Abstract
In this review we will focus on the current status and views concerning the production of antibody fragments and antibody fusion proteins by yeasts and filamentous fungi. We will focus on single-chain antibody fragment production (scFv and VHH) by these lower eukaryotes and the possible applications of these proteins. Also the coupling of fragments to relevant enzymes or other components will be discussed. As an example of the fusion protein strategy, the 'magic bullet' approach for industrial applications, will be highlighted.
Similar content being viewed by others
Introduction
Antibodies (also called immunoglobulins) are glycoproteins, which specifically recognise foreign molecules. These recognised foreign molecules are called antigens. When antigens invade humans or animals, an immunological response is triggered which involves the production of antibodies by B-lymphocytes. By this immunological response, microorganisms, larger parasites, viruses and bacterial toxins can be rendered harmless. The unique ability of antibodies to specifically recognise and bind with high affinity to virtually any type of antigen, made them interesting molecules for medical and scientific research.
In 1975 Köhler and Milstein developed the monoclonal antibody technology [1] by immortalising mouse cell lines that secreted only one single type of antibody with unique antigen specificity, called monoclonal antibodies (mAbs). With this technology, isolation and production of mAbs against protein, carbohydrate, nucleic acids and hapten antigens was achieved. The technology resulted in a rapid development of the use of antibodies in diagnostics (e.g. pregnancy tests; [2]), human therapeutics and as fundamental research tools.
More applications outside research and medicine can be considered, such as consumer applications. Examples are the use of antibodies in shampoos to prevent the formation of dandruff [3] or in toothpaste to protect against tooth decay caused by caries [4]. For these purposes large quantities of antibodies are required. However, for these applications on a larger scale there were some major problems concerning the expensive production system based on mammalian expression, the difficulty of producing antibodies in bulk amounts and the low stability and solubility of some antibodies under specific (harsh) conditions.
In this review we will discuss the possibilities of large-scale production of antibodies and fragments thereof by relevant expression systems. Requirements are that the system used for production is cheap, accessible for genetic modifications, easily scaled up for greater demands and safe for use in consumer applications.
First, structure and characteristics of antibodies and antibody fragments generated thereof will be discussed, followed by the impact of recombinant DNA technology and antibody engineering techniques on the generation and modification of antibodies and antibody fragments. The modification of antibodies is of major interest since changes in their functionality and physico-chemical properties will broaden their application area. For most applications only the antigen-binding site of the native antibody molecule is required and even preferred. By the development of recombinant DNA technology and the increasing knowledge on the structure of antibody molecules created the opportunity to clone and engineer smaller fragments of antibody genes [5, 6] and subsequent alter their functions, for example improve the affinity for their antigen. Besides that, recombinant DNA technology provides the possibility to generate fusion proteins or 'Magic bullets', consisting of an antibody fragment fused to an effector molecule.
In this review the various expression systems for these type of protein will be outlined. We will detail on using yeasts and filamentous fungi as suitable expression systems for antibody fragments and antibody fusion proteins.
Antibodies and their unique antigen binding domains
Whole antibodies
In vertebrates five immunoglobulin classes are described (IgG, IgM, IgA, IgD and IgE), which differ in their function in the immune system. IgGs are the most abundant immunoglobulins in the blood and these molecules have a molecular weight of approximately 160 kDa. They have a basic structure of two identical heavy (H) chain polypeptides and two identical light (L) chain polypeptides (Figure 1). The H and L chains, which are all β-barrels, are kept together by disulfide bridges and non-covalent bonds (for a review about antibody structure see [7]). The chains themselves can be divided in variable and constant domains. The variable domains of the heavy and light chain (VH and VL) which are extremely variable in amino acid sequences are located at the N-terminal part of the antibody molecule. VH and VL together form the unique antigen-recognition site. The amino acid sequences of the remaining C-terminal domains are much less variable and are called CH1, CH2, CH3 and CL.
Schematical representation of the structure of a conventional IgG and fragments that can be generated thereof. The constant heavy-chain domains CH1, CH2 and CH3 are shown in yellow, the constant light-chain domain (CL) in green and the variable heavy-chain (VH) or light-chain (VL) domains in red and orange, respectively. The antigen binding domains of a conventional antibody are Fabs and Fv fragments. Fab fragments can be generated by papain digestion. Fvs are the smallest fragments with an intact antigen-binding domain. They can be generated by enzymatic approaches or expression of the relevant gene fragments (the recombinant version). In the recombinant single-chain Fv fragment, the variable domains are joined by a peptide linker. Both possible configurations of the variable domains are shown, i.e. the carboxyl terminus of VH fused to the N-terminus of VL and vice versa.
Fc fragment
The non-antigen binding part of an antibody molecule, the constant domain Fc mediates several immunological functions, such as binding to receptors on target cells and complement fixation (triggering effector functions that eliminate the antigen). The Fc domain is not essential for most biotechnical applications, relying on antigen binding. The Fc fragment, which is glycosylated, can have different effector functions in the different classes of immunoglobulins.
Antigen binding region
The unique antigen-binding site of an antibody consists of the heavy and light chain variable domains (VH and VL). Each domain contains four conserved framework regions (FR) and three regions called CDRs (complementarity determining regions) or hypervariable regions. The CDRs strongly vary in sequence and determine the specificity of the antibody. VL and VH domains together form a binding site, which binds a specific antigen.
Antibody fragments generated thereof
Several functional antigen-binding antibody fragments could be engineered by proteolysis of antibodies (papain digestion, pepsin digestions or other enzymatic approaches), yielding Fab, Fv or single domains (Figure 1).
Fab fragments
Fab fragments (fragment antigen binding) are the antigen-binding domains of an antibody molecule, containing VH + CH1 and CL + VL. Between CL and CH1 an interchain disulfide bond is present. The molecular weight of the heterodimer is usually around 50 kDa [8]. Fab fragments can be prepared by papain digestions of whole antibodies.
Fv fragments
The minimal fragment (~30 kDa) that still contains the whole antigen-binding site of a whole IgG antibody is composed of both the variable heavy chain (VH) and variable light chain (VL) domains. This heterodimer, called Fv fragment (for fragment variable) is still capable of binding the antigen [9]. Normally, native Fv fragments are unstable since the non-covalently associated VL and VH domains tend to dissociate from one another at low protein concentrations.
Single domains
Single domain antigen binding fragments (dAbs) or VHs were generated in the past [10, 11]. They have good antigen-binding affinities, but exposure of the hydrophobic surface of the VH to the solvent, which normally interacts with the VL, causes a sticky behaviour of the isolated VHs. It turned out to be difficult to produce them in soluble form, although replacement of certain amino acids increased solubility of these single domains (see also Llama Heavy-chain antibody fragments) Besides that, their affinity for the antigen was much less compared with other antibody fragments [12].
Heavy-chain antibodies in Camelidae
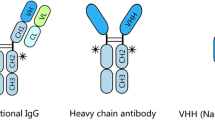
In 1993 Hamers-Casterman et al. [13] discovered a novel class of IgG antibodies in Camelidae (camels, dromedaries and llamas). These antibodies are devoid of light chains and therefore called 'heavy-chain' IgGs or HCAb (for heavy-chain antibody; Figure 2). HCAbs have a molecular weight of ~95 kDa instead of the ~160 kDa for conventional IgG antibodies. Their binding domains consist only of the heavy-chain variable domains, referred to as VHHs [14] to distinguish it from conventional VHs. Since the first constant domain (CH1) is absent (spliced out during mRNA processing due to loss of a splice consensus signal; [15, 16]), the variable domain (VHH) is immediately followed by the hinge region, the CH2 and the CH3 domains. Although the HCAbs are devoid of light chains, they have an authentic antigen-binding repertoire. The current knowledge about the genetic generation mechanism of HCAbs is reviewed by Nguyen et al. [17, 18].
Schematical representation of the structure of a conventional IgG, a heavy-chain IgG antibody and the variable heavy-chain antibody fragment (VHH) that can be generated of the latter. Heavy-chain antibodies found in llama and camel are only composed of heavy-chains and lack the light chain completely, as shown in this Figure. The antigen-binding domain consists of only the VH domain, which is referred to as VHH (variable heavy-chain antibody fragment), to distinguish it from a normal VH. The constant heavy-chain domains CH1, CH2 and CH3 are shown in yellow, the constant light-chain domain (CL) in green and the variable heavy-chain (VH or VHH) or light-chain (VL) domains in red and orange, respectively.
Recombinant antibodies, antibody fragments and antibody fusion proteins
The development and applications of recombinant DNA technology led to the design of several new antibodies and antibody fragments. Firstly, functionalities of these proteins may be altered resulting in novel and improved functions. One of the possible applications of recombinant whole antibodies is the use in human therapeutics (see also Recombinant whole antibodies). Secondly, smaller antibody fragments may be synthesised having the advantage over whole antibodies in applications requiring tissue penetration and rapid clearance from the blood or kidney. Moreover, the use of recombinant expression systems could also be the solution for large-scale production of antibody (fragments).
Recombinant whole antibodies
The development of human(ised) antibody molecules is mostly aimed at reduction of unwanted immunological properties in medical applications [19]. Repeated doses of foreign (murine) antibody molecules could lead to an immune response in patients recognising the mouse antibody as foreign. This so-called HAMA (human anti-mouse antibody) response can lead to severe health problems.
Two strategies are developed to reduce the antigenicity of therapeutic antibodies (see also [20]). One of these strategies is chimerisation. In this case the constant murine domains are replaced by human constant domains [21, 22]. The second strategy is grafting of only the murine CDRs onto existing human antibody framework regions, which is called humanisation [22].
At present there are more than 10 recombinant antibodies approved by the US Food and Drug Administration (FDA) for use in medicine and many more are in a late stage of clinical trials. FDA approved recombinant mAbs are e.g. Herceptin™ (Genetech, San Francisco, CA), which targets and blocks the growth factor Her2 on the surface of breast cancer cells and Rituxan™ (IDEC Pharmaceuticals Inc., San Diego, CA) used against non-Hodgkin's lymphoma (see for more examples [23, 24]). The use of recombinant antibodies for medical purposes does not require a cheap large-scale production process per se, since only a limited amount of pure preparations is needed.
Production of recombinant antibody fragments by Escherichia coli
Much work on antibody fragment production has been focussed on Escherichia coli as an expression system (reviewed in [25]). The advantage of this system is the ability to produce proteins in relative large amounts. Besides that, E. coli is easily accessible for genetic modifications, requires simple inexpensive media for rapid growth and they can easily be cultured in fermentors permitting large-scale production of proteins of interest. Several antibody fragments have been produced in functional form (e.g. [8, 9, 26, 27]) and expression of relevant gene segments also permitted the production of the recombinant antibody fragments. The problem of stability has been tackled by generation of single-chain Fv (scFv) or disulfide stabilised Fv (dsFv) fragments.
Selection of antibody fragments with improved functionalities
In 1990 McCafferty et al. [28] showed that antibody fragments could be displayed on the surface of filamentous phages, called phage-display. This technology is based on the fusion of the antibody variable genes to a phage coat protein gene (e.g. [20]). After displaying an antibody fragment on the protein surface of the phage, antigen specific phages can be selected and enriched by multiple rounds of affinity panning (e.g. reviewed in [29, 30]). This technique makes it possible to select phages that bind almost any antigen, including those previously considered to be difficult, such as self-antigens or cell surface proteins.
Libraries can be prepared from variable genes isolated from immunised animals, non-immunised sources (naïve libraries, thus avoiding the need for immunisation) or even (semi-) synthetic libraries can be constructed. The V genes can be subjected to random mutagenesis, chain or DNA shuffling methods [31], mimicking the natural hypermutation mechanism.
Single-chain Fv fragments and multimers
An attractive recombinant antibody fragment is the single-chain Fv (scFv) fragment (reviewed in [32, 33]). It has a high affinity for its antigen and can be expressed in a variety of hosts [34]. These and other properties make scFv fragments not only applicable in medicine (reviewed in [35]), but also of potential for biotechnological applications. In the scFv fragment the VH and VL domains are joined with a hydrophilic and flexible peptide linker, which improves expression and folding efficiency [36, 37]. Usually linkers of about 15 amino acids are used, of which the (Gly4Ser)3 linker has been used most frequently [35]. Unfortunately, some scFv molecules have a reduced affinity compared to the parental whole antibody or Fab molecule [12, 38, 39]. Besides that, scFv molecules can be easily proteolytically degraded, depending on the linker used [40]. With the development of genetic engineering techniques these limitations could be practically overcome by research focussed on improvement of function and stability, as discussed in [32]. An example is the generation of disulfide-stabilised Fv fragments where the VH-VL dimer is stabilised by an interchain disulfide bond [38, 41, 42]. Cysteïnes are introduced at the interface between the VL and VH domains, forming a disulfide bridge, which holds the two domains together (reviewed in [43]).
Dissociation of scFvs results in monomeric scFvs, which can be complexed into dimers (diabodies), trimers (triabodies) or larger aggregates ([44], reviewed in [45]). The simplest designs are diabodies that have two functional antigen-binding domains that can be either similar (bivalent diabodies) or have specificity for distinct antigens (bispecific diabodies). These bispecific antibodies allow for example the recruitment of novel effector functions (such as cytotoxic T cells) to the target cells, which make them very useful for applications in medicine (reviewed in [46, 47]).
Llama Heavy-chain antibody fragments (VHHs)
The other type of interesting antibody fragments are VHHs (see Figure 2) comprising the smallest available intact antigen-binding fragment (~15 kDa, 118–136 residues [48, 49]). The affinities found for VHHs were in the nanomolecular range and comparable with those of Fab and single chain Fv (scFv) fragments [50, 51]. Besides that VHHs are highly soluble and more stable than the corresponding derivatives of scFv and Fab fragments [50, 52]. VHHs carry amino acid substitutions that make them more hydrophilic and prevent the prolonged interaction with BiP (Immunoglobulin heavy-chain binding protein), which normally binds to the H-chain in the Endoplasmic Reticulum (ER) during folding and assembly, until it is displaced by the L-chain [53]. There are indications that this increased hydrophilicity improves secretion of the VHHs from the ER. Hence, production of VHHs in commercially attractive microorganisms may be favourable.
Several ways are described to obtain functional VHHs: from proteolysed HCAb of an immunised camelid, direct cloning of VHH genes from B-cells of an immunised camelid resulting in recombinant VHHs or from naïve or synthetic libraries [49]. VHHs with desired antigen specificity could be selected by phage display (see Selection of antibody fragments with improved functionalities). Using VHHs in phage display is much simpler and more efficient as compared with Fabs or scFvs, since only one domain needs to be cloned and expressed to obtain a functional antigen-binding fragment [52, 54].
As already noted before (see Antibody fragments generated thereof), classical VHs were difficult to produce in soluble form. To improve their solubility and prevent non-specific binding, residues located on the VL side of VHs were replaced by 'VHH-like' residues, mimicking the more soluble VHH fragments. This process has been termed camelisation [55–57] and these camelised VH fragments, particularly those based on the human framework, are expected to have significant advantages for therapeutical purposes in humans (reviewed in [58]).
Fusion proteins ('Magic bullets')
A completely new use of the binding capacity of antibody fragments is the design of a fusion approach, in which an effector protein is coupled to an antigen recognising antibody fragment. In human medicine this approach is referred to 'Magic bullet'. All kinds of molecules can be used as effector molecule only limited by the imagination. The gene encoding the effector may be directly fused to the gene of the antibody fragment of interest, resulting in novel bifunctional proteins [59]. Examples of the use of this approach will be given in the section Antibody fragments and antibody fusion proteins for large-scale applications and consumer products.
Applications of antibody fragments and antibody fusion proteins
Applications of antibody fragments in human medicine
The smaller the better
Most applications of recombinant antibody fragments are related to diagnosis and therapy in human medicine, which is especially focussed on the use of antibodies as the ideal cancer-targeting reagent (reviewed in [19, 60–62]). For some clinical applications small antibody fragments have advantages over whole antibodies. The small size permits them to penetrate tissues and solid tumours more rapidly than whole antibodies [63] which recently also was shown for VHHs [64]. Smaller antibody fragments have also a much faster clearance rate in the blood circulation, which leads to differences of selectivity [63]. Nowadays there are also promising pre-clinical and clinical trials with antibody fragments as diagnostic or therapeutical agents [61, 65]. Another application of antibody fragments is to treat viral infections with so-called intrabodies, which are intracellular antibodies synthesised by the cell and targeted to inactivate specific proteins within the cell [66].
'Magic bullets' in medicine
The use of bi-functional molecules in medicine is aimed at delivery of a protein drug, which is only active where it is required. It thereby limits the dose of the drug, resulting in less side effects of the drug towards healthy tissue and/or less immunogenic response to the protein drug itself. Also the physical interaction between the target and the effector molecule increases the potency of the effector. Fusion proteins are ideal immuno agents for cancer diagnosis [67] and cancer therapeutics. An example is the use of cancer-specific bi-functional antibodies targeting potent cytotoxic molecules to tumour cells and subsequently eliminate these tumour cells without harming healthy cells [68].
Potential applications of VHHs
Specific applications of VHHs are foreseen in the following direction:
VHHs as drug carriers
It is expected that VHHs are also applicable in diagnosis and therapy in human medicine, especially when an economically feasible production, small size and stability are required (reviewed in [49]). Cortez-Retamozo et al. [64] recently showed that VHHs specifically could be targeted to tumour cells, which together with the possibility of generation of bispecific VHH constructs [69] is of major interest for cancer therapy.
VHHs as delivery carriers in the brain
Antibodies and many other water soluble compounds are excluded from the brain by the blood-brain barrier (BBB), thus making treatment of brain-related disease very difficult. Recently, Muruganandam et al. [70] showed that VHH were able to selectively bind to and transmigrate across the BBB in a human in vitro BBB model and partly in vivo in mice. This property can be exploited for the development of efficient antibody carriers suitable for delivery of macromolecules across the human BBB and subsequently for treatment of neurological diseases.
VHHs as potent enzyme inhibitors
Hypervariable regions in VHHs are on average longer than those of VHs [71, 72]. The extended hypervariable regions of VHHs are capable of penetrating deep into the cleft of active sites of enzymes, binding to novel epitopes that are not recognised by conventional antibodies [51, 73, 74]. Because of this property VHHs may act as better potent enzyme inhibitors [51, 75, 76].
VHHs in consumer products
Since llama VHHs are very stable, even at high temperature, applications can be envisaged in which a high temperature step is involved (e.g. pasteurisation), without losing antigen-binding properties [50]. Recently it was shown that VHHs could be used to prevent phage infection in cheese production processes [77], by recognising a structural protein of the phage, which is involved in recognition of the host Lactococcus lactis.
Antibody fragments and antibody fusion proteins for large-scale applications and consumer products
Many additional applications can be envisaged if an inexpensive and simple production system is available, yielding large amounts of antibody fragments that can be purified easily. The highly specific antigen-binding ability could be used for inactivating bacteria or specific enzymes that can cause spoilage of food. Other suggested applications are the use in biosensors, treatment of wastewater [78], industrial scale separation processes such as separation of chiral molecules [79], purification of specific components (proteins) from biological materials or the use as abzymes [80, 81]. They have also been considered as components of novel consumer goods with new improved functionalities, in oral care and personal hygiene (e.g. in toothpaste or mouthwashes [82]). For dental applications antibody fragments can be coupled to enzymes to increase the concentration of antimicrobials like hypothiocyanate and hypohalites, for example glucose oxidase (GOX; [83]), galactose oxidase (GaOX; [84]) or lactate oxidase (LOX; [85]). Other examples are targeted bleach in laundry washing (e.g. detergents containing antibodies coupled to molecules that specifically remove difficult stains) or the use in shampoos where antibodies act to prevent dandruff by inhibiting growth of specific microorganisms causing this [3].
Suitable expression systems for the large-scale production of antibody fragments and antibody fusion proteins
To be able to use antibody fragments and antibody fusion proteins in these large scale applications, a suitable expression system has to be chosen. Several expression systems are available, both from prokaryotic (Table 1) and eukaryotic (Table 2) origin. Our main interest goes out to these systems that are able to economically produce large amount of proteins into the culture medium. Several of these systems can be considered as suitable (both from prokaryotic and eukaryotic origin). Hereafter several of these systems will be discussed, with an emphasis on yeast and fungal systems.
Drawbacks using E. coli as a host for antibody fragment production
As described in the section Production of recombinant antibody fragments by Escherichia coli, this micro-organism has shown to be a potential expression host for antibody fragments and fusion proteins. Although the general production yields in shake-flask cultures are low (several mg/L), in fermentation processes several g/L could be obtained (reviewed in [86]). There are two possibilities of antibody fragment production in E. coli, either by secretion of the fragments into the culture medium and/or periplasmic space (the compartment between the inner and outer membrane) or preparation of inclusion bodies with subsequent in vitro folding. However, both strategies have disadvantages that make the use of this prokaryote not attractive for the large-scale production of antibody fragments and antibody fusion proteins. Firstly, the secretion of folded and fully assembled fragments in the medium or periplasmic space is often accompanied with cell lysis and subsequent product loss. Secondly, 'toxicity' of the antibody sequence and concomitant plasmid loss is frequently observed, which hamper high production levels (reviewed in [25]). Thirdly, expression of the fragments in inclusion bodies, which often results in insoluble protein aggregates [87], demands laborious and cost-intensive in vitro refolding (denaturation and renaturation) and purification steps. Hence, the final yield of fragments is only a small percentage of the protein that was initially present in the inclusion bodies even though purification steps are nowadays facilitated by affinity chromatography using C-terminal polypeptide tails, like poly-His6 or FLAG [88, 89]. Recently, production of soluble and functional scFv by E. coli could be increased by improving disulfide bond formation activity in the cytoplasm, using mutants and overexpression of disulfide-bond isomerase [90]. Finally, E. coli is unable to carry out eukaryotic post-translational modifications and is therefore not suitable when glycosylation of antibody fragments or more importantly the fusion proteins is required.
Alternative prokaryotic expression systems
E. coli is not the only available prokaryotic expression system, although it is rather dominant in the field. Alternative prokaryotic expression systems are available for antibody fragment production (Table 1). However, these will encounter similar limitations as E. coli, even though most organisms described in Table 1 secrete the investigated antibody fragment into the culture medium. A field where production of antibody fragments in prokaryotic cells could still be interesting, is in food grade organisms used for delivery passive immunisation in humans, by means of functional foods. In a recent article, Kruger et al. [91] reported the production of scFv antibody fragments against Streptococcus mutans by the Gram positive food grade bacteria Lactobacillus zeae. In experimental animals a decrease of S. mutans and reduced development of caries was observed.
Eukaryotic expression systems
Also several eukaryotic systems can be envisaged for large-scale production of antibody fragments and antibody fusion proteins (see also [34]), like mammalian cells, insect cells, plants, transgenic animals and lower eukaryotes (see Table 2).
The production of therapeutical whole antibodies is well established in mammalian cells. However, large-scale production is expensive and time-consuming.
'Plantibodies' can be produced in several plant target organs (reviewed in [92]). Roots, storage organs (seeds and tubers) and fruiting bodies can be suitable for mass oral (edible) applications (see [93] and references therein). Expression of scFv in transgenic plants has been proposed as a way to produce and store pharmaceutical antibodies [94, 95] and as means to block physiological processes in the plant itself [96] or establish plant pathogen resistance [97]. Plants show several advantages as large-scale antibody production systems, like the ease and low costs of growing plants, even in large quantities. However, the generation of transgenic plants that express antibodies is a time consuming process and the downstream processing to isolate the expressed antibodies from the plant parts is relatively expensive and laborious.
Production of antibody fragments by lower eukaryotes
An attractive possibility for the cost-effective large-scale production of antibody fragments and antibody fusion proteins are yeast or fungal fermentations. Large-scale fermentation of these organisms is an established technology already used for bulk production of several other recombinant proteins and extensive knowledge is available on downstream processes. Besides that, yeasts and filamentous fungi are accessible for genetic modifications and the protein of interest may be secreted into the culture medium. In addition, some of their products have the so-called GRAS (Generally Regarded As Safe) status and they do not harbour pyrogens, toxins or viral inclusions.
Methylotrophic and other yeasts
The methylotrophic and other yeasts like Candida boidinii, Hansenula polymorpha, Pichia methanolica and Pichia pastoris are well known systems for the production of heterologous proteins (reviewed in [98]). High levels of heterologous proteins (milligram-to-gram quantities) can be obtained and scaling up to fermentation for industrial applications is possible [99–101].
Especially the P. pastoris system is used in several industrial-scale production processes [102]. Ridder et al. [103] were the first to report the expression of a scFv fragment by P. pastoris. From then on several papers reported about the use of P. pastoris for the production of recombinant antibodies and fragments thereof [104, 105]. In shake-flask cultures a level of 250 mg/L scFv was obtained [106] and Freyre et al. [107] were able to obtain even an expression level of 1.2 g/L scFv fragment under fermentation conditions. However, Cupit et al. [108] also showed that the production of antibody fragments by P. pastoris is not always a success story.
Based on the described results the commercial recombinant antibody production by P. pastoris is promising. However, products currently obtained from P. pastoris are not regarded as GRAS, which may limit its use.
Wood et al. [109] were the first to report the production of mouse IgM by the baker's yeast S. cerevisiae, although only unassembled chains were detected in the culture medium. However, the production of Fab fragments was possible as was first shown by Horwitz et al. [110]. Although the obtained levels were low, functional Fab fragments were secreted in the culture medium. Davis et al. [111] expressed scFv antibody fragments in Schizosaccharomyces pombe. Studies on the scFv production in the non conventional yeasts Yarrowia lipolytica and Kluyveromyces lactis resulted in 10–20 mg/L functional and soluble anti-Ras scFv [112].
Filamentous fungi: Trichoderma reesei and Aspergillus spp
Filamentous fungi, in particular species from the genera Trichoderma and Aspergillus have the capacity to secrete large amounts of proteins, metabolites and organic acids into their culture medium. This property has been widely exploited by the food and beverage industries where compounds secreted by these filamentous fungal species have been used for decades. This has led to the GRAS status for some of their products. Filamentous fungi like A. awamori, A. niger and A. oryzae are therefore suitable organisms for the production of commercially interesting homologous and heterologous proteins [113–115]. Strategies to improve protein secretion by filamentous fungi are extensively reviewed in [116–119].
Production strains of Trichoderma reesei (Hypocrea jecorina) have an exceptional secretion capacity up to 35 g protein/L, where half of the secreted protein consists of the cellulase cellobiohydrolase I (CBH1; [120]). Therefore, Trichoderma is considered as an excellent host for the production of heterologous proteins (reviewed in [121, 122]). Nyyssönen et al. [123] reported a production of 1 mg/L in shake-flasks of Fab antibody fragments by T. Reesei Rut-C30. More strikingly, when the Fab antibody fragment chain was fused to the core-linker region of CBH1, a production level of 40 mg/L in shake-flasks and 150 mg/L in bioreactor cultivations was obtained [123, 124].
The use of S. cerevisiae and A. awamori for the large-scale production of antibody fragments and fusion proteins
In our own laboratory at TNO Nutrition and Food Research in Zeist (The Netherlands) and in collaboration with Unilever Research Vlaardingen (The Netherlands) research on antibody fragment production in S. cerevisiae and A. awamori has been carried out [125, 126]. The aim of this project was a detailed comparison of both expression systems, in relation to their possible large-scale production process of antibody fragments and fusion proteins. In the framework of this collaboration also a new A. awamori expression system, based on xylose induction was developed [127].
The use of S. cerevisiae and A. awamori for the large-scale production of scFv
To investigate the feasibility of a large-scale cost-effective process for the extracellular production of (functionalised) scFv fragments initially S. cerevisiae was used. However, it was shown that S. cerevisiae was a poor host for the production of scFv, since the secretion of scFv was hampered by improper folding of the fragments, because large aggregates were formed in the ER and vacuolar-like organelles. It was hypothesised that the exposure of the hydrophobic surfaces on the VL and VH chains of scFv plays an important role in the accumulation of scFv in the cell [128]. Shusta et al. [129] reported the increase of scFv production up to 20 mg/L in S. cerevisiae by optimising the expression system by overexpression of two ER resident chaperones and reduction of growth temperature. Kauffman et al. [130] showed that overexpression of scFv in S. cerevisiae resulted in cellular stress, displayed by decreased growth rates and induction of the Unfolded Protein Response (UPR). It was hypothesised that a functional UPR was required to decrease the malfolded scFv in the ER, leading to a recovery from cell stress.
As further improved levels were desired also a fungal expression system was considered [4]. In shake-flask cultures a production level of 10 mg/L was achieved by using A. awamori as production host. As secretion of a heterologous protein can be greatly enhanced by fusing it to a "carrier" protein such as glucoamylase (GLA; [117, 118]), also this fusion-approach was employed. Analysis of the culture medium of transformants carrying the fusion construct revealed a production of approximately 50 mg/L scFv in the culture medium [4]. Several commercially interesting scFv fragments were investigated for their ability to be produced by A. awamori using the GLA-fusion strategy. The results showed that the production levels differed significantly between the different scFv transformants. Interestingly, in some cases increased levels of scFv detected in the culture medium corresponded to an increase of transcription level of the ER chaperone BiPA [131], indicating that the antibody fragments, like in S. cerevisiae, may have problems with correct folding and aggregate in the fungal cell.
To increase production levels, successful 10 L and 1,5 × 104 L scale fermentations were carried out resulting in 200 mg/L scFv under optimal conditions. However, variable amounts of scFv dimers and other multimers were observed. Recent fermentation experiments performed by Sotiriadis et al. [132] showed that the highest scFv level was observed when induction was started in the late exponential phase. An increase of the carbon and nitrogen source concentrations and a decreased of the concentration of the inducer, resulted in increased product yields.
Production of Llama VHH antibody fragments by S. cerevisiae and A. awamori
Although the production of scFv fragments by S. cerevisiae and A. awamori was successful, levels up to several g/L were not achieved. Possibly the hydrophobic regions of the scFv, responsible for keeping the variable regions of the heavy and light chains together, could also interact with other molecules in the cell. Aggregation of scFv in S. cerevisiae may result in accumulation and subsequent degradation (of a part) of the antibody fragment molecules [128] as frequently observed when expressing heterologous proteins that exhibit hydrophobic surfaces [133]. Interestingly, antibody fragments devoid of these hydrophobic surfaces could be obtained from camels, dromedaries and llamas (VHHs, see Llama Heavy-chain antibody fragments (V HH s) and [13]), providing an option to improve production levels in relevant microorganisms [126].
VHHs could be produced in E. coli up to levels of 6 mg/L, were found to be extremely stable, highly soluble and reacted specifically and with high affinity with antigens [52]. VHHs were produced in S. cerevisiae at levels over 100 mg/L in shake-flask cultures [134], although considerable amounts of VHHs were detected intracellularly. From a 1,5 × 104 L fed-batch fermentation, 1.3 kg of VHHs was obtained, which clearly showed that these fragments could be produced in this host more efficiently than scFv fragments [135]. For a cost-effective large-scale process for the production of VHHs in S. cerevisiae further improvement is required. Van der Linden et al. [54] showed that production of VHHs by S. cerevisiae could be improved by DNA shuffling techniques, in which three homologous VHH genes were randomly fragmentated and reassembled subsequently.
Based on the fact that A. awamori performed superior for scFv also the possibility of VHH production by A. awamori was investigated. As a model VHHs against the hapten RR6 were chosen [134]. Gene fragments coding for anti-RR6 VHHs were cloned in an expression vector containing the highly inducible endoxylanase promoter. Recent experiments (Joosten et al. submitted) showed that functional VHHs could be produced in the culture medium in shake-flask cultures, albeit at relatively low levels. For further optimisation a carrier strategy and controlled fermentations will be carried out.
Production of 'Magic bullets' by A. awamori
A major research interest is the production of fusion proteins or 'Magic bullets', consisting of an antibody fragment (scFv or VHH fragment) fused to an enzyme of interest. In our laboratory research has been carried out with a few examples of scFv fragments coupled to glucose oxidase (GOX). GOX is already for many years an interesting enzyme for coupling to antibodies for killing cells [136]. A scFv, which recognises for example oral Streptomycetes, when fused to GOX, which is an antimicrobial enzyme, may kill bacteria by generation of the bactericidal hydrogen peroxide. In activity assays it was shown that the fusion protein produced by A. awamori was functional, both in binding to the antigen and GOX activity [4].
In the detergent industry enzymatic bleaching may be a good alternative to the current chemical bleaching used. To make these laundry-cleaning products more effective, the production of Magic bullets by filamentous fungi or yeasts is of interest. An enzyme coupled to an antibody fragment recognising persistent stains from e.g. azo-dyes [134] results in a more directed bleaching process, resulting in lower amounts of required detergent, reduction of harmful effects of the enzyme to the textile and lower environmental burden (see Figure 3).
Schematical example of the 'Magic bullet' approach in consumer applications, where an antibody fragment (in this case a llama variable heavy-chain antibody fragment; VHH) recognising a spot on textile, is coupled to an effector molecule (in this case A. ramosus peroxidase; ARP). ARP is a peroxidase, which utilises hydrogen peroxide to catalyse the oxidation of a wide range of organic and inorganic compounds, which makes the enzyme suitable for use in bleaching processes [139].
Currently we are investigating the feasibility of production of VHH-enzyme fusions by A. awamori. One of the VHHs used is a model llama VHH, recognising the azo-dye Reactive Red 6 (RR6 [134]). As a bleaching enzyme, the Arthromyces ramosus peroxidase (ARP) [137, 138] was genetically linked to the VHH fragment. This peroxidase utilises hydrogen peroxide to catalyse the oxidation of a wide range of organic and inorganic compounds, which makes the enzyme suitable for use in bleaching processes [139]. ARP alone could be produced in high amounts by A. awamori (800 mg/L; Lokman et al. submitted). Preliminary results showed the feasibility of fusion protein production by A. awamori, yielding high levels of ARP-VHH fusion protein in controlled fermentation experiments (Joosten et al. manuscript in preparation). The fusion protein showed both ARP activity and azo-dye binding activity.
In future experiments VHHs fragments can be replaced by other more relevant antibody fragments, for example those binding tomato or blood spots. Also the peroxidase part of the fusion can be further optimised.
Conclusions and future prospects
Recent developments in the fields of antibody engineering and expression systems have enabled the engineering and production of antibodies and antibody fragments for a wide variety of applications. A lot of examples are already mentioned, but presumably more applications can be envisaged. The development of the 'Magic bullet' approach will even increase the interest in antibodies and their related products, also for applications in human medicine. A recently envisioned application that is of much interest, is the use of antibody fragments in micro-arrays. Antibody arrays can be used for proteomic analysis by comparing the differences in presence of proteins in healthy and diseased cells. For this purpose antibody fragments derived from large phage-antibody libraries can be used as probes to capture proteins on chips in a high-throughput system (reviewed in [140, 141]). In this respect, VHHs fragments are of great interest, due to their simple and stable structure.
In this review we evaluated whether the yeast S. cerevisiae and the filamentous fungus A. awamori are suitable expression systems for the large-scale production of antibody fragments and antibody fusion proteins. Although A. awamori is not the best expression system for the production single antibody domains (scFv and VHH fragments), in particular for the production of antibody fusion proteins filamentous fungi offer significant potential. In particular in those cases where specific post-translational modification (e.g. N-glycosylation) is required for functional expression of the effector protein (also in relation to pharmaceutical applications). In contrast to S. cerevisiae, filamentous fungi do not show extensive hyperglycosylation [142].
Both the scFv-GOX as well as results from ARP-VHH fusion proteins showed that the filamentous fungal system is a promising candidate for the production of antibody fusion proteins. In the future production of other fusion proteins can be investigated in this or other fungal expression systems, allowing a potential breakthrough for antibody technology in producing large amounts of specific recognition units coupled to effector molecules for consumer applications.
References
Köhler G, Milstein C: Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975, 256: 495-497.
Porter P, Coley J, Gani M: Immunochemical criteria for successful matching of monoclonal antibodies to immunoassays of peptide hormones for assessment of pregnancy and ovulation. Prog Clin Biol Res. 1988, 285: 181-200.
Van der Linden RHJ: Unique characteristics of llama heavy chain antibodies. Ph.D. thesis Utrecht University. Utrecht (The Netherlands). 1999
Frenken LG, Hessing JG, Van den Hondel CA, Verrips CT: Recent advances in the large-scale production of antibody fragments using lower eukaryotic microorganisms. Res Immunol. 1998, 149: 589-599.
Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR: Making antibodies by phage display technology. Annu Rev Immunol. 1994, 12: 433-455.
Winter G, Milstein C: Man-made antibodies. Nature. 1991, 349: 293-299.
Padlan EA: Anatomy of the antibody molecule. Mol Immunol. 1994, 31: 169-217.
Better M, Chang CP, Robinson RR, Horwitz AH: Escherichia coli secretion of an active chimeric antibody fragment. Science. 1988, 240: 1041-1043.
Skerra A, Pluckthun A: Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988, 240: 1038-1041.
Cai X, Garen A: A melanoma-specific VH antibody cloned from a fusion phage library of a vaccinated melanoma patient. Proc Natl Acad Sci U S A. 1996, 93: 6280-6285.
Ward ES, Gussow D, Griffiths AD, Jones PT, Winter G: Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature. 1989, 341: 544-546.
Borrebaeck CA, Malmborg AC, Furebring C, Michaelsson A, Ward S, Danielsson L, Ohlin M: Kinetic analysis of recombinant antibody-antigen interactions: relation between structural domains and antigen binding. Biotechnology (N Y). 1992, 10: 697-698.
Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N, Hamers R: Naturally occurring antibodies devoid of light chains. Nature. 1993, 363: 446-448.
Muyldermans S, Lauwereys M: Unique single-domain antigen binding fragments derived from naturally occurring camel heavy-chain antibodies. J Mol Recognit. 1999, 12: 131-140.
Nguyen VK, Hamers R, Wyns L, Muyldermans S: Loss of splice consensus signal is responsible for the removal of the entire C(H)1 domain of the functional camel IgG2A heavy-chain antibodies. Mol Immunol. 1999, 36: 515-524.
Woolven BP, Frenken LG, van der Logt P, Nicholls PJ: The structure of the llama heavy chain constant genes reveals a mechanism for heavy-chain antibody formation. Immunogenetics. 1999, 50: 98-101.
Nguyen VK, Desmyter A, Muyldermans S: Functional heavy-chain antibodies in Camelidae. Adv Immunol. 2001, 79: 261-296.
Nguyen VK, Su C, Muyldermans S, van der Loo W: Heavy-chain antibodies in Camelidae; a case of evolutionary innovation. Immunogenetics. 2002, 54: 39-47.
Colcher D, Pavlinkova G, Beresford G, Booth BJ, Choudhury A, Batra SK: Pharmacokinetics and biodistribution of genetically-engineered antibodies. Q J Nucl Med. 1998, 42: 225-241.
Kipriyanov SM, Little M: Generation of recombinant antibodies. Mol Biotechnol. 1999, 12: 173-201.
Neuberger MS, Williams GT, Mitchell EB, Jouhal SS, Flanagan JG, Rabbitts TH: A hapten-specific chimaeric IgE antibody with human physiological effector function. Nature. 1985, 314: 268-270.
Riechmann L, Clark M, Waldmann H, Winter G: Reshaping human antibodies for therapy. Nature. 1988, 332: 323-327.
Holliger P, Hoogenboom H: Antibodies come back from the brink. Nat Biotechnol. 1998, 16: 1015-1016.
Gura T: Therapeutic antibodies: magic bullets hit the target. Nature. 2002, 417: 584-586.
Pluckthun A: Escherichia coli producing recombinant antibodies. Bioprocess Technol. 1994, 19: 233-252.
Le Gall F, Bove JM, Garnier M: Engineering of a single-chain variable-fragment (scFv) antibody specific for the stolbur phytoplasma (Mollicute) and its expression in Escherichia coli and tobacco plants. Appl Environ Microbiol. 1998, 64: 4566-4572.
Zhu Z, Zapata G, Shalaby R, Snedecor B, Chen H, Carter P: High level secretion of a humanized bispecific diabody from Escherichia coli. Biotechnology (N Y). 1996, 14: 192-196.
McCafferty J, Griffiths AD, Winter G, Chiswell DJ: Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990, 348: 552-554.
Hoogenboom HR, Chames P: Natural and designer binding sites made by phage display technology. Immunol Today. 2000, 21: 371-378.
Hoogenboom HR, de Bruine AP, Hufton SE, Hoet RM, Arends JW, Roovers RC: Antibody phage display technology and its applications. Immunotechnology. 1998, 4: 1-20.
Marks JD, Griffiths AD, Malmqvist M, Clackson TP, Bye JM, Winter G: By-passing immunization: building high affinity human antibodies by chain shuffling. Biotechnology (N Y). 1992, 10: 779-783.
Worn A, Pluckthun A: Stability engineering of antibody single-chain Fv fragments. J Mol Biol. 2001, 305: 989-1010.
Raag R, Whitlow M: Single-chain Fv s. Faseb J. 1995, 9: 73-80.
Verma R, Boleti E, George AJ: Antibody engineering: comparison of bacterial, yeast, insect and mammalian expression systems. J Immunol Methods. 1998, 216: 165-181.
Huston JS, McCartney J, Tai MS, Mottola-Hartshorn C, Jin D, Warren F, Keck P, Oppermann H: Medical applications of single-chain antibodies. Int Rev Immunol. 1993, 10: 195-217.
Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, Lee T, Pope SH, Riordan GS, Whitlow M: Single-chain antigen-binding proteins. Science. 1988, 242: 423-426.
Huston JS, Levinson D, Mudgett-Hunter M, Tai MS, Novotny J, Margolies MN, Ridge RJ, Bruccoleri RE, Haber E, Crea R: Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988, 85: 5879-5883.
Glockshuber R, Malia M, Pfitzinger I, Pluckthun A: A comparison of strategies to stabilize immunoglobulin Fv-fragments. Biochemistry. 1990, 29: 1362-1367.
Mallender WD, Carrero J, Voss EW: Comparative properties of the single chain antibody and Fv derivatives of mAb 4-4-20. Relationship between interdomain interactions and the high affinity for fluorescein ligand. J Biol Chem. 1996, 271: 5338-5346.
Whitlow M, Bell BA, Feng SL, Filpula D, Hardman KD, Hubert SL, Rollence ML, Wood JF, Schott ME, Milenic DE: An improved linker for single-chain Fv with reduced aggregation and enhanced proteolytic stability. Protein Eng. 1993, 6: 989-995.
Brinkmann U, Reiter Y, Jung SH, Lee B, Pastan I: A recombinant immunotoxin containing a disulfide-stabilized Fv fragment. Proc Natl Acad Sci U S A. 1993, 90: 7538-7542.
Reiter Y, Brinkmann U, Kreitman RJ, Jung SH, Lee B, Pastan I: Stabilization of the Fv fragments in recombinant immunotoxins by disulfide bonds engineered into conserved framework regions. Biochemistry. 1994, 33: 5451-5459.
Reiter Y, Brinkmann U, Lee B, Pastan I: Engineering antibody Fv fragments for cancer detection and therapy: disulfide-stabilized Fv fragments. Nat Biotechnol. 1996, 14: 1239-1245.
Atwell JL, Breheney KA, Lawrence LJ, McCoy AJ, Kortt AA, Hudson PJ: scFv multimers of the anti-neuraminidase antibody Nc10: length of the linker between VH and VL domains dictates precisely the transition between diabodies and triabodies. Protein Eng. 1999, 12: 597-604.
Hudson PJ, Kortt AA: High avidity scFv multimers; diabodies and triabodies. J Immunol Methods. 1999, 231: 177-189.
Cao Y, Suresh MR: Bispecific antibodies as novel bioconjugates. Bioconjug Chem. 1998, 9: 635-644.
Kriangkum J, Xu B, Nagata LP, Fulton RE, Suresh MR: Bispecific and bifunctional single chain recombinant antibodies. Biomol Eng. 2001, 18: 31-40.
Sheriff S, Constantine KL: Redefining the minimal antigen-binding fragment. Nat Struct Biol. 1996, 3: 733-736.
Muyldermans S: Single domain camel antibodies: current status. J Biotechnol. 2001, 74: 277-302.
van der Linden RH, Frenken LG, de Geus B, Harmsen MM, Ruuls RC, Stok W, de Ron L, Wilson S, Davis P, Verrips CT: Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochim Biophys Acta. 1999, 1431: 37-46.
Lauwereys M, Arbabi Ghahroudi M, Desmyter A, Kinne J, Holzer W, De Genst E, Wyns L, Muyldermans S: Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. Embo J. 1998, 17: 3512-3520.
Ghahroudi M, Desmyter A, Wyns L, Hamers R, Muyldermans S: Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997, 414: 521-526.
Knarr G, Gething MJ, Modrow S, Buchner J: BiP binding sequences in antibodies. J Biol Chem. 1995, 270: 27589-27594.
van der Linden RH, de Geus B, Frenken GJ, Peters H, Verrips CT: Improved production and function of llama heavy chain antibody fragments by molecular evolution. J Biotechnol. 2000, 80: 261-270.
Davies J, Riechmann L: 'Camelising' human antibody fragments: NMR studies on VH domains. FEBS Lett. 1994, 339: 285-290.
Davies J, Riechmann L: Single antibody domains as small recognition units: design and in vitro antigen selection of camelized, human VH domains with improved protein stability. Protein Eng. 1996, 9: 531-537.
Tanha J, Xu P, Chen Z, Ni F, Kaplan H, Narang SA, MacKenzie CR: Optimal design features of camelized human single-domain antibody libraries. J Biol Chem. 2001, 276: 24774-24780.
Riechmann L, Muyldermans S: Single domain antibodies: comparison of camel VH and camelised human VH domains. J Immunol Methods. 1999, 231: 25-38.
Neuberger MS, Williams GT, Fox RO: Recombinant antibodies possessing novel effector functions. Nature. 1984, 312: 604-608.
Hazra DK, Britton KE, Lahiri VL, Gupta AK, Khanna P, Saran S: Immunotechnological trends in radioimmunotargeting: from 'Magic bullet' to 'smart bomb'. Nucl Med Commun. 1995, 16: 66-75.
Hudson PJ: Recombinant antibody constructs in cancer therapy. Curr Opin Immunol. 1999, 11: 548-557.
von Mehren M, Weiner LM: Monoclonal antibody-based therapy. Curr Opin Oncol. 1996, 8: 493-498.
Yokota T, Milenic DE, Whitlow M, Schlom J: Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992, 52: 3402-3408.
Cortez-Retamozo V, Lauwereys M, Hassanzadeh Gh G, Gobert M, Conrath K, Muyldermans S, De Baetselier P, Revets H: Efficient tumor targeting by single-domain antibody fragments of camels. Int J Cancer. 2002, 98: 456-462.
Maynard JA, Maassen CB, Leppla SH, Brasky K, Patterson JL, Iverson BL, Georgiou G: Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat Biotechnol. 2002, 20: 597-601.
Marasco WA: Intracellular antibodies (intrabodies) as research reagents and therapeutic molecules for gene therapy. Immunotechnology. 1995, 1: 1-19.
Spooner RA, Murray S, Rowlinson-Busza G, Deonarain MP, Chu A, Epenetos AA: Genetically engineered antibodies for diagnostic pathology. Hum Pathol. 1994, 25: 606-614.
Boleti E, Deonarain MP, Spooner RA, Smith AJ, Epenetos AA, George AJ: Construction, expression and characterisation of a single chain anti-tumour antibody (scFv)-IL-2 fusion protein. Ann Oncol. 1995, 6: 945-947.
Conrath K, Lauwereys M, Wyns L, Muyldermans S: Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J Biol Chem. 2001, 276: 7346-7350.
Muruganandam A, Tanha J, Narang S, Stanimirovic D: Selection of phage-displayed llama single-domain antibodies that transmigrate across human blood-brain barrier endothelium. Faseb J. 2002, 16: 240-242.
Vu KB, Ghahroudi MA, Wyns L, Muyldermans S: Comparison of llama VH sequences from conventional and heavy chain antibodies. Mol Immunol. 1997, 34: 1121-1131.
Harmsen MM, Ruuls RC, Nijman IJ, Niewold TA, Frenken LG, de Geus B: Llama heavy-chain V regions consist of at least four distinct subfamilies revealing novel sequence features. Mol Immuno. 2000, 37: 579-590.
Desmyter A, Transue TR, Ghahroudi MA, Thi MH, Poortmans F, Hamers R, Muyldermans S, Wyns L: Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nat Struct Biol. 1996, 3: 803-811.
Transue TR, De Genst E, Ghahroudi MA, Wyns L, Muyldermans S: Camel single-domain antibody inhibits enzyme by mimicking carbohydrate substrate. Proteins. 1998, 32: 515-522.
Conrath KE, Lauwereys M, Galleni M, Matagne A, Frere JM, Kinne J, Wyns L, Muyldermans S: Beta-lactamase inhibitors derived from single-domain antibody fragments elicited in the Camelidae. Antimicrob Agents Chemother. 2001, 45: 2807-2812.
Desmyter A, Spinelli S, Payan F, Lauwereys M, Wyns L, Muyldermans S, Cambillau C: Three camelid VHH domains in complex with porcine pancreatic alpha-amylase. Inhibition and versatility of binding topology. J Biol Chem. 2002, 277: 23645-23650.
Ledeboer AM, Bezemer S, de Hiaard JJ, Schaffers IM, Verrips CT, van Vliet C, Dusterhoft EM, Zoon P, Moineau S, Frenken LG: Preventing phage lysis of Lactococcus lactis in cheese production using a neutralizing heavy-chain antibody fragment from llama. J Dairy Sci. 2002, 85: 1376-1382.
Graham BM, Porter AJ, Harris WJ: Cloning, expression and characterization of a single-chain antibody fragment to the herbicide paraquat. J Chem Technol Biotechnol. 1995, 63: 279-289.
Got PA, Scherrmann JM: Stereoselectivity of antibodies for the bioanalysis of chiral drugs. Pharm Res. 1997, 14: 1516-1523.
Janda KD, Lo LC, Lo CH, Sim MM, Wang R, Wong CH, Lerner RA: Chemical selection for catalysis in combinatorial antibody libraries. Science. 1997, 275: 945-948.
Wade H, Scanlan TS: The structural and functional basis of antibody catalysis. Annu Rev Biophys Biomol Struct. 1997, 26: 461-493.
Beggs T, Hammond K, Klugkist J: Oral compositions. WO 95/01155. 1995
Hill KJ, Kaszuba M, Creeth JE, Jones MN: Reactive liposomes encapsulating a glucose oxidase-peroxidase system with antibacterial activity. Biochim Biophys Acta. 1997, 1326: 37-46.
Lis M, Kuramitsu HK: Galactose oxidase-glucan binding domain fusion proteins as targeting inhibitors of dental plaque bacteria. Antimicrob Agents Chemother. 1997, 41: 999-1003.
Hayes ML: A lactate oxidase salivary peroxidase thiocyanate antibacterial enzyme system. Microb Ecol Health Dis. 1996, 9: 321-328.
Harrison JS, Keshavarz-Moore E: Production of antibody fragments in Escherichia coli. Ann N Y Acad Sci. 1996, 782: 143-158.
Skerra A: Bacterial expression of immunoglobulin fragments. Curr Opin Immunol. 1993, 5: 256-262.
Kortt AA, Malby RL, Caldwell JB, Gruen LC, Ivancic N, Lawrence MC, Howlett GJ, Webster RG, Hudson PJ, Colman PM: Recombinant anti-sialidase single-chain variable fragment antibody. Characterization, formation of dimer and higher-molecular-mass multimers and the solution of the crystal structure of the single-chain variable fragment/sialidase complex. Eur J Biochem. 1994, 221: 151-157.
Casey JL, Keep PA, Chester KA, Robson L, Hawkins RE, Begent RH: Purification of bacterially expressed single chain Fv antibodies for clinical applications using metal chelate chromatography. J Immunol Methods. 1995, 179: 105-116.
Jurado P, Ritz D, Beckwith J, de Lorenzo V, Fernandez LA: Production of functional single-chain Fv antibodies in the cytoplasm of Escherichia coli. J Mol Biol. 2002, 320: 1-10.
Kruger C, Hu Y, Pan Q, Marcotte H, Hultberg A, Delwar D, Van Dalen PJ, Pouwels PH, Leer RJ, Kelly CG, Van Dollenweerd C, Ma JK, Hammarstrom L: In situ delivery of passive immunity by lactobacilli producing single-chain antibodies. Nat Biotechnol. 2002, 20: 702-706.
Conrad U, Fiedler U: Compartment-specific accumulation of recombinant immunoglobulins in plant cells: an essential tool for antibody production and immunomodulation of physiological functions and pathogen activity. Plant Mol Biol. 1998, 38: 101-109.
Peeters K, De Wilde C, De Jaeger G, Angenon G, Depicker A: Production of antibodies and antibody fragments in plants. Vaccine. 2001, 19: 2756-2761.
Stöger E, Vaquero C, Torres E, Sack M, Nicholson L, Drossard J, Williams S, Keen D, Perrin Y, Christou P, Fischer R: Cereal crops as viable production and storage systems for pharmaceutical scFv antibodies. Plant Mol Biol. 2000, 42: 583-590.
Yuan Q, Hu W, Pestka JJ, He SY, Hart LP: Expression of a functional antizearalenone single-chain Fv antibody in transgenic Arabidopsis plants. Appl Environ Microbiol. 2000, 66: 3499-3505.
De Jaeger G, De Wilde C, Eeckhout D, Fiers E, Depicker A: The plantibody approach: expression of antibody genes in plants to modulate plant metabolism or to obtain pathogen resistance. Plant Mol Biol. 2000, 43: 419-428.
Ziegler A, Torrance L: Applications of recombinant antibodies in plant pathology. Mol Plant Pathol. 2002, 3: 401-407.
Gellissen G: Heterologous protein production in methylotrophic yeasts. Appl Microbiol Biotechnol. 2000, 54: 741-750.
Cregg JM, Vedvick TS, Raschke WC: Recent advances in the expression of foreign genes in Pichia pastoris. Biotechnology (N Y). 1993, 11: 905-910.
Faber KN, Harder W, Ab G, Veenhuis M: Review: methylotrophic yeasts as factories for the production of foreign proteins. Yeast. 1995, 11: 1331-1344.
Fischer R, Drossard J, Emans N, Commandeur U, Hellwig S: Towards molecular farming in the future: Pichia pastoris-based production of single-chain antibody fragments. Biotechnol Appl Biochem. 1999, 30: 117-120.
Cregg JM, Cereghino JL, Shi J, Higgins DR: Recombinant protein expression in Pichia pastoris. Mol Biotechnol. 2000, 16: 23-52.
Ridder R, Schmitz R, Legay F, Gram H: Generation of rabbit monoclonal antibody fragments from a combinatorial phage display library and their production in the yeast Pichia pastoris. Biotechnology (N Y). 1995, 13: 255-260.
Andrade EV, Albuquerque FC, Moraes LM, Brigido MM, Santos-Silva MA: Single-Chain Fv with Fc Fragment of the Human IgG1 Tag: Construction, Pichia pastoris Expression and Antigen Binding Characterization. J Biochem (Tokyo). 2000, 128: 891-895.
Pennell CA, Eldin P: In vitro production of recombinant antibody fragments in Pichia pastoris. Res Immunol. 1998, 149: 599-603.
Eldin P, Pauza ME, Hieda Y, Lin G, Murtaugh MP, Pentel PR, Pennell CA: High-level secretion of two antibody single chain Fv fragments by Pichia pastoris. J Immunol Methods. 1997, 201: 67-75.
Freyre FM, Vazquez JE, Ayala M, Canaan-Haden L, Bell H, Rodriguez I, Gonzalez A, Cintado A, Gavilondo JV: Very high expression of an anti-carcinoembryonic antigen single chain Fv antibody fragment in the yeast Pichia pastoris. J Biotechnol. 2000, 76: 157-163.
Cupit PM, Whyte JA, Porter AJ, Browne MJ, Holmes SD, Harris WJ, Cunningham C: Cloning and expression of single chain antibody fragments in Escherichia coli and Pichia pastoris. Lett Appl Microbiol. 1999, 29: 273-277.
Wood CR, Boss MA, Kenten JH, Calvert JE, Roberts NA, Emtage JS: The synthesis and in vivo assembly of functional antibodies in yeast. Nature. 1985, 314: 446-449.
Horwitz AH, Chang CP, Better M, Hellstrom KE, Robinson RR: Secretion of functional antibody and Fab fragment from yeast cells. Proc Natl Acad Sci U S A. 1988, 85: 8678-8682.
Davis GT, Bedzyk WD, Voss EW, Jacobs TW: Single chain antibody (SCA) encoding genes: one-step construction and expression in eukaryotic cells. Biotechnology (N Y). 1991, 9: 165-169.
Swennen D, Paul MF, Vernis L, Beckerich JM, Fournier A, Gaillardin C: Secretion of active anti-Ras single-chain Fv antibody by the yeasts Yarrowia lipolytica and Kluyveromyces lactis. Microbiology. 2002, 148: 41-50.
Radzio R, Kuck U: Synthesis of biotechnologically relevant heterologous proteins in filamentous fungi. Process-biochem. 1997, 32: 529-539.
Punt PJ, van Biezen N, Conesa A, Albers A, Mangnus J, van den Hondel C: Filamentous fungi as cell factories for heterologous protein production. Trends Biotechnol. 2002, 20: 200-206.
van den Hondel CAJJM, Punt PJ, van Gorcum RFM: Heterologous gene expression in filamentous fungi. In: More genetic manipulations of filamentous fungi. Edited by: Bennet JW, Lasure LL. 1991, 396-428. Orlando, Academic Press
Verdoes JC, Punt PJ, van den Hondel CAMJJ: Molecular genetic strain improvement for the overproduction of fungal proteins by filamentous fungi. Appl Microbiol Biotechnol. 1995, 43: 195-205.
Gouka RJ, Punt PJ, van den Hondel CAMJJ: Efficient production of secreted proteins by Aspergillus: progress, limitations and prospects. Appl Microbiol Biotechnol. 1997, 47: 1-11.
Ward M, Wilson LJ, Kodama KH, Rey MW, Berka RM: Improved production of chymosin in Aspergillus by expression as a glucoamylase-chymosin fusion. Biotechnology (N Y). 1990, 8: 435-440.
Archer DB, Jeenes DJ, Mackenzie DA: Strategies for improving heterologous protein production from filamentous fungi. Antonie Van Leeuwenhoek. 1994, 65: 245-250.
Durand H, Clanet M, Tiraby G: Genetic improvement of Trichoderma reesei for large scale cellulase production. Enzyme Microb Technol. 1988, 6: 341-346.
Keranen S, Penttila M: Production of recombinant proteins in the filamentous fungus Trichoderma reesei. Curr Opin Biotechnol. 1995, 6: 534-537.
Nevalainen H, Suominen P, Taimisto K: On the safety of Trichoderma reesei. J Biotechnol. 1994, 37: 193-200.
Nyyssönen E, Penttila M, Harkki A, Saloheimo A, Knowles JK, Keranen S: Efficient production of antibody fragments by the filamentous fungus Trichoderma reesei. Biotechnology (N Y). 1993, 11: 591-595.
Nyyssonen E, Keranen K, Penttila M, Takkinen K, Knowles JKC: Immunoglobulin production by Trichoderma. World Patent Application WO 92/01797. 1992
Hessing JGM, van Gorcom RFM, Verbakel JMA, Musters W, Frenken LGJ, van den Hondel CAMJJ, Verrips CT: Process for producing fusion proteins comprising scFv fragments by a transformed mould. World Patent Application WO 94/29457. 1994
Hamers R, Hamers-Casterman C, Muyldermans S, Frenken LGJ, Verrips CT: Production of antibodies or (functionalized) fragments thereof derived from heavy chain immunoglobulins of Camelidae. World Patent Application WO 94/25591. 1994,
Gouka RJ, Hessing JG, Punt PJ, Stam H, Musters W, van den Hondel CAMJJ: An expression system based on the promoter region of the Aspergillus awamori 1, 4-beta-endoxylanase A gene. Appl Microbiol Biotechnol. 1996, 46: 28-35.
Frenken L, van Tuijl E, Bos JW, Müller WH, Verkleij AJ, Verrips CT: ScFv antibody fragments produced in Saccharomyces cerevisiae accumulate in the endoplasmic reticulum and the vacuole. Biological membranes: structure biogenesis and dynamics. Edited by: NATO ASI Series. 1994, H82: 223-236. Springer-Verlag Berlin, Heidelberg,
Shusta EV, Raines-R T, Pluckthun A, Wittrup-K D: Increasing the secretory capacity of Saccharomyces cerevisiae for production of single chain antibody fragments. Nat Biotechnol. 1998, 16: 773-777.
Kauffman KJ, Pridgen EM, Doyle FJ, Dhurjati PS, Robinson AS: Decreased protein expression and intermittent recoveries in BiP levels result from cellular stress during heterologous protein expression in Saccharomyces cerevisiae. Biotechnol Prog. 2002, 18: 942-950.
Punt PJ, van Gemeren IA, Drint-Kuijvenhoven J, Hessing JG, van Muijlwijk-Harteveld GM, Beijersbergen A, Verrips CT, van den Hondel CA: Analysis of the role of the gene bipA, encoding the major endoplasmic reticulum chaperone protein in the secretion of homologous and heterologous proteins in black Aspergilli. Appl Microbiol Biotechnol. 1998, 50: 447-454.
Sotiriadis A, Keshavarz T, Keshavarz-Moore E: Factors Affecting the Production of a Single-Chain Antibody Fragment by Aspergillus awamori in a Stirred Tank Reactor. Biotechnol Prog. 2001, 17: 618-623.
Sagt CM, Muller WH, Boonstra J, Verkleij AJ, Verrips CT: Impaired secretion of a hydrophobic cutinase by Saccharomyces cerevisiae correlates with an increased association with immunoglobulin heavy-chain binding protein (BiP). Appl Environ Microbiol. 1998, 64: 316-324.
Frenken LG, van der Linden RH, Hermans PW, Bos JW, Ruuls RC, de Geus B, Verrips CT: Isolation of antigen specific llama VHH antibody fragments and their high level secretion by Saccharomyces cerevisiae. J Biotechnol. 2000, 78: 11-21.
Thomassen YE, Meijer W, Sierkstra L, Verrips CT: Large-scale production of VHH antibody fragments by Saccharomyces cerevisiae. Enzyme Microb Technol. 2002, 30: 273-278.
Verhoeyen ME, van der Logt CP, Beggs TS, Davis PJ: Antibody fragments for controlled delivery of therapeutic agents. Biochem Soc Trans. 1995, 23: 1067-1073.
Akimoto K, Shinmen Y, Sumida M, Asami S, Amachi T, Yoshizumi H, Saeki Y, Shimizu S, Yamada H: Luminol chemiluminescence reaction catalyzed by a microbial peroxidase. Anal Biochem. 1990, 189: 182-185.
Fukuyama K, Kunishima N, Amada F, Kubota T, Matsubara H: Crystal structures of cyanide- and triiodide-bound forms of Arthromyces ramosus peroxidase at different pH values. Perturbations of active site residues and their implication in enzyme catalysis. J Biol Chem. 1995, 270: 21884-21892.
Kjalke M, Andersen MB, Schneider P, Christensen B, Schulein M, Welinder KG: Comparison of structure and activities of peroxidases from Coprinus cinereus, Coprinus macrorhizus and Arthromyces ramosus. Biochim Biophys Acta. 1992, 1120: 248-256.
Borrebaeck CA: Antibodies in diagnostics – from immunoassays to protein chips. Immunol Today. 2000, 21: 379-382.
Holt LJ, Enever C, de Wildt RM, Tomlinson IM: The use of recombinant antibodies in proteomics. Curr Opin Biotechnol. 2000, 11: 445-449.
Maras M, van Die I, Contreras R, van den Hondel CA: Filamentous fungi as production organisms for glycoproteins of bio-medical interest. Glycoconj J. 1999, 16: 99-107.
Wu XC, Ng SC, Near RI, Wong SL: Efficient production of a functional single-chain antidigoxin antibody via an engineered Bacillus subtilis expression-secretion system. Biotechnology (N Y). 1993, 11: 71-76.
Wu SC, Yeung JC, Duan Y, Ye R, Szarka SJ, Habibi HR, Wong SL: Functional production and characterization of a fibrin-specific single-chain antibody fragment from Bacillus subtilis: effects of molecular chaperones and a wall-bound protease on antibody fragment production. Appl Environ Microbiol. 2002, 68: 3261-3269.
Binnie C, Cossar JD, Stewart DI: Heterologous biopharmaceutical protein expression in Streptomyces. Trends Biotechnol. 1997, 15: 315-320.
Ueda Y, Tsumoto K, Watanabe K, Kumagai I: Synthesis and expression of a DNA encoding the Fv domain of an anti-lysozyme monoclonal antibody, HyHEL10, in Streptomyces lividans. Gene. 1993, 129: 129-134.
Pschorr J, Bieseler B, Fritz HJ: Production of the immunoglobulin variable domain REIv via a fusion protein synthesized and secreted by Staphylococcus carnosus. Biol Chem Hoppe Seyler. 1994, 375: 271-280.
Kujau MJ, Hoischen C, Riesenberg D, Gumpert J: Expression and secretion of functional miniantibodies McPC603scFvDhlx in cell-wall-less L-form strains of Proteus mirabilis and Escherichia coli: a comparison of the synthesis capacities of L-form strains with an E. coli producer strain. Appl Microbiol Biotechnol. 1998, 49: 51-58.
Rippmann JF, Klein M, Hoischen C, Brocks B, Rettig WJ, Gumpert J, Pfizenmaier K, Mattes R, Moosmayer D: Procaryotic expression of single-chain variable-fragment (scFv) antibodies: secretion in L-form cells of Proteus mirabilis leads to active product and overcomes the limitations of periplasmic expression in Escherichia coli. Appl Environ Microbiol. 1998, 64: 4862-4869.
Neuberger MS: Making novel antibodies by expressing transfected immunoglobulin genes. Trends Biochem Sci. 1985, 9: 347-349.
King DJ, Byron OD, Mountain A, Weir N, Harvey A, Lawson AD, Proudfoot KA, Baldock D, Harding SE, Yarranton GT: Expression, purification and characterization of B72.3 Fv fragments. Biochem J. 1993, 290: 723-729.
Riechmann L, Foote J, Winter G: Expression of an antibody Fv fragment in myeloma cells. J Mol Biol. 1988, 203: 825-828.
Dorai H, McCartney JE, Hudziak RM, Tai MS, Laminet AA, Houston LL, Huston JS, Oppermann H: Mammalian cell expression of single-chain Fv (sFv) antibody proteins and their C-terminal fusions with interleukin-2 and other effector domains. Biotechnology (N Y). 1994, 12: 890-897.
Jost CR, Kurucz I, Jacobus CM, Titus JA, George AJ, Segal DM: Mammalian expression and secretion of functional single-chain Fv molecules. J Biol Chem. 1994, 269: 26267-26273.
Ailor E, Betenbaugh MJ: Modifying secretion and post-translational processing in insect cells. Curr Opin Biotechnol. 1999, 10: 142-145.
Bei R, Schlom J, Kashmiri SV: Baculovirus expression of a functional single-chain immunoglobulin and its IL-2 fusion protein. J Immunol Methods. 1995, 186: 245-255.
Carayannopoulos L, Max EE, Capra JD: Recombinant human IgA expressed in insect cells. Proc Natl Acad Sci U S A. 1994, 91: 8348-8352.
Hasemann CA, Capra JD: High-level production of a functional immunoglobulin heterodimer in a baculovirus expression system. Proc Natl Acad Sci U S A. 1990, 87: 3942-3946.
Kretzschmar T, Aoustin L, Zingel O, Marangi M, Vonach B, Towbin H, Geiser M: High-level expression in insect cells and purification of secreted monomeric single-chain Fv antibodies. J Immunol Methods. 1996, 195: 93-101.
Mahiouz DL, Aichinger G, Haskard DO, George AJ: Expression of recombinant anti-E-selectin single-chain Fv antibody fragments in stably transfected insect cell lines. J Immunol Methods. 1998, 212: 149-160.
Hiatt A, Cafferkey R, Bowdish K: Production of antibodies in transgenic plants. Nature. 1989, 342: 76-78.
Fischer R, Vaquero-Martin C, Sack M, Drossard J, Emans N, Commandeur U: Towards molecular farming in the future: transient protein expression in plants. Biotechnol Appl Biochem. 1999, 30: 113-116.
Kuroiwa Y, Kasinathan P, Choi YJ, Naeem R, Tomizuka K, Sullivan EJ, Knott JG, Duteau A, Goldsby RA, Osborne BA, Ishida I, Robl JM: Cloned transchromosomic calves producing human immunoglobulin. Nat Biotechnol. 2002, 20: 889-894.
Little M, Kipriyanov SM, Le Gall F, Moldenhauer G: Of mice and men: hybridoma and recombinant antibodies. Immunol Today. 2000, 21: 364-370.
Pollock DP, Kutzko JP, Birck-Wilson E, Williams JL, Echelard Y, Meade HM: Transgenic milk as a method for the production of recombinant antibodies. J Immunol Methods. 1999, 231: 147-157.
Young MW, Meade H, Curling JM, Ziomek CA, Harvey M: Production of recombinant antibodies in the milk of transgenic animals. Res Immunol. 1998, 149: 609-610.
Acknowledgements
The authors would like to thank Theo Verrips and Nicole van Luijk for critical reading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Joosten, V., Lokman, C., van den Hondel, C.A. et al. The production of antibody fragments and antibody fusion proteins by yeasts and filamentous fungi. Microb Cell Fact 2, 1 (2003). https://doi.org/10.1186/1475-2859-2-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2859-2-1