individuals (as deduced from clinical studies of malariotherapy patients) suggests that all released merozoites successfully reinvade [2, 9]. An analysis of patients data indicates that infection is not restricted by the availability of RBC [58] although disease contributes to increased destruction of both RBC and PRBC [1].
The case of rosetting is somewhat different: the emerging merozoites will encounter uninfected RBC of the rosette that are available for invasion. Rosettes may obstruct microvessels to the flow of uninfected RBC more efficiently than cytoadhering PRBC, but this is compensated for by the availablilty of rosetting RBC for reinvasion. If the entire microvessel is filled with rosettes, quantitative considerations imply that the rate of parasite propagation will be lower compared to non-rosetting parasites: PRBC is surrounded in the rosette by 4–7 RBC, while each schizont can release up to 20 merozoites. Once again, since the evolution of parasitaemia indicates that all merozoites successfully invade, rosetting cannot be a quantitatively important phenomenon in falciparum malaria.
The availability of uninfected RBC to merozoites is not restrained
As we have just argued, if total clogging of venules never occurs, does the availability of uninfected RBC limit the evolution of parasitaemia in non-immune patients as suggested by mathematical models of within-host parasite dynamics (see Introduction)? Such limitation is based on the assumption of the mass action law that is applicable to a closed system. The anatomy of post capillary venules, the regulation of blood flow and cytoadherence of PRBC to the endothelium of the venules in conjunction with the parasite cycle, implies that reinvasion occurs in an open system. In this system merozoites emerge from sequestered segmenters and are exposed to a continuous flow of uninfected RBC within the venule. In fact, the partial clogging of the capillaries and the decreased deformability of uninfected RBC could reduce the velocity of flow thereby increasing the chances of invasion. Merozoites could also be entrained by the blood flow to second generation venules where they would meet even larger numbers of RBC coming from merging first generation venules that may not contain sequestered PRBC at all. Therefore the merozoites enjoy a pure source of RBC, with much higher concentration than that in the total bloodstream. Thus, it must be concluded that reinvasion is not limited by the availability of uninfected RBC. The reduction of parasite propagation observed in natural infections that parallels increasing anaemia [1] must result from other factors such as increased immune response, virulence of the parasite and its preference for sub-sets of RBC [59, 60]. Merozoites released from schizonts lose their invasive capability within minutes [61, 62], and from studies in cultures of P. falciparum it is known that agitation reduces the levels of reinvasion [63]. This would imply that reinvasion must occur before meorozoites reach large veins where the velocity of blood flow is considerably accelerated. Here again, the reduced rate of blood flow in venules lined with cytoadhering PRBC is an advantage for efficacious reinvasion that may be favored by decreased agitation.
The general observation that anaemia is associated with the severity of infection [64], implies that parasitaemia is not limited by anaemia, i.e, the reduced availability of RBC. The ability of virulent parasite strains to multiply so rapidly as to outpace the evolution of effective defense by the host [59] indicates that the supply of RBC is not restricted. The slower evolution of parasitaemia with avirulent strains that have a preference for a sub-set of RBC [59, 60] may result from the loss of the ability to invade due to encounter with unsusceptible RBC, and not from a general limited supply of RBC.
Models of within host dynamics describing the dynamics of parasite evolution and auto-regulation that do not assume limitation of RBC availability were shown to successfully describe the clinical picture [65, 66].
References
White NJ, Ho M: The pathophysiology of malaria. Adv Parasitol. 1992, 31: 83-173.
Gravenor MB, Kwiatkowski D: An analysis of the temperature effects of fever on the intra-host population dynamics of Plasmodium falciparum. Parasitology. 1998, 117: 97-105. 10.1017/S0031182098002893.
Gravenor MB, van Hensbroek MB, Kwiatkowski D: Estimating sequestered parasite population dynamics in cerebral malaria. Proc Natl Acad Sci USA. 1998, 95: 7620-7624. 10.1073/pnas.95.13.7620.
Gravenor MB, McLean AR, Kwiatkowski D: The regulation of malaria parasitaemia: parameter estimates for a population model. Parasitology. 1995, 110: 115-122.
Gupta S, Hill AV, Kwiatkowski D, Greenwood AM, Greenwood BM, Day KP: Parasite virulence and disease patterns in Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1994, 91: 3715-3719.
Hetzel C, Anderson RM: The within-host cellular dynamics of bloodstage malaria: theoretical and experimental studies. Parasitology. 1996, 113: 25-38.
Saul A: Models for the in-host dynamics of malaria revisited: errors in some basic models lead to large over-estimates of growth rates. Parasitology. 1998, 117: 405-407. 10.1017/S0031182098003230.
Anderson RM, May RM, Gupta S: Non-linear phenomena in host-parasite interactions. Parasitology. 1989, 99 (Suppl): S59-S79.
Newton PN, Chotivanich K, Chierakul W, Ruangveerayuth R, Teerapong P, Silamut K, Looareesuwan S, White NJ: A comparison of the in vivo kinetics of Plasmodium falciparum ring-infected erythrocyte surface antigen-positive and -negative erythrocytes. Blood. 2001, 98: 450-457. 10.1182/blood.V98.2.450.
Jakeman GN, Saul A, Hogarth WL, Collins WE: Anaemia of acute malaria infections in non-immune patients primarily results from destruction of uninfected erythrocytes. Parasitology. 1999, 119: 127-133. 10.1017/S0031182099004564.
Wickramasinghe SN, Abdalla SH: Blood and bone marrow changes in malaria. Baillieres Best Pract Res Clin Haematol. 2000, 13: 277-299. 10.1053/beha.1999.0072.
Reeder JC, Brown GV: Antigenic variation and immune evasion in Plasmodium falciparum malaria. Immunol Cell Biol. 1996, 74: 546-554.
Udomsangpetch R, Taylor BJ, Looareesuwan S, White NJ, Elliott JF, Ho M: Receptor specificity of clinical Plasmodium falciparum isolates: nonadherence to cell-bound E-selectin and vascular cell adhesion molecule-1. Blood. 1996, 88: 2754-2760.
Ho M, Schollaardt T, Niu X, Looareesuwan S, Patel KD, Kubes P: Characterization of Plasmodium falciparum-infected erythrocyte and P-selectin interaction under flow conditions. Blood. 1998, 91: 4803-4809.
Newbold C, Craig A, Kyes S, Rowe A, Fernandez-Reyes D, Fagan T: Cytoadherence, pathogenesis and the infected red cell surface in Plasmodium falciparum. Int J Parasitol. 1999, 29: 927-937. 10.1016/S0020-7519(99)00049-1.
Ho M, White NJ: Molecular mechanisms of cytoadherence in malaria. Am J Physiol. 1999, 276: C1231-C1242.
MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA: Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985, 119: 385-401.
Pongponratn E, Riganti M, Punpoowong B, Aikawa M: Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg. 1991, 44: 168-175.
Warrell DA: Cerebral malaria: clinical features, pathophysiology and treatment. Ann Trop Med Parasitol. 1997, 91: 875-884. 10.1080/00034989760644.
Turner G: Cerebral malaria. Brain Pathol. 1997, 7: 569-582.
Silamut K, Phu NH, Whitty C, Turner GD, Louwrier K, Mai NT, Simpson JA, Hien TT, White NJ: A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol. 1999, 155: 395-410.
Dondorp AM, Kager PA, Vreeken J, White NJ: Abnormal blood flow and red blood cell deformability in severe malaria. Parasitol Today. 2000, 16: 228-232. 10.1016/S0169-4758(00)01666-5.
Sitprija V, Napathorn S, Laorpatanaskul S, Suithichaiyakul T, Moollaor P, Suwangool P, Sridama V, Thamaree S, Tankeyoon M: Renal and systemic hemodynamics, in falciparum malaria. Am J Nephrol. 1996, 16: 513-519.
Looareesuwan S, Wilairatana P, Krishna S, Kendall B, Vannaphan S, Viravan C, White NJ: Magnetic resonance imaging of the brain in patients with cerebral malaria. Clin Infect Dis. 1995, 21: 300-309.
Charoenpan P, Indraprasit S, Kiatboonsri S, Suvachittanont O, Tanomsup S: Pulmonary edema in severe falciparum malaria. Hemodynamic study and clinicophysiologic correlation. Chest. 1990, 97: 1190-1197.
Lee MV, Ambrus JL, DeSouza JM, Lee RV: Diminished red blood cell deformability in uncomplicated human malaria. A preliminary report. J Med. 1982, 13: 479-485.
Areekul S, Yamarat P: Alterations in the viscosity and deformability of red cells in patients with Plasmodium falciparum. J Med Assoc Thai. 1988, 71: 196-202.
Nash GB, O'Brien E, Gordon-Smith EC, Dormandy JA: Abnormalities in the mechanical properties of red blood cells caused by Plasmodium falciparum. Blood. 1989, 74: 855-861.
Paulitschke M, Nash GB: Membrane rigidity of red blood cells parasitized by different strains of Plasmodium falciparum. J Lab Clin Med. 1993, 122: 581-589.
Dondorp AM, Angus BJ, Chotivanich K, Silamut K, Ruangveerayuth R, Hardeman MR, Kager PA, Vreeken J, White NJ: Red blood cell deformability as a predictor of anaemia in severe falciparum malaria. Am J Trop Med Hyg. 1999, 60: 733-737.
Udomsangpetch R, Webster HK, Pattanapanyasat K, Pitchayangkul S, Thaithong S: Cytoadherence characteristics of rosette-forming Plasmodium falciparum. Infect Immun. 1992, 60: 4483-4490.
Fernandez V, Wahlgren M: Rosetting and autoagglutination in Plasmodium falciparum. Chem Immunol. 2002, 80: 163-187.
Nash GB, Cooke BM, Marsh K, Berendt A, Newbold C, Stuart J: Rheological analysis of the adhesive interactions of red blood cells parasitized by Plasmodium falciparum. Blood. 1992, 79: 798-807.
Nash GB, Cooke BM, Carlson J, Wahlgren M: Rheological properties of rosettes formed by red blood cells parasitized by Plasmodium falciparum. Br J Haematol. 1992, 82: 757-763.
Chotivanich K, Udomsangpetch R, Dondorp A, Williams T, Angus B, Simpson JA, Pukrittayakamee S, Looareesuwan S, Newbold CI, White NJ: The mechanisms of parasite clearance after antimalarial treatment of Plasmodium falciparum malaria. J Infect Dis. 2000, 182: 629-633. 10.1086/315718.
Cooke BM, Berendt AR, Craig AG, MacGregor J, Newbold CI, Nash GB: Rolling and stationary cytoadhesion of red blood cells parasitized by Plasmodium falciparum : separate roles for ICAM-1, CD36 and thrombospondin. Br J Haematol. 1994, 87: 162-170.
Chu Y, Haigh T, Nash GB: Rheological analysis of the formation of rosettes by red blood cells parasitized by Plasmodium falciparum. Br J Haematol. 1997, 99: 777-783. 10.1046/j.1365-2141.1997.4643268.x.
Raventos-Suarez C, Kaul DK, Macaluso F, Nagel RL: Membrane knobs are required for the microcirculatory obstruction induced by Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci U S A. 1985, 82: 3829-3833.
Kaul DK, Liu XD, Nagel RL, Shear HL: Microvascular hemodynamics and in vivo evidence for the role of intercellular adhesion molecule-1 in the sequestration of infected red blood cells in a mouse model of lethal malaria. Am J Trop Med Hyg. 1998, 58: 240-247.
Kaul DK, Nagel RL, Llena JF, Shear HL: Cerebral malaria in mice: demonstration of cytoadherence of infected red blood cells and microrheologic correlates. Am J Trop Med Hyg. 1994, 50: 512-521.
Kaul DK, Roth EF, Nagel RL, Howard RJ, Handunnetti SM: Rosetting of Plasmodium falciparum-infected red blood cells with uninfected red blood cells enhances microvascular obstruction under flow conditions. Blood. 1991, 78: 812-819.
Cooke BM, Morris-Jones S, Greenwood BM, Nash GB: Adhesion of parasitized red blood cells to cultured endothelial cells: a flow-based study of isolates from Gambian children with falciparum malaria. Parasitology. 1993, 107: 359-368.
Eiam-Ong S, Sitprija V: Falciparum malaria and the kidney: a model of inflammation. Am J Kidney Dis. 1998, 32: 361-375.
Lasky LA: Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu Rev Biochem. 1995, 64: 113-139. 10.1146/annurev.biochem.64.1.113.
Areekul S: Renal plasma flow in rhesus monkeys infected with Plasmodium knowlesi. Southeast Asian J Trop Med Public Health. 1987, 18: 186-192.
Sitprija V, Vongsthongsri M, Poshyachinda V, Arthachinta S: Renal failure in malaria: a pathophysiologic study. Nephron. 1977, 18: 277-287.
Molyneux ME, Looareesuwan S, Menzies IS, Grainger SL, Phillips RE, Wattanagoon Y, Thompson RP, Warrell DA: Reduced hepatic blood flow and intestinal malabsorption in severe falciparum malaria. Am J Trop Med Hyg. 1989, 40: 470-476.
Pukrittayakamee S, White NJ, Davis TM, Looareesuwan S, Supanaranond W, Desakorn V, Chaivisuth B, Williamson DH: Hepatic blood flow and metabolism in severe falciparum malaria: clearance of intravenously administered galactose. Clin Sci (Lond). 1992, 82: 63-70.
Warrell DA, White NJ, Veall N, Looareesuwan S, Chanthavanich P, Phillips RE, Karbwang J, Pongpaew P, Krishna S: Cerebral anaerobic glycolysis and reduced cerebral oxygen transport in human cerebral malaria. Lancet. 1988, 2: 534-538. 10.1016/S0140-6736(88)92658-X.
Newton CR, Marsh K, Peshu N, Kirkham FJ: Perturbations of cerebral hemodynamics in Kenyans with cerebral malaria. Pediatr Neurol. 1996, 15: 41-49. 10.1016/0887-8994(96)00115-4.
Arbeille P, Carles G, Bousquet F, Body G, Lansac J: Fetal cerebral and umbilical artery blood flow changes during pregnancy complicated by malaria. J Ultrasound Med. 1998, 17: 223-229.
Franz DR, Lee M, Seng LT, Young GD, Baze WB, Lewis GE: Peripheral vascular pathophysiology of Plasmodium berghei infection: a comparative study in the cheek pouch and brain of the golden hamster. Am J Trop Med Hyg. 1987, 36: 474-480.
Clavier N, Rahimy C, Falanga P, Ayivi B, Payen D: No evidence for cerebral hypoperfusion during cerebral malaria. Crit Care Med. 1999, 27: 628-632. 10.1097/00003246-199903000-00047.
Krishna S, Waller DW, ter Kuile F, Kwiatkowski D, Crawley J, Craddock CF, Nosten F, Chapman D, Brewster D, Holloway PA: Lactic acidosis and hypoglycaemia in children with severe malaria: pathophysiological and prognostic significance. Trans R Soc Trop Med Hyg. 1994, 88: 67-73.
Newton CR, Taylor TE, Whitten RO: Pathophysiology of fatal falciparum malaria in African children. Am J Trop Med Hyg. 1998, 58: 673-683.
Newton CR, Krishna S: Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacol Ther. 1998, 79: 1-53. 10.1016/S0163-7258(98)00008-4.
Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N: Indicators of life-threatening malaria in African children. N Engl J Med. 1995, 332: 1399-1404. 10.1056/NEJM199505253322102.
White NJ: Pathophysiology. In Clinics in Tropical Medicine and Communicable Diseases. Edited by: Strickland GT. 1986, London, Saunders, Malaria, 1: 59-90.
Simpson JA, Silamut K, Chotivanich K, Pukrittayakamee S, White NJ: Red cell selectivity in malaria: a study of multiple-infected erythrocytes. Trans R Soc Trop Med Hyg. 1999, 93: 165-168.
Chotivanich K, Udomsangpetch R, Simpson JA, Newton P, Pukrittayakamee S, Looareesuwan S, White NJ: Parasite multiplication potential and the severity of Falciparum malaria. J Infect Dis. 2000, 181: 1206-1209. 10.1086/315353.
Dennis ED, Mitchell GH, Butcher GA, Cohen S: In vitro isolation of Plasmodium knowlesi merozoites using polycarbonate sieves. Parasitology. 1975, 71: 475-481.
Miller LH: Hypothesis on the mechanism of erythrocyte invasion by malaria merozoites. Bull World Health Organ. 1977, 55: 157-162.
Butcher GA: A comparison of static thin layer and suspension cultures for the maintenance in vitro of Plasmodium falciparum. Ann Trop Med Parasitol. 1981, 75: 7-17.
Slutsker L, Taylor TE, Wirima JJ, Steketee RW: In-hospital morbidity and mortality due to malaria-associated severe anaemia in two areas of Malawi with different patterns of malaria infection. Trans R Soc Trop Med Hyg. 1994, 88: 548-551.
Hoshen MB, Heinrich R, Stein WD, Ginsburg H: Mathematical modelling of the within-host dynamics of Plasmodium falciparum. Parasitology. 2000, 121: 227-235. 10.1017/S0031182099006368.
Molineaux L, Diebner HH, Eichner M, Collins WE, Jeffery GM, Dietz K: Plasmodium falciparum parasitaemia described by a new mathematical model. Parasitology. 2001, 122: 379-391. 10.1017/S0031182001007533.
Acknowledgement
This research was supported by the Israel Science Foundation (grant No. 187/98).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ginsburg, H., Hoshen, M.B. Is the development of falciparum malaria in the human host limited by the availability of uninfected erythrocytes?. Malar J 1, 18 (2002). https://doi.org/10.1186/1475-2875-1-18
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-1-18