Abstract
Background
Metabolic syndrome (MetS) is associated with an increased risk of major cardiovascular events. Increased high-sensitivity C-reactive protein (hsCRP) levels are associated with MetS and its components. Changes in gamma-glutamyl transferase (GGT) levels in response to oxidative stress are also associated with MetS, and the levels could be modulated by hsCRP.
Methods
From a single community, we recruited 822 men (mean age, 61 ± 14 years) and 1,097 women (63 ± 12 years) during their annual health examination. We investigated whether increased hsCRP and GGT levels are synergistically associated with MetS and insulin resistance evaluated by Homeostasis of model assessment of insulin resistance (HOMA-IR).
Results
Of these subjects, 141 men (17.2%) and 170 women (15.5%) had MetS. Participants with MetS had a higher hsCRP and GGT level than those without MetS in both genders, and the HOMA-IR increased significantly in correlation with an increase in hsCRP and GGT. In men, the adjusted odds ratios (95% confidence interval) for MetS across tertiles of hsCRP and GGT were 1.00, 1.69 (1.01-2.80), and 2.13 (1.29-3.52), and 1.00, 3.26 (1.84-5.78) and 6.11 (3.30-11.3), respectively. In women, the respective corresponding values were 1.00, 1.54 (0.92-2.60), and 3.08 (1.88-5.06), and 1.00, 1.70 (1.04-2.79) and 2.67 (1.66-4.30). The interaction between increased hsCRP and GGT was a significant and independent determinant for MetS and insulin resistance in both genders.
Conclusions
These results suggested that higher CRP and GGT levels were synergistically associated with MetS and insulin resistance, independently of other confounding factor in the general population.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS), a clustering of cardiovascular risk factors such as insulin resistance, hypertension, glucose intolerance, hypertriglyceridemia, and low high-density lipoprotein cholesterol (HDL-C) levels, is a major worldwide public health problem. MetS increases the risk of atherosclerotic disease, diabetes [1, 2], and cardiovascular disease (CVD) [3]. MetS affects 13.3% to 24.4% of Japanese men ≥ 30 years of age [4, 5]. With the continuous increase in obesity prevalence in Japan, MetS may become even more common.
Recent data support the concept that high-sensitivity C-reactive protein (hsCRP) is an inflammatory marker and independent predictor reflecting the early stage of CVD [6]. Several studies have demonstrated that hsCRP is induced by cytokines produced by accumulated adipocytes, and then increases in subjects with MetS [7, 8].
Serum Gamma-Glutamyl Transferase is an enzyme present on cell surfaces and in serum that contributes to the extracellular catabolism of glutathione (GSH), but most serum GGT is derived from the liver [9]. Gamma-Glutamyl Transferase (GGT) is also a clinical marker of several factors: alcohol consumption, body fat content [10], plasma lipid/lipoproteins [11, 12] and glucose levels [12–14], blood pressure [12, 14], and metabolic syndrome [14, 15]. It is also associated with CVD [14, 15] and CVD mortality [14–16]. In addition, Taki et al. [17] reported that GGT showed a significant correlation with hsCRP, suggesting a possible interaction between these two key makers. However, there are few reports on the relationship between CRP, GGT and MetS in Japan.
The aim of this study was to determine whether increased hsCRP and GGT levels are interactively associated with MetS, and we examined cross-sectional data from Japanese community-dwelling participants.
Methods
Subjects
Participants were recruited at the time of their annual health examination in a rural town located in Ehime prefecture, Japan. Participants were recruited at the time of their annual health examination in a rural town with a total population of 11,136 (as of April 2002) and located in Ehime prefecture, Japan, in 2002. Among the 9,133 adults (4,395 male) aged 19 to 90 years in this population, a random sample of 3,164 (34.6%) subjects was recruited. Other characteristics such as smoking and alcohol habits, and medication, were investigated by individual interviews that were conducted using a structured questionnaire. The final study sample included 1,919 eligible persons. All procedures were approved by the Ethics Committee of Ehime University School of Medicine and each subject gave informed consent to participate.
Evaluation of Risk Factors
Information on demographic characteristics and risk factors was collected using the clinical files. Body mass index was calculated by dividing weight (in kilograms) by the square of the height (in meters). We measured blood pressure with an appropriate-sized cuff on the right upper arm of the subjects in a sedentary position using an automatic oscillometric blood pressure recorder (BP-103i; Colin, Aichi, Japan) while they were seated after having rested for at least 5 min. Smoking status was defined as the number of cigarette packs per day multiplied by the number of years smoked (pack year), and the participants were classified into never smokers, past smokers, light smokers (<30 pack year) and heavy smokers (≥30 pack year). The daily alcohol consumption was measured using the Japanese liquor unit in which a unit corresponds to 22.9 g of ethanol, and the participants were classified into never drinkers, occasional drinkers (<1 unit/day), light drinkers (1-1.9 unit/day), and heavy drinkers (≥2 unit/day). Total cholesterol (T-C), triglycerides (TG), HDL-C, fasting blood glucose (FBG), creatinine (enzymatic method), uric acid, immuno-reactive insulin (IRI), plasma high molecular weight (HMW) adiponectin (FUJIREBIO, Tokyo, Japan), hsCRP, and GGT were measured during fasting. Plasma hsCRP concentration was measured using a Behring BN II nephelometer (Dade Behring Inc., Marburg, Germany) and this inter- and intraassay coefficient variations was 3.2 and 6.7%, respectively. Serum GGT concentration was assayed with an automatic analyzer (TBA-c6000, TOSHIBA, Tokyo) and this intraassay-coefficient of variation was 0.87 to 2.11%. Low-density lipoprotein cholesterol (LDL-C) level was calculated by the Friedewald formula. Participants with TG levels ≥400 mg/dL were excluded. Estimated GFR was calculated using the following equation: eGFR = 194×Cr-1.094×Age-0.287×0.739 (if female) [18]. Participants with an eGFR <30 mL/min/1.73 m2 were excluded. Homeostasis of model assessment of insulin resistance (HOMA-IR) was calculated from FBG and IRI levels using the following formula: {FBG (mg/dL) × IRI (mU/mL)}/405 [19]. Insulin resistance was defined as a HOMA-IR ≥2.6.
Metabolic Syndrome
We applied condition-specific cutoff points for MetS based on the modified criteria of the National Cholesterol Education Program's Adult Treatment Panel (NCEP-ATP) III report [20]. Metabolic syndrome was defined as subjects with at least three or more of the following five conditions: 1) obesity: BMI ≥25.0 kg/m2 according to the guidelines of the Japanese Society for the Study of Obesity (waist circumference was not available in this study) [21, 22]; 2) raised BP with systolic blood pressure (SBP) ≥130 mmHg and/or diastolic blood pressure (DBP) ≥85 mmHg, and/or current treatment for hypertension; 3) Hypertriglyceridemia with a TG level ≥1.69 mmol/L (150 mg/dL); 4) low HDL cholesterolemia with a HDL-C <1.04 mmol/L (40 mg/dL) in men and 1.30 mmol/L (50 mg/dL) in women; and 5) IFG with a FBG level ≥6.1 mmol/L (110 mg/dL) or current treatment for diabetes mellitus.
Statistical Analysis
Data are presented as the mean ± standard deviation (SD) unless otherwise specified, and in the cases of parameters with non-normal distributions (TG, IRI, FBG, HOMA-IR, and GGT) the data are shown as median (interquartile range) values. In all analyses, parameters with non-normal distributions were used after log-transformation. As several background differences between men and women were demonstrated by previous studies [2, 16, 22], statistical analysis was performed according to sex using PASW Statistics 17.0 (Statistical Package for Social Science Japan, Inc., Tokyo, Japan). The differences among groups categorized by sex and presence of MetS were analyzed by Student's t-test for continuous variables or the χ2 -test for categorical variables. Correlations between various characteristics and HOMA-IR were determined using Pearson's correlation. Subjects were divided into three groups based on tertiles of serum GGT and hsCRP within sex and then combined to avoid the gender differences. Multiple logistic regression analysis was used to evaluate the contribution of confounding factors for MetS and each component of MetS. The synergistic effect of CRP and GGT was evaluated using a general linear model adjusted for the following parameters: age, smoking status, alcohol consumption, uric acid, and estimated glomerular filtration rate. In addition, we demonstrated whether the ORs for MetS and insulin resistance dose-dependently increased in relation to hsCRP and GGT in subgroups of confounding factors which effected on MetS and insulin resistance (e.g., age, alcohol consumption, uric acid, medication, HMW adiponectin). A p-value < 0.05 was considered significant.
Results
Characteristics of subjects
The characteristics of the study participants in relation to sex are illustrated in Table 1. The study included 822 men, aged 61 ± 14 (range, 20-89) years, and 1,097 women, aged 63 ± 12 (range, 21-88) years. Smoking status, alcohol consumption, history of CVD, DBP, TG, uric acid, FBG, hsCRP, and GGT were higher in men than in women, but age, HDL-C, LDL-C, presence of antilipidemic medication, IRI, HOMA-IR, and HMW adiponectin were higher in women than in men. There was no inter-group difference in BMI, SBP, presence of antihypertensive medication, eGFR, and diabetic medication.
Association between various characteristics, and MetS and insulin resistance
Table 2 shows the risk of MetS and abnormalities of its components in relation to hsCRP and GGT among 822 men and 1,097 women. Of these, 141 men (17.2%) and 170 women (15.5%) had MetS. As shown Table 2, BMI, SBP, DBP, TG, LDL-C, FBG, HOMA-IR, presence of diabetic medication, hsCRP, and GGT showed a higher level in participants with MetS than those without in both genders, but HDL-C and HMW adiponectin showed a lower level in those with MetS. Age, presence of antilipidemic medication, and uric acid were higher only in women with MetS, but eGFR was lower in women with MetS. In men, the HOMA-IR increased significantly in correlation with an increase in BMI, SBP, DBP, presence of antihypertensive medication, TG, LDL-C, presence of antilipidemic medication, uric acid, FBG, presence of diabetic medication, hsCRP, and GGT, but decrease in age, HDL-C, and HMW adiponectin. In women, the HOMA-IR increased significantly in correlation with an increase in age, BMI, SBP, DBP, presence of antihypertensive medication, TG, LDL-C, presence of antilipidemic medication, uric acid, FBG, presence of diabetic medication, hsCRP, and GGT, but decrease in HDL-C, eGFR, and HMW adiponectin.
The adjusted odds ratio for MetS. its components, and insulin resistance in relation to tertiles of hsCRP and GGT
As shown Table 3, after adjustments for age, smoking status, alcohol consumption, uric acid, and eGFR, the prevalence rate of MetS increased significantly in relation to hsCRP and GGT in both genders. In men, the ORs (95% CI) for MetS across tertiles of hsCRP and GGT were 1.00, 1.69 (1.01-2.80), and 2.13 (1.29-3.52), and 1.00, 3.26 (1.84-5.78) and 6.11 (3.30-11.3), respectively. In women, the ORs (95% CI) for MetS across tertiles of hsCRP and GGT were 1.00, 1.54 (0.92-2.60), and 3.08 (1.88-5.06), and 1.00, 1.70 (1.04-2.79) and 2.67 (1.66-4.30), respectively. In men, the ORs of hsCRP were significantly high for MetS components of obesity, low HDL cholesterolemia, and impaired fasting glucose, and the ORs of GGT were significantly high for obesity, raised blood pressure, hypertriglyceridemia, and impaired fasting glucose. In women, the ORs of hsCRP were significantly high for MetS components of obesity, hypertriglyceridemia, low HDL cholesterolemia, and impaired fasting glucose, and the ORs of GGT were significantly high for obesity, hypertriglyceridemia, and impaired fasting glucose. The ORs for HOMA-IR ≥2.6 also increased significantly in relation to hsCRP and GGT in both genders.
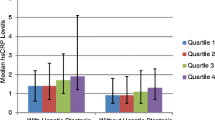
Synergistic effect of GGT and hsCRP on mean accumulating number of MetS components and insulin resistance
In addiction to their direct associations, we observed a synergistic effect between hsCRP and GGT (Figure 1). In Figure 1 subjects were divided into three groups (tertiles) according to CRP and GGT levels within sex. We assessed the statistical significance of the synergistic relationship using a general linear model with the following confounding factors: age, smoking status, alcohol consumption, uric acid, eGFR (Table 4). The interaction between increased hsCRP and GGT was a significant and independent determinant for MetS and HOMA-IR ≥2.6 in both genders.
Synergistic effect of GGT and hsCRP. A, Mean accumulating number of metabolic syndrome components: obesity, raised blood pressure, hypertriglyceridemia, low high-density lipoprotein cholesterolemia, and impaired fasting glucose. B, HOMA-IR. Study subjects were divided into three groups (tertiles) according to GGT and hsCRP levels. Each tertile was calculated within sex and then combined to avoid the gender differences.
Association between hsCRP and GGT levels, and metabolic syndrome and HOMA-IR, within selected subgroups
Next, to control potential confounding by MetS and insulin resistance, the data were further stratified by age, alcohol consumption, uric acid, HMW adiponectin, and medication (e.g., antihypertensive, antilipidemic, and diabetic medication) (Table 5). The ORs for MetS and HOMA-IR ≥2.6 increased significantly in relation to hsCRP and GGT in almost all the subgroups.
Discussion
In 1,919 community-dwelling subjects, we determined the prevalence rate of MetS, as defined by modified NCEP-ATPIII criteria [20], and examined the association between hsCRP and GGT, and MetS and its components. MetS was common, occurring in 17.2% of men and 15.5% of women. In both men and women, the prevalence rate of MetS increased significantly in relation to hsCRP and GGT, even after adjusting for age, smoking status, drinking status, uric acid, and eGFR. The OR of MetS increased dose-dependently with increasing tertiles of hsCRP and GGT. In addition, we demonstrated that there is an interaction between increased hsCRP and GGT. The ORs of MetS and HOMA-IR ≥2.6 were significantly increased in relation to hsCRP and GGT in almost all the subgroups stratified by age, alcohol consumption, uric acid, HMW adiponectin, and medication. To our knowledge, this is the first study to indicate these associations of CRP and GGT with MetS and insulin resistance in about 2,000 community-dwelling subjects.
Systemic inflammation is closely associated with the pathogenesis of MetS. Several previous studies have demonstrated that elevated CRP was associated with increased odds of MetS after adjusting for potential confounding factors [23–26]. In a rural Chinese population, compared with subjects without components of MetS, those with 1, 2, 3, 4, and 5 components of MetS had ORs of 1.39, 1.08, 1.84, 2.65, and 1.21 for elevated CRP in men and 1.91, 2.06, 3.10, 4.06, and 6.01 in women, respectively [27].
In our study, higher GGT levels were also positively associated with MetS, independent of other confounders. Similar results have been reported in recent studies [28, 29]. Nakanishi et al [28] demonstrated that serum GGT may be an important predictor for developing MetS in 2,957 metabolic syndrome-free men and 3,260 nondiabetic men aged 35-59 years. After adjustments for age, family history of diabetes, BMI, alcohol intake, smoking status, regular physical activity, and white blood cell count, increased serum GGT was related to the risk of developing MetS, even among individuals with normal GGT concentrations, this is a finding consistent with previous prospective reports looking at GGT. Among a total 3246 Korean adults, the number of MetS components, prevalence of MetS, and insulin resistance (HOMA-IR) increased closely as the quartile of serum GGT increased [30]. André et al also demonstrated that serum GGT is an important predictor for developing MetS in 1,659 men and 1,889 women without MetS at baseline [31]. Moreover, in a pooled logistic analysis after adjustments for age, alcohol intake, smoking status, physical activity, alanine aminotransferase, fasting insulin and HOMA-IR, high baseline GGT concentrations predicted future development of MetS defined by the IDF and AHA/NHLBI criteria after 4 y of follow-up [32]. In a community-based cohort study of 9,148 Korean adults that included 1056 men, the risk of MetS occurring increased across the baseline GGT quartiles, independent of age plus the time elapsed from visit 1 to visit 2, baseline MetS, uric acid, regular exercise, alcohol consumption, and smoking, and even after further updating GGT values during the follow-up [33]. We also suggested that higher serum GGT was significantly associated with MetS and its components in the same population and this association was related with insulin resistance, independent of other confounding factors [34]. A unique point of this result is that hsCRP and GGT were independently and synergistically associated with MetS, its components and insulin resistance.
The mechanisms by which hsCRP and GGT reflect the risk for MetS are not completely understood. However, systemic inflammation is closely involved in the pathogenesis of MetS, and thus, both elevated hsCRP and GGT may also reflect inflammation, which impairs insulin signaling in the liver, muscle, and adipose tissues [35]. Fat accumulation in the liver or adipose tissues can induce inflammatory cytokines such as tumor necrosis factor-α, interleukin-6, and interleukin-8 [36]. These cytokines produced by adipocytes stimulate the hepatic synthesis of CRP, which is an acute-phase protein, and influence insulin resistance, lipid and glucose metabolism. [37]. Moreover, high GGT is strongly associated with higher CRP levels [38], suggesting that this enzyme represents the expression of subclinical inflammation, and has a role in cellular stress [39]. On the other hand, we have been also reported that increased hsCRP and decreased high molecular weight (HMW) adiponectin are synergistically associated with the accumulation of metabolic disorders [40], however, both hsCRP and GGT were associated with insulin resistance also in subgroups stratified by HMW adiponectin. Furthermore, It has been demonstrated that increased GGT levels might be an antioxidant marker (defensive response) to oxidative stress or a direct marker of oxidative stress, which is involved directly in the generation of reactive oxygen species (ROS), especially in the presence of iron or other transition metals, inducing lipid peroxidation in human biological membranes [41, 42].
There are some limitations to this study. First, our cross-sectional study design does not eliminate potential causal relationships between CRP, GGT and MetS. Second, the prevalence rate of MetS, GGT, and hsCRP categories is based on a single assessment of blood, which may introduce a misclassification bias. Third, we used BMI ≥25 to classify individuals with visceral obesity because waist circumference measurements were not available, which might have caused an under or over estimation of the effect of visceral obesity on MetS [43]. In fact, the prevalence rate of MetS in women was higher than those of the general reports in Japanese [1, 44]. Fourth, serum GGT levels are various for same alcoholic consumption, and the possible association of the fatty liver with the presence of MetS, and with the elevated GGT could not be accurately explored. Thus, we demonstrated that GTT are independently associated with MetS after adjustment for alcoholic consumption and also in subgroup of drinker and non-drinker, suggesting drinking status does not dramatically affect the usefulness of γ-GTP as a biomarker for MetS risk. Fifth, the presence of viral hepatitis must be considered, but examinations of hepatitis B surface antigen and antibody to hepatitis C were not performed. In addition, the rate of taking antilipidemic medication in men with Mets was a little low; however we cannot explain that background. Therefore the demographics and referral source may limit generalizability.
Conclusion
In conclusion, the present study showed that hsCRP and GGT levels are strongly associated with MetS or its components in the general population. The underlying mechanism behind this relationship is unclear, but seems to be independent of traditional cardiovascular risk factors such as age, smoking status, alcohol consumption, uric acid, or renal function. For community-dwelling healthy persons, prospective population-based studies are needed to investigate the mechanisms underlying this association to determine whether intervention, such as effective lifestyle modifications or medication (e.g., antihypertensive, antilipidemic, and diabetic medication) that decrease hsCRP and GGT in adults [45], will decrease the risk of MetS.
References
Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM, San Antonio Heart Study: The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care. 2003, 26: 3153-3159. 10.2337/diacare.26.11.3153.
McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, Ballantyne CM, Heiss G: The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005, 28: 385-390. 10.2337/diacare.28.2.385.
Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT: The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002, 288: 2709-2716. 10.1001/jama.288.21.2709.
Kekeuchi H, Saitoh S, Takagi S, Ohnishi H, Ohata J, Isobe T, Fujiwara T, Akasaka K, Shimada K: Metabolic syndrome and insulin resistance in Japanese Male-Tanno-Soubetsu Study. J Japan Diab Soc. 2003, 46: 739-744. (in Japanese, Abstract in English)
Shiwaku K, Nogi A, Kitajima K, Anuurad E, Enkhmaa B, Yamasaki M, Kim JM, Kim IS, Lee SK, Oyunsuren T, Yamane Y: Prevalence of the metabolic syndrome using the modified ATP III definitions for workers in Japan, Korea and Mongolia. J Occup Health. 2005, 47: 126-135. 10.1539/joh.47.126.
Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH: Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998, 98: 731-733.
Ford ES: The metabolic syndrome and C-reactive protein, fibrinogen, and leukocyte count: findings from the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2003, 168: 351-358. 10.1016/S0021-9150(03)00134-5.
Tomiyama H, Koji Y, Yambe M, Motobe K, Shiina K, Gulnisa Z, Yamamoto Y, Yamashina A: Elevated C-reactive protein augments increased arterial stiffness in subjects with the metabolic syndrome. Hypertension. 2005, 45: 997-1003. 10.1161/01.HYP.0000165018.63523.8a.
Goldberg DM: Structural, functional, and clinical aspects of gamma-glutamyltransferase. CRC Crit Rev Clin Lab Sci. 1980, 12: 1-58. 10.3109/10408368009108725.
Devries MC, Samjoo IA, Hamadeh MJ, Tarnopolsky MA: Effect of endurance exercise on hepatic lipid content, enzymes, and adiposity in men and women. Obesity (Silver Spring). 2008, 16: 2281-2288. 10.1038/oby.2008.358.
Lee DS, Evans JC, Robins SJ, Wilson PW, Albano I, Fox CS, Wang TJ, Benjamin EJ, D'Agostino RB, Vasan RS: Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007, 27: 127-133. 10.1161/01.ATV.0000251993.20372.40.
Jimba S, Nakagami T, Oya J, Wasada T, Endo Y, Iwamoto Y: Increase in gamma-glutamyltransferase level and development of established cardiovascular risk factors and diabetes in Japanese adults. Metab Syndr Relat Disord. 2009, 7: 411-418. 10.1089/met.2008.0082.
Nakanishi N, Nishina K, Li W, Sato M, Suzuki K, Tatara K: Serum gamma-glutamyltransferase and development of impaired fasting glucose or type 2 diabetes in middle-aged Japanese men. J Intern Med. 2003, 254: 287-295. 10.1046/j.1365-2796.2003.01198.x.
Lee DS, Evans JC, Robins SJ, Wilson PW, Albano I, Fox CS, Wang TJ, Benjamin EJ, D'Agostino RB, Vasan RS: Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007, 27: 127-133. 10.1161/01.ATV.0000251993.20372.40.
Mason JE, Starke RD, Van Kirk JE: Gamma-glutamyl transferase: a novel cardiovascular risk biomarker. Prev Cardiol. 2010, 13: 36-41. 10.1111/j.1751-7141.2009.00054.x.
Strasak AM, Kelleher CC, Klenk J, Brant LJ, Ruttmann E, Rapp K, Concin H, Diem G, Pfeiffer KP, Ulmer H: Vorarlberg Health Monitoring and Promotion Program Study Group. Longitudinal change in serum gamma-glutamyltransferase and cardiovascular disease mortality: a prospective population-based study in 76,113 Austrian adults. Arterioscler Thromb Vasc Biol. 2008, 28: 1857-1865. 10.1161/ATVBAHA.108.170597.
Taki K, Nishio K, Hamajima N, Niwa T: Metabolic syndrome defined by new criteria in Japanese is associated with increased liver enzymes and C-reactive protein. Nagoya J Med Sci. 2008, 70: 1-9.
The Japanese Society of Nephrology: Japanese version of GFR estimation. 2007,Updated Oct 29., [http://www.jsn.or.jp]
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985, 28: 412-419. 10.1007/BF00280883.
Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C, American Heart Association; National Heart, Lung and Blood Institute: Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004, 109: 433-438. 10.1161/01.CIR.0000111245.75752.C6.
The examination Committee of Criteria for "Obesity Disease" in Japan: Japan Society for Study of Obesity. New criteria for 'obesity disease' in Japan. Circ J. 2002, 66: 987-992. 10.1253/circj.66.987.
Ota T, Takamura T, Hirai N, Kobayashi K: Preobesity in World Health Organization classification involves the metabolic syndrome in Japanese. Diabetes Care. 2002, 25: 1252-1253. 10.2337/diacare.25.7.1252.
Ye X, Yu Z, Li H, Franco OH, Liu Y, Lin X: Distributions of C-reactive protein and its association with metabolic syndrome in middle-aged and older Chinese people. J Am Coll Cardiol. 2007, 49: 1798-1805. 10.1016/j.jacc.2007.01.065.
Okosun IS: Metabolic syndrome and C-reactive protein in American adults: the impact of abdominal obesity. Metab Syndr Relat Disord. 2008, 6: 289-297. 10.1089/met.2008.0040.
Wen J, Liang Y, Wang F, Sun L, Guo Y, Duan X, Liu X, Tao Q, Wong TY, Lu X, Wang N: Association of C-reactive protein and metabolic syndrome in a rural Chinese population. Clin Biochem. 2009, 42: 976-983. 10.1016/j.clinbiochem.2009.03.026.
Seet RC, Lee CY, Lim EC, Quek AM, Huang SH, Khoo CM, Halliwell B: Markers of oxidative damage are not elevated in otherwise healthy individuals with the metabolic syndrome. Diabetes Care. 2010, 33: 1140-1142. 10.2337/dc09-2124.
Wen J, Liang Y, Wang F, Sun L, Guo Y, Duan X, Liu X, Tao Q, Wong TY, Lu X, Wang N: Association of C-reactive protein and metabolic syndrome in a rural Chinese population. Clin Biochem. 2009, 42: 976-983. 10.1016/j.clinbiochem.2009.03.026.
Nakanishi N, Suzuki K, Tatara K: Serum gamma-glutamyl transferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2004, 27: 1427-1432. 10.2337/diacare.27.6.1427.
Steinvil A, Shapira I, Ben-Bassat OK, Cohen M, Vered Y, Berliner S, Rogowski O: The association of higher levels of within-normal-limits liver enzymes and the prevalence of the metabolic syndrome. Cardiovasc Diabetol. 2010, 9: 30-10.1186/1475-2840-9-30.
Kang YH, Min HK, Son SM, Kim IJ, Kim YK: The association of serum gamma glutamyltransferase with components of the metabolic syndrome in the Korean adults. Diabetes Res Clin Pract. 2007, 77: 306-313. 10.1016/j.diabres.2006.11.009.
André P, Balkau B, Vol S, Charles MA, Eschwège E, DESIR Study Group: Gamma-glutamyltransferase activity and development of the metabolic syndrome (International Diabetes Federation Definition) in middle-aged men and women: Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) cohort. Diabetes Care. 2007, 30: 2355-2361.
Jo SK, Lee WY, Rhee EJ, Won JC, Jung CH, Park CY, Oh KW, Park SW, Kim SW: Serum gamma-glutamyl transferase activity predicts future development of metabolic syndrome defined by 2 different criteria. Clin Chim Acta. 2009, 403: 234-240. 10.1016/j.cca.2009.03.035.
Ryu S, Chang Y, Woo HY, Yoo SH, Choi NK, Lee WY, Kim I, Song J: Longitudinal increase in gamma-glutamyltransferase within the reference interval predicts metabolic syndrome in middle-aged Korean men. Metabolism. 2010, 59: 683-689. 10.1016/j.metabol.2009.08.024.
Kawamoto R, Kohara K, Tabara Y, Miki T, Otsuka N: Serum gamma-glutamyl transferase levels are associated with metabolic syndrome in community-dwelling individuals. J Atheroscler Thromb. 2009, 16: 355-362.
Hotamisligil GS: Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003, 27 (Suppl 3): S53-S55. 10.1038/sj.ijo.0802502.
Fain JN: Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006, 74: 443-477. full_text.
Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G: Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001, 280: E745-751.
Bo S, Gambino R, Durazzo M, Guidi S, Tiozzo E, Ghione F, Gentile L, Cassader M, Pagano GF: Associations between g-glutamyl transferase, metabolic abnormalities and inflammation in healthy subjects from a population-based cohort: A possible implication for oxidative stress. World J Gastroenterol. 2005, 11: 7109-7117.
Lee DH, Jacobs DR: Association between serum gammaglutamyltransferase and C-reactive protein. Atherosclerosis. 2005, 178: 327-330. 10.1016/j.atherosclerosis.2004.08.027.
Tabara Y, Osawa H, Kawamoto R, Tachibana-Iimori R, Yamamoto M, Nakura J, Miki T, Makino H, Kohara K: Reduced high-molecular-weight adiponectin and elevated high-sensitivity C-reactive protein are synergistic risk factors for metabolic syndrome in a large-scale middle-aged to elderly population: the Shimanami Health Promoting Program Study. J Clin Endocrinol Metab. 2008, 93: 715-722. 10.1210/jc.2007-0397.
Lim JS, Yang JH, Chun BY, Kam S, Jacobs DR, Lee DH: Is serum gamma-glutamyltransferase inversely associated with serum antioxidants as a marker of oxidative stress?. Free Radic Biol Med. 2004, 37: 1018-1023. 10.1016/j.freeradbiomed.2004.06.032.
Drozdz R, Parmentier C, Hachad H, Leroy P, Siest G, Wellman M: gamma-Glutamyltransferase dependent generation of reactive oxygen species from a glutathione/transferrin system. Free Radic Biol Med. 1998, 25: 786-792. 10.1016/S0891-5849(98)00127-0.
Kawamoto R, Ohtsuka N, Ninomiya D, Nakamura S: Carotid atherosclerosis in normal-weight metabolic syndrome. Intern Med. 2007, 46: 1771-1777. 10.2169/internalmedicine.46.0261.
Oda E, Kawai R: Reproducibility of high-sensitivity C-reactive protein as an inflammatory component of metabolic syndrome in Japanese. Circ J. 2010, 74: 1488-1493. 10.1253/circj.CJ-10-0156.
Farah R, Shurtz-Swirski R, Lapin O: Intensification of oxidative stress and inflammation in type 2 diabetes despite antihyperglycemic treatment. Cardiovasc Diabetol. 2008, 7: 20-10.1186/1475-2840-7-20.
Acknowledgements
This work was supported in part by a grant-in-aid for Scientific Research from the Foundation for Development of Community (2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
RK, YT, and KK participated in the design of the study, performed the statistical analysis and drafted the manuscript. NO, TaK, and ToK contributed to acquisition of data and its interpretation. ST and MA contributed to conception and design of the statistical analysis. TM conceived of the study, participated in its design, coordination and helped to draft the manuscript. All authors read and approved the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kawamoto, R., Tabara, Y., Kohara, K. et al. High-sensitivity c-reactive protein and gamma-glutamyl transferase levels are synergistically associated with metabolic syndrome in community-dwelling persons. Cardiovasc Diabetol 9, 87 (2010). https://doi.org/10.1186/1475-2840-9-87
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2840-9-87