Abstract
Background
Angiotensin receptor blockers (ARBs) are reported to provide direct protection to many organs by controlling inflammation and decreasing oxidant stress. Pioglitazone, an anti-diabetic agent that improves insulin resistance, was also reported to decrease inflammation and protect against atherosclerosis. This study aimed to evaluate the utility of combination therapy with both medicines from the viewpoint of anti-inflammatory effects.
Methods
We administered candesartan (12 mg daily) and pioglitazone (15 mg daily) simultaneously for 6 months to hypertensive patients with type 2 diabetes mellitus (T2DM) and evaluated whether there were improvements in the serum inflammatory parameters of high-molecular-weight adiponectin (HMW-ADN), plasminogen activator inhibitor-1 (PAI-1), highly sensitive C-reactive protein (Hs-CRP), vascular cell adhesion molecule-1 (VCAM-1), and urinary-8-hydroxydeoxyguanosine (U-8-OHdG). We then analyzed the relationship between the degree of reductions in blood pressure and HbA1c values and improvements in inflammatory factors. Furthermore, we analyzed the relationship between pulse pressure and the degree of lowering of HbA1c and improvements in inflammatory factors. Finally, we examined predictive factors in patients who received benefits from the co-administration of candesartan with pioglitazone from the viewpoint of inflammatory factors.
Results
After 6 months of treatment, in all patients significant improvements from baseline values were observed in HMW-ADN and PAI-1 but not in VCAM-1, Hs-CRP, and U-8-OHdG. Changes in HbA1c were significantly correlated with changes in HMW-ADN and PAI-1 in all patients, but changes in blood pressure were not correlated with any of the parameters examined. Correlation and multilinear regression analyses were performed to determine which factors could best predict changes in HbA1c. Interestingly, we found a significant positive correlation of pulse pressure values at baseline with changes in HbA1c.
Conclusions
Our data suggest that the pulse pressure value at baseline is a key predictive factor of changes in HbA1c. Co-administration of candesartan with pioglitazone, which have anti-inflammatory (changes in HMW-ADN and PAI-1) effects and protective effects on organs, could be an effective therapeutic strategy for treating hypertensive patients with type 2 diabetes mellitus.
Trial registration
UMIN-CTR: UMIN000010142
Similar content being viewed by others
Background
It is well established that hypertension complicated with T2DM results in an increased incidence of cardiovascular disease [1]. Hence, to reduce cardiovascular events in patients with T2DM, treatment of hypertension in addition to glycemic control is important [2, 3]. Angiotensin receptor blockers (ARBs) are regarded as first-line therapy for hypertensive patients with T2DM. Moreover, a large-scale clinical trial showed that the use of ARBs prevented the onset of diabetes mellitus. Recently, ARBs received much attention in terms of decreasing oxidant stress and controlling inflammation in organs. Previously we reported that candesartan improved inflammatory parameters (HMW-ADN and PAI-1) in hypertensive patients with T2DM of long duration independently of blood pressure changes [4].
Pioglitazone is a diabetic medicine that improves insulin resistance. That pioglitazone directly reduces the incidence of cardiovascular events and stroke was shown by the PROactive Study, a large-scale clinical trial [5, 6]. The protective action of pioglitazone on organs is thought to be through an anti-inflammatory action that reduces oxidant stress similar to ARBs as previously noted [7–10]. Therefore, using medicines such as pioglitazone in conjunction with an ARB might be useful not only with respect to blood pressure and plasma glucose control but also from the viewpoint of protection of organs.
Because the prevalence of metabolic syndrome has increased worldwide and the number of hypertensive patients with diabetes is also expected to increase, the opportunities for clinicians to use an ARB in conjunction with an anti-diabetic medication are expected to grow. Therefore, verifying the effectiveness of this combination therapy in patients with hypertension and diabetes is of clinical significance. However, the utility of such combination therapy from the viewpoint of their anti-inflammatory effects has not been clarified.
In this study, we administered as an ARB candesartan, which is the only sartan approved for use in chronic heart failure patients in Japan, and pioglitazone, an anti-diabetic medicine that improves insulin resistance, for 6 months to hypertensive patients with T2DM of long duration but without a history of cardiovascular events. We evaluated whether there was improvement in the serum inflammatory parameters of high-molecular-weight adiponectin (HMW-ADN), plasminogen activator inhibitor-1 (PAI-1), highly sensitive C-reactive protein (Hs-CRP), vascular cell adhesion molecule-1 (VCAM-1), and urinary-8-hydroxydeoxyguanosine (U-8-OHdG). We then analyzed the relationship between the degree of lowering of HbA1c and blood pressure and changes in inflammatory factors. Furthermore, the relationship between pulse pressure and the degree of lowering of HbA1c and changes in inflammatory factors was analyzed. Finally, we analyzed predictors of which patients would benefit from co-administration of candesartan with pioglitazone through their organ protective effects.
Methods
Participants
In this prospective study, patients were targeted for enrollment among hypertensive patients with T2DM (defined according to ADA criteria [11] or the use of anti-diabetic agents) who regularly attended the Jikei University School of Medicine affiliated hospital for treatment. We enrolled 41 patients (34 males and 7 females, 25–75 years old, average 60 years) who had hypertension (defined as diastolic blood pressure [DBP] ≧80 mmHg or systolic blood pressure [SBP] ≧130 mmHg, average 141/86) or who were taking anti-hypertensive agents (Table 1). Patients with secondary hypertension were excluded, as were patients with impaired liver function defined by plasma aminotransferase (or aspartate aminotransferase) >39 mUml (normal values: 11–39 mUml) and alanine aminotransferase >34 mUml (normal values: 11–34 mUml) or impaired kidney function (defined as serum creatinine level >1.3 mg/100 ml (normal values: 0.6–1.3 mg/100 ml). Also excluded were those with unstable cardiovascular conditions (e.g., New York Heart Association class I–IV congestive heart failure or a history of myocardial infarction or stroke) or who had a cerebrovascular incident within 6 months of study enrollment. Women who were pregnant, lactating or who might become pregnant due to inadequate contraceptive precautions were also excluded. Patients with known contraindications or intolerance to candesartan or pioglitazone were also excluded. Patients were simultaneously administered 12 mg candesartan and 15 mg pioglitazone daily for a duration of 6 months. If at the beginning of the study patients were taking an ARB or ACE-I, that drug was replaced with 12 mg candesartan. Anti-hypertensive and anti-diabetic agents were not changed during this study. The study protocol was approved by the institutional review board at Jikei University School of Medicine and conducted in accordance with the Declaration of Helsinki and its amendments. After a full explanation of the study, all patients gave written informed consent.
Baseline assessment of participants
Before starting the study, all patients underwent an initial screening assessment that included a medical history and physical examination. We evaluated patients at the start of the study to establish baseline values, then again after the 6th month of treatment. Parameters examined were as follows: body weight, body mass index (BMI), SBP, DBP, HbA1c, fasting plasma glucose (FPG), HMW-ADN, PAI-1, Hs-CRP, VCAM-1, and U-8-OHdG. To evaluate tolerability of candesartan and pioglitazone, all adverse events were recorded. All plasmatic parameters were measured after a 12-h overnight fast. In all cases, venous blood samples were taken between 800 and 900 h. We used plasma obtained by the addition of Na2-EDTA (1 mgml_1) and centrifuged at 3000 g for 15 min at 4°C. All measurements were performed in a central laboratory. BMI was calculated as weight (kg) divided by the square of height (m). Height and weight were determined using a standard scale (SYSTEM 502, TANITA, Tokyo, Japan). Blood pressure measurements were obtained from the right arm while patients were in a seated position using a standard sphygmomanometer (ADVANCE BP-203RVIIIC/D, OMRON colin, Tokyo, Japan) (Korotkoff I and V) with an appropriately-sized cuff. Furthermore, the same investigator measured patients’ blood pressure at each visit, always in the morning and after the patient had rested for at least 10 min in a quiet room. Three successive blood pressure readings were obtained at 1-min intervals, and the mean of the three readings was calculated. HbA1c level was measured by high-performance liquid chromatography (HPLC) (HLC723-G9, TOSOH, Tokyo, Japan), with intra- and interassay coefficients of variation (CsV) of 1%. Plasma glucose was assayed by the glucose-oxidase method (GA08II, A&T, Yokohama, Japan) with intra- and inter-assay CsV of 0.8%. Plasma HMW-ADN level was determined using a chemiluminescent enzyme immunoassay (CLEIA) (Fuji Rebio, Tokyo, Japan). Plasma VCAM-1 level (normal values 277–836 ng/ml) was determined using an enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Inc., Minneapolis, MN, USA). Plasma PAI-1 levels (normal values ≤50 ng/ml) were determined using a latex photometric immunoassay (LPIA) (Mitsubishi Chemical Medience, Tokyo, Japan). The U-8-OHdG level (normal values l: 6.1-16.3 ng/mg·cr) was measured using HPLC (Mitsubishi Chemical Medience, Tokyo, Japan). Plasma Hs-CRP level (normal values <0.3 mg/dl) was measured using the latex agglutination nephelometry method (Siemens Healthcare Diagnostics, Inc., Marburg, Germany).
Statistical analysis
Statistical analysis of data was performed using the Statistical Package for Social Sciences software, version 19.0 (SPSS, Chicago, IL, USA). Data are presented as the mean ± s.e. For all statistical analyses, P<0.05 was considered statistically significant.
Results
Characteristics of study sample
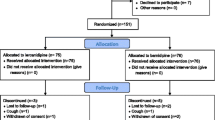
Of the 41 patients who were enrolled in the study, 39 completed the study. The reason for premature withdrawal was lost-to-follow-up. Characteristics of the patient population upon entering the study and the antidiabetic treatments taken before and during the study are shown in Table 1. Patient data at baseline and after the 6th month of the study are shown in Table 2. Significant improvements from baseline values were observed in both SBP and DBP after 6 months *P<0.01, Table 2 and Figure 1). HbA1c and FPG values were significantly improved from baseline after the 6-month treatment period *P<0.01, Table 2 and Figure 2).
Furthermore, significant improvements in baseline values for HMW-ADN and PAI-1 were recorded in all patients after 6 months of treatment (*P<0.05, **P<0.01, respectively). VCAM-1, U-8-OHdG, and Hs-CRP values did not decrease from baseline after 6 months of candesartan with pioglitazone treatment (Figure 3).
Changes from baseline of inflammatory parameters after treatment. HMW-ADN, high molecular weight adiponectin; Hs-CRP, highly sensitive C-reactive protein; PAI-1, plasminogen activator inhibitor-1; U-8-OHdG, urinary 8-hydroxydeoxyguanosine; VCAM-1, vascular cell adhesion molecule-1. Error bars indicate SEM for HMW-AND. *P<0.05 vs Pre. **P<0.01 *P=0.002 vs Pre. Error bars indicate SEM for PAI-1.
Correlations
Stepwise multilinear regression analysis was performed to establish which factors could best predict changes in patients’ inflammatory factors. Co-administration of candesartan with pioglitazone improved inflammatory parameters (HMW-ADN and PAI-1) in hypertensive patients with T2DM of long duration independent of blood pressure changes. In addition, the following positive correlations with changes in HbA1c (⊿HbA1c) were revealed: ⊿HbA1c vs. ⊿HMW-ADN (r=−0.382, P=0.018, Figure 4A) and ⊿HbA1c vs. ⊿PAI-1(r=0.404, P=0.015, Figure 4B). Results of other correlation analyses did not show patterns of associations with regard to ⊿HbA1c with ⊿VCAM-1, ⊿8-OHdG, and ⊿Hs-CRP (Additional file 1: Figure S1).
There were no correlations between changes in SBP and DBP (⊿SBP and ⊿DBP) and the following parameters: ⊿SBP vs. ⊿HMW-ADN, ⊿SBP vs. ⊿PAI-1, ⊿DBP vs. ⊿HMW-ADN, and ⊿DBP vs. ⊿PAI-1 (Figure 5A, B, C, and D, respectively). And there were no correlations between ⊿SBP, ⊿DBP and the following parameters: ⊿SBP vs. ⊿VCAM-1, ⊿SBP vs. ⊿U-8-OHdG, ⊿SBP vs. ⊿Hs-CRP, ⊿DBP vs. ⊿VCAM-1, ⊿DBP vs. ⊿U-8-OHdG, ⊿DBP vs. ⊿Hs-CRP (Additional file 2: Figure S2, Additional file 3: Figure S3). Furthermore, correlation analyses indicated that no associations existed between ⊿SBP and ⊿DBP and ⊿HbA1c (Figure 6A, B).
Discussion
In this prospective trial of patients with hypertension and T2DM, we observed that co-administration of candesartan with pioglitazone had beneficial effects with regard to hypertension, inflammation, and adipose tissue metabolism. In particular, patients receiving treatment with both agents experienced a significant improvement in blood pressure, HbA1c, HMW-ADN, and PAI-1. Furthermore, we analyzed predictive factors in patients who received the greatest benefits from the co-administration of candesartan with pioglitazone.
Co-administration of candesartan with pioglitazone
Both candesartan and pioglitazone were clinically proven to provide direct cardiovascular protection through pleiotropic effects [12–16] not only in basic studies. Furthermore, the anti-inflammatory effect and suppression of oxidative stress in tissues have been the center of attention as the pleiotropic action of both drugs. The combination of candesartan and pioglitazone was more effective than candesartan monotherapy in pigs and rats with cardiovascular risks [17, 18]. Pioglitazone and candesartan in combination were demonstrated to present additive protective effects on renal fibrosis in an experimental mouse model, suggesting that their use in combination would be an effective treatment for chronic kidney disease [19]. Moreover, in patients with T2DM in whom prior hypoglycemic therapies failed, pioglitazone co-administration improved blood lipid and glycemic profiles, thus decreasing the proportion of patients with a high microvascular or macrovascular risk [20]. Therefore, we retrospectively analyzed the effects of co-administration therapy compared to the use of candesartan monotherapy on HMW-ADN and PAI-1, which we addressed previously [4]. After adjustment for age, BMI, HbA1c and blood pressure values, the co-administration arm compared to the candesartan administration only arm had a significant improvement in ⊿HMW-ADN and a tendency toward improvement in ⊿PAI-1 (%) (Additional file 4: Figure S4).
That there has been an increase in hypertensive patients with T2DM supports the clinical importance of this research. Our data suggest that this therapy was effective from the viewpoint of inflammatory parameters (HMW-ADN and PAI-1) for hypertensive patients with T2DM with a cardiovascular risk. However, these data were not investigated at the same time for the same purpose, which might be considered a limitation of this study. Large-scale prospective multi-arm studies are expected in the future.
Correlation between ⊿HbA1c and inflammatory factors ⊿HMW-ADN and ⊿PAI-1
A positive correlation was observed in ⊿HMW-ADN and ⊿PAI-1 and changes in HbA1c (Figure 4A, B). Our data might be thought to indicate that the improvement in HMW-ADN·PAI-1 with plasma glucose (HbA1c) created a virtuous circle. Did the improvement in plasma glucose cause the improvement in inflammatory parameters or was the opposite correct? The truth is unknown. But our results showed close relationships between inflammation and blood glucose levels. Pioglitazone has a dose–response effect on insulin sensitivity and insulin secretion in T2DM [21–24]. We are inclined to the opinion that if these patients would take pioglitazone 30–45 mg daily, further improvement in the inflammatory parameters in addition to HbA1c would be achieved by a virtuous circle.
Correlation between ⊿SBP,⊿DBP and inflammatory factors ⊿HMW-ADN and ⊿PAI-1
⊿SBP and ⊿DBP correlation analyses did not indicate patterns of associations with these parameters (Figure 5A, B, C, D). These results might indicate that candesartan improved these parameters directly and not through changes in blood pressure or that it had lesser effects in patients with diabetes mellitus of a long duration.
Correlation between ⊿HbA1c and pulse pressure values at baseline
Interestingly, by correlation analysis we observed a significant correlation between the degree of lowering of HbA1c (⊿HbA1c) and pulse pressure values at baseline. Furthermore, multiple regression analysis showed that pulse pressure was independent of age and BMI (Table 3 and Figure 7). In summary, these data suggest that pulse pressure values at baseline can become a key predictive factor of changes in patients’ HbA1c. There is evidence that pulse pressure is an index parameter of arteriosclerosis [25, 26]. In brief, our data suggest that the patients in our study who did not have advanced atherosclerosis were those who could benefit most from this treatment. These results are unique. We think that there is an important relationship between blood pressure and plasma glucose, and the reason for this should be examined in the future.
Benefits and risks of pioglitazone
Among our study patients, we did not observe increases in body weight, exacerbation of heart failure, and the development of bladder cancer after 6 months of treatment. In France, analysis revealed a significant association between pioglitazone and bladder cancer in men [27]. But in Asia, two reports showed no relation of thiazolidinediones with bladder cancer. [28, 29]. Cariou et al. noted that the thiazolidinediones, mainly pioglitazone, remain effective and useful anti-diabetic drugs with a unique insulin-sensitizing action. This therapeutic option must be validated by a decrease in HbA1c and the lack of serious adverse events [30]. Also, with regard to cardiovascular outcomes, pioglitazone is useful for patients with cardiovascular and microvascular risks [12, 13, 20]. Therefore, pioglitazone has benefits in hypertensive patients with T2DM who are at high risk for cardiovascular disease.
Study limitations
This was a pilot study. It was a single group pre-post study of a small number of patients with unbalanced distribution between men and women. Co-administration therapy for 6 months was less effective with regard to VCAM-1, U-8-OHdG, and Hs-CRP. The precise reason is unknown. Results of larger clinical trials evaluating the anti-inflammatory effects of the co-administration of candesartan with pioglitazone are awaited.
Conclusion
In conclusion, the simultaneous co-administration of candesartan with pioglitazone improved inflammatory parameters (HMW-ADN and PAI-1), and improvements in inflammatory parameters were correlated with ⊿HbA1c. Pulse pressure values at baseline can become a key predictive factor for changes in patients’ HbA1c. The co-administration of candesartan with pioglitazone could be an effective therapeutic strategy for treating hypertensive patients with T2DM.
Abbreviations
- BMI:
-
Body mass index
- DPB:
-
Diastolic blood pressure
- ELISA:
-
Enzyme-linked immunosorbent assay
- FPG:
-
Fasting plasma glucose
- HbA1c:
-
Glycated hemoglobin A1c
- HMW-ADN:
-
High-molecular-weight adiponectin
- Hs-CRP:
-
Highly sensitive C-reactive protein
- PAI-1:
-
Plasminogen activator inhibitor-1
- SBP:
-
Systolic blood pressure
- T2DM:
-
Type 2 diabetes mellitus
- U-8-OHdG:
-
Urinary 8-hydroxydeoxyguanosine
- VCAM-1:
-
Vascular cell adhesion molecule-1.
References
Chen G, McAlister FA, Walker RL, Hemmelgarn BR, Campbell NR: Cardiovascular outcomes in framingham participants with diabetes: the importance of blood pressure. Hypertension. 2011, 57 (5): 891-897. 10.1161/HYPERTENSIONAHA.110.162446.
Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK prospective diabetes study group. BMJ (Clinical research ed). 1998, 317 (7160): 703-713. 10.1136/bmj.317.7160.703.
Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR: Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ (Clinical research ed). 2000, 321 (7258): 412-419. 10.1136/bmj.321.7258.412.
Sakamoto M, Suzuki H, Hayashi T, Iuchi H, Isaka T, Sakamoto N, Kayama Y, Tojo K, Yoshimura M, Utsunomiya K: Effects of candesartan in hypertensive patients with type 2 diabetes mellitus on inflammatory parameters and their relationship to pulse pressure. Cardiovasc Diabetol. 2012, 11: 118-10.1186/1475-2840-11-118.
Charbonnel B, Dormandy J, Erdmann E, Massi-Benedetti M, Skene A: The prospective pioglitazone clinical trial in macrovascular events (PROactive): can pioglitazone reduce cardiovascular events in diabetes? Study design and baseline characteristics of 5238 patients. Diabetes Care. 2004, 27 (7): 1647-1653. 10.2337/diacare.27.7.1647.
Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD: Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone clinical trial in macroVascular events): a randomised controlled trial. Lancet. 2005, 366 (9493): 1279-1289. 10.1016/S0140-6736(05)67528-9.
Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Gravina A, Mereu R, Palumbo I, Fogari E, D'Angelo A, Cicero AF: Differential effects of candesartan and olmesartan on adipose tissue activity biomarkers in type II diabetic hypertensive patients. Hypertension research : official journal of the Japanese Society of Hypertension. 2010, 33 (8): 790-795. 10.1038/hr.2010.85.
Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Gravina A, Mereu R, Palumbo I, D'Angelo A, Cicero AF: Candesartan effect on inflammation in hypertension. Hypertension research : official journal of the Japanese Society of Hypertension. 2010, 33 (3): 209-213. 10.1038/hr.2009.212.
Koh KK, Han SH, Chung WJ, Ahn JY, Jin DK, Kim HS, Park GS, Kang WC, Ahn TH, Shin EK: Comparison of effects of losartan, irbesartan, and candesartan on flow-mediated brachial artery dilation and on inflammatory and thrombolytic markers in patients with systemic hypertension. Am J Cardiol. 2004, 93 (11): 1432–-1435. A1410
Yano Y, Hoshide S, Ishikawa J, Noguchi C, Tukui D, Takanori H, Tada M, Kanemaru Y, Yano A, Ishikawa S: The differential effects of angiotensin II type 1 receptor blockers on microalbuminuria in relation to low-grade inflammation in metabolic hypertensive patients. Am J Hypertens. 2007, 20 (5): 565-572. 10.1016/j.amjhyper.2006.12.008.
Gillett MJ: International expert committee report on the role of the A1c assay in the diagnosis of diabetes: diabetes care 2009; 32(7): 1327–1334. The Clinical biochemist Reviews/Australian Association of Clinical Biochemists. 2009, 30 (4): 197-200.
Abbas A, Blandon J, Rude J, Elfar A, Mukherjee D: PPAR- gamma agonist in treatment of diabetes: cardiovascular safety considerations. Cardiovasc Hematol Agents Med Chem. 2012, 10 (2): 124-134. 10.2174/187152512800388948.
Hanefeld M, Pfutzner A, Forst T, Kleine I, Fuchs W: Double-blind, randomized, multicentre, and active comparator controlled investigation of the effect of pioglitazone, metformin, and the combination of both on cardiovascular risk in patients with type 2 diabetes receiving stable basal insulin therapy: the PIOCOMB study. Cardiovasc Diabetol. 2011, 10: 65-10.1186/1475-2840-10-65.
McMurray J, Ostergren J, Pfeffer M, Swedberg K, Granger C, Yusuf S, Held P, Michelson E, Olofsson B: Clinical features and contemporary management of patients with low and preserved ejection fraction heart failure: baseline characteristics of patients in the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur J Heart Fail. 2003, 5 (3): 261-270. 10.1016/S1388-9842(03)00052-7.
Abrahamsson P, Dobson J, Granger CB, McMurray JJ, Michelson EL, Pfeffer M, Pocock S, Solomon SD, Yusuf S, Swedberg K: Impact of hospitalization for acute coronary events on subsequent mortality in patients with chronic heart failure. Eur Heart J. 2009, 30 (3): 338-345.
McCarty CA, Dowrick A, Cameron J, McGrath B, Robman LD, Dimitrov P, Tikellis G, Nicolas C, McNeil J, Guymer R: Novel measures of cardiovascular health and its association with prevalence and progression of age-related macular degeneration: the CHARM Study. BMC Ophthalmol. 2008, 8: 25-10.1186/1471-2415-8-25.
Dohi T, Miyauchi K, Iesaki T, Tsuruta R, Tsuboi S, Ogita M, Kubota N, Kasai T, Yokoyama T, Daida H: Candesartan with pioglitazone protects against endothelial dysfunction and inflammatory responses in porcine coronary arteries implanted with sirolimus-eluting stents. Circulation journal : official journal of the Japanese Circulation Society. 2011, 75 (5): 1098-1106. 10.1253/circj.CJ-10-0917.
Nakamura T, Yamamoto E, Kataoka K, Yamashita T, Tokutomi Y, Dong YF, Matsuba S, Ogawa H, Kim-Mitsuyama S: Beneficial effects of pioglitazone on hypertensive cardiovascular injury are enhanced by combination with candesartan. Hypertension. 2008, 51 (2): 296-301. 10.1161/HYPERTENSIONAHA.107.099044.
Higashi K, Oda T, Kushiyama T, Hyodo T, Yamada M, Suzuki S, Sakurai Y, Miura S, Kumagai H: Additive antifibrotic effects of pioglitazone and candesartan on experimental renal fibrosis in mice. Nephrology (Carlton). 2010, 15 (3): 327-335. 10.1111/j.1440-1797.2009.01253.x.
Rodriguez A, Reviriego J, Karamanos V, del Canizo FJ, Vlachogiannis N, Drossinos V, Group ES: Management of cardiovascular risk factors with pioglitazone combination therapies in type 2 diabetes: an observational cohort study. Cardiovasc Diabetol. 2011, 10: 18-10.1186/1475-2840-10-18.
Miyazaki Y, Matsuda M, DeFronzo RA: Dose–response effect of pioglitazone on insulin sensitivity and insulin secretion in type 2 diabetes. Diabetes Care. 2002, 25 (3): 517-523. 10.2337/diacare.25.3.517.
Kutoh E, Fukushima T: Insulin-dependent actions of pioglitazone in newly diagnosed, drug naive patients with type 2 diabetes. Endocrine. 2009, 35 (3): 333-340. 10.1007/s12020-009-9174-2.
Bays H, McElhattan J, Bryzinski BS: A double-blind, randomised trial of tesaglitazar versus pioglitazone in patients with type 2 diabetes mellitus. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2007, 4 (3): 181-193.
Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL: Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose–response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000, 23 (11): 1605-1611. 10.2337/diacare.23.11.1605.
Bangalore S, Messerli FH, Franklin SS, Mancia G, Champion A, Pepine CJ: Pulse pressure and risk of cardiovascular outcomes in patients with hypertension and coronary artery disease: an INternational VErapamil SR-trandolapril STudy (INVEST) analysis. Eur Heart J. 2009, 30 (11): 1395-1401. 10.1093/eurheartj/ehp109.
Russo D, Morrone LF, Brancaccio S, Napolitano P, Salvatore E, Spadola R, Imbriaco M, Russo CV, Andreucci VE: Pulse pressure and presence of coronary artery calcification. Clinical journal of the American Society of Nephrology : CJASN. 2009, 4 (2): 316-322. 10.2215/CJN.02580508.
Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H: Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012, 55 (7): 1953-1962. 10.1007/s00125-012-2538-9.
Song SO, Kim KJ, Lee BW, Kang ES, Cha BS, Lee HC: The risk of bladder cancer in korean diabetic subjects treated with pioglitazone. Diabetes & metabolism journal. 2012, 36 (5): 371-378. 10.4093/dmj.2012.36.5.371.
Tseng CH: Pioglitazone and bladder cancer: a population-based study of Taiwanese. Diabetes Care. 2012, 35 (2): 278-280. 10.2337/dc11-1449.
Cariou B, Charbonnel B, Staels B: Thiazolidinediones and PPARgamma agonists: time for a reassessment. Trends in endocrinology and metabolism: TEM. 2012, 23 (5): 205-215. 10.1016/j.tem.2012.03.001.
Acknowledgments
We are grateful to Y. Takada for excellent research assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HS and MS conceptualized the research hypothesis and analyses, researched the data, performed all of the statistical analyses and wrote the manuscript. TH, HI, TI, KO, NS and YK reviewed and edited the manuscript. KT, MY and KU assisted in conceptualizing the research question and reviewed and edited the manuscript. All authors read and approved the final manuscript.
Hirofumi Suzuki, Masaya Sakamoto contributed equally to this work.
Electronic supplementary material
12933_2013_658_MOESM1_ESM.pptx
Additional file 1: Figure S1: Inflammatory factors vs. ⊿HbA1c. (A). ⊿VCAM-1 vs. ⊿HbA1c: r=0.318, P=0.058; (B). ⊿8-OHdG vs. ⊿HbA1c: r= 0.215, P=.201; (C). ⊿Hs-CRP vs. ⊿HbA1c; r= 0.239; P=0.203. (PPTX 74 KB)
12933_2013_658_MOESM2_ESM.pptx
Additional file 2: Figure S2: Inflammatory factors vs. ⊿SBP. (A). ⊿VCAM-1 vs. ⊿SBP: r=−0.085, P=0.642; (B). ⊿U-8-OHdG vs. ⊿SBP: r=0.043, P=0.823; (C). ⊿Hs-CRP vs. ⊿SBP: r=−0.278, P=0.170. (PPTX 73 KB)
12933_2013_658_MOESM3_ESM.pptx
Additional file 3: Figure S3: Inflammatory factors vs. ⊿DBP. (A). ⊿VCAM-1 vs. ⊿DBP: r=−0.066, P=0.726; (B). ⊿U-8-OHdG vs. ⊿DBP: r=−0.132, P=0.494; (C). ⊿Hs-CRP vs. ⊿DBP: r=−0.286, P=0.156. (PPTX 73 KB)
12933_2013_658_MOESM4_ESM.pptx
Additional file 4: Figure S4: Co-administration vs. single candesartan. After adjusted HbA1c in both patients, co-administration arm compared to candesartan administration only arm significantly improved in ⊿HMW-ADN and ⊿PAI-1 (%). *P<0.05 vs Candesartan (Can). Error bars indicate SEM for Can and for Candesartan + Pioglitazone (Can + Pio). (PPTX 52 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Suzuki, H., Sakamoto, M., Hayashi, T. et al. Effects of co-administration of candesartan with pioglitazone on inflammatory parameters in hypertensive patients with type 2 diabetes mellitus: a preliminary report. Cardiovasc Diabetol 12, 71 (2013). https://doi.org/10.1186/1475-2840-12-71
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2840-12-71