Abstract
Background
Adiponectin and resistin are adipokines which modulate insulin action, energy, glucose and lipid homeostasis. Meta-analyses showed that hypoadiponectinemia and hyperresistinemia are strongly associated with increased risk of insulin resistance, type 2 diabetes (T2DM), metabolic syndrome (MS) and cardiovascular disease. The aim of this study was to propose a novel adiponectin-resistin (AR) index by taking into account both adiponectin and resistin levels to povide a better indicator of the metabolic homeostasis and metabolic disorders. In addition, a novel insulin resistance (IRAR) index was proposed by integration of the AR index into an existing insulin resistance index to provide an improved diagnostic biomarker of insulin sensitivity.
Methods
In this case control study, anthropometric clinical and metabolic parameters including fasting serum total adiponectin and resistin levels were determined in 809 Malaysian men (208 controls, 174 MS without T2DM, 171 T2DM without MS, 256 T2DM with MS) whose ages ranged between 40-70 years old. Significant differences in continuous variables among subject groups were confirmed by ANCOVA or MANCOVA test using 1,000 stratified bootstrap samples with bias corrected and accelerated (BCa) 95% CI. Spearman's rho rank correlation test was used to test the correlation between two variables.
Results
The AR index was formulated as 1+log10(R0)-log10(A0). The AR index was more strongly associated with increased risk of T2DM and MS than hypoadiponectinemia and hyperresistinemia alone. The AR index was more strongly correlated with the insulin resistance indexes and key metabolic endpoints of T2DM and MS than adiponectin and resistin levels alone. The AR index was also correlated with a higher number of MS components than adiponectin and resistin levels alone. The IRAR index was formulated as log10(I0G0)+log10(I0G0)log10(R0/A0). The normal reference range of the IRAR index for insulin sensitive individuals was between 3.265 and 3.538. The minimum cut-off values of the IRAR index for insulin resistance assessment were between 3.538 and 3.955.
Conclusions
The novel AR and IRAR indexes are cost-effective, precise, reproducible and reliable integrated diagnostic biomarkers of insulin sensitivity for screening subjects with increased risk of future development of T2DM and MS.
Similar content being viewed by others
Background
The world prevalence of diabetes among adults will be 6.4%, affecting 285 million adults, in year 2010, and will increase to 7.7%, and 439 million adults by year 2030 [1]. Malaysia is listed as the top 10 countries with the highest prevalence of diabetes in recent global estimate of the prevalence of diabetes for years 2010 and 2030 [1]. In addition, a recent nationwide survey showed that Malaysia has a much higher prevalence of metabolic syndrome (MS) compared with other Asian countries [2]. Insulin resistance is a prerequisite root factor for development of type 2 diabetes (T2DM) [3]. It is also the most unifying parameter to characterize the pathophysiology of the MS [3]. The MS drives the twin global epidemics of T2DM and cardiovascular disease [4]. T2DM itself is accompanied by increased risk for cardiovascular disease which is aggravated by the concomitant risk factors of the MS [4]. Adiponectin [5] and resistin [6] hormones are thought to link T2DM and MS with cardiovascular risk.
Adiponectin is an adipocyte-secreted polypeptide hormone with molecular weight 30 kDa (244 amino acids) which modulates a number of metabolic processes, and regulates insulin sensitivity and energy homeostasis, as well as glucose and lipid metabolism [7]. The hormone plays a principal role in the suppression of the metabolic derangements that may result in insulin resistance, T2DM, MS, and cardiovascular disease [5, 8, 9].
Resistin is a macrophage-derived signalling polypeptide hormone with molecular weight 12.5 kDa and its length is 108 amino acids in humans [10]. In contrast with adiponectin, resistin has low circulating levels [10]. However, the blood circulating levels of resistin have been shown to be up-regulated in subjects with insulin resistance, T2DM, MS, and cardiovascular disease [6, 11].
The concurrence of hypoadiponectinemia [5, 8, 9] and hyperresistinemia [6, 10] in subjects with insulin resistance, T2DM and MS risk are well-established. A significant inverse correlation between adiponectin and resistin levels has also been reported in the literatures [12, 13]. The overall structure of multimeric assembly or oligomerization of resistin is similar to that of adiponectin [14]. Taking these studies together, it may be speculated that adiponectin and resistin share a common regulatory mechanism to mediate the body metabolism (e.g. energy, glucose and lipid homeostasis). Thus, a novel adiponectin-resistin (AR) index was proposed by taking into account both adiponectin and resistin levels to povide a better indicator of the metabolic homeostasis and metabolic disorders.
Established direct methods to quantify insulin sensitivity, such as euglycemic hyperinsulinemic clamp technique, are complex, troublesome, expensive, time-consuming, laborious and impractical in clinical practice. Surrogate indexes are available, but there are no universal cutoff points to define insulin resistance. Moreover, the existing surrogates indexes have low sensitivity and lack robustness for early diagnosis of insulin resistance in the general population. It is therefore of great interest to establish a convenient, cost-effective and reliable insulin sensitivity index. Thus, a novel insulin resistance (IRAR) index was proposed by integration of the AR index into an existing insulin resistance index to provide a more promising biomarker of insulin sensitivity for early diagnosis of T2DM and MS in the daily clinical practice and for large-scale clinical investigation. It also allows early treatment to prevent or to delay the onset of long-term severe complications including cardiovascular risk.
Methods
Subjects
All subjects were native to Malaysia and were males to avoid confounding effect of gender. The ages for all subjects were restricted to 40-70 years old because individuals with 40-70 years old contribute the majority cases of T2DM and MS. Subjects comprised three primary ethnic groups which were Malay, Chinese and Indian. Ethical clearance (reference number of Ethical Approval Letter was 612.17) to undertake this study was obtained from the University Malaya Medical Centre (UMMC) Ethics Committee. Informed consent was obtained from each subject, to whom possible consequences of the studies were explained. Each subject received a detailed questionnaire about the personal and family disease history and demographic data.
A case control study was designed. The subjects were classified into 208 controls, 174 MS without T2DM, 171 T2DM without MS, 256 T2DM with MS for a total 809 subjects. The controls were non-diabetic subjects who had no personal and family history and had no first degree relatives such as parent and sibling with T2DM and MS. The fasting plasma glucose levels for a control was in the normal range (<5.60 mmol/L) according to American Diabetes Association (ADA) 2003 diagnostic criteria [15]. Type 2 diabetes (T2DM) were identified as diabetic subjects who had fasting plasma glucose levels of ≥7.0 mmol/L and had been diagnosed by a diabetic physician with T2DM or had been taking diabetic medication.
Metabolic syndrome (MS) was defined according to International Diabetes Federation (IDF) 2005 diagnostic criteria [4]. According to the International Diabetes Federation (IDF) 2005 diagnostic criteria, for a person to be defined as having the MS they must have: central obesity (≥90 cm for South Asians male population), plus any two of the following four factors, which were raised triglycerides (≥1.70 mmol/L), reduced HDL cholesterol (<1.03 mmol/L in males), raised blood pressure (SBP ≥130 mmHg or DBP ≥85 mmHg or hypertension) and raised fasting plasma glucose (≥5.60 mmol/L or previously diagnosed T2DM).
The controls and subjects with T2DM or MS were selected from those attending the University Malaya Medical Centre (UMMC) for routine medical check-up or treatment. All subjects had not been diagnosed with other hereditary (e.g. cancer and cardiovascular disease) and infectious diseases (e.g. hepatitis) (Additional file 1, Supplementary Methods).
Determination of anthropometric clinical and metabolic parameters
The metabolic parameters including fasting serum total adiponectin (Additional file 1, Figure S1A), resistin (Additional file 1, Figure S1B), insulin, total cholesterol, LDL cholesterol, HDL cholesterol, triglyceride, plasma glucose, and whole blood HbA1C levels were tested. The anthropometric parameters including the blood pressure, body mass index (BMI), waist circumference, and waist-to-hip ratio (WHR), were also measured or calculated. Surrogate indexes of insulin sensitivity including quantitative insulin sensitivity check index (QUICKI), homeostasis model assessment of insulin resistance (HOMA-IR) index, Bennett index, McAuley (1) index and McAuley (2) index were calculated (Additional file 1, Table S1).
Statistical analysis
Significant differences in continuous variables among subject groups were confirmed by univariate analysis of covariance (ANCOVA) or multivariate analysis of covariance (MANCOVA) with PASW Statistics 18 Program (SPSS Inc, Chicago, Illinois, USA). General linear model was used, in which each subject group was included as a fixed factor. The models included ages as covariate. Type III sum-of-squares method was used. Then, 1,000 stratified bootstrap samples with bias corrected and accelerated (BCa) 95% confident interval were used for pair wise comparisons. Stratified bootstrapping method was used for stratified ethnicity and for multiple testing bias corrections due to deviation of normality. Spearman's rho rank correlation test was used to test the correlation between two variables. Nonparametric correlation test was used because most of the variables are not normally distributed. All p-values were two-tailed, and p-values below 0.05 were considered statistically significant.
Results
Clinical features of T2DM and MS subjects
The clinical characteristics of the controls, and subjects with type 2 diabetes (T2DM), and metabolic syndrome (MS) are shown in Table 1 and Table 2, which reflect the criteria used to define the subject groups. There was homogeneity for the covariate in terms of ages and ethnicity to match the case-control groups (Table 1). Subjects with T2DM and MS had higher (df = 3; F = 15.096; P = 1.45 × 10-9) serum insulin levels than the healthy subjects (Table 2). Serum insulin levels were the highest in subjects presenting with both T2DM and MS (Table 2).
Serum adiponectin levels
Hypoadiponectinemia was strongly associated (df = 3; F = 13.900; P = 7.65 × 10-9) with increased risk of type 2 diabetes (T2DM) and metabolic syndrome (MS) in Malaysian men (Figure 1). Serum adiponectin levels were significantly lower in MS subjects who do not yet manifest T2DM as compared to the healthy subjects (Figure 1). Serum adiponectin levels were also lower in T2DM subjects who do not yet manifest MS as compared to the healthy subjects (Figure 1). Interestingly, serum adiponectin levels were further down-regulated in subjects presenting with both T2DM and MS (Figure 1). These findings were consistent with previous reports on adiponectin in most epidemiological studies [5, 8, 16] and meta-analyses [9].
Association of fasting serum adiponectin levels with T2DM and MS susceptibility (n = 809). Data are expressed as mean (95% confident interval). ANCOVA test was used, followed by pairwise comparison using 1,000 stratified bootstrap samples with bias corrected and accelerated (BCa) 95% CI for multiple testing bias corrections due to deviation of normality. The p-value was also adjusted for covariate ages with stratified ethnicity. The location of statistically significant differences are displayed as the double arrow. Significant levels: P* < 0.05, P** < 0.01, P*** < 0.001. Note: T2DM = type 2 diabetes; MS = metabolic syndrome.
Serum adiponectin levels were positively correlated with serum HDL cholesterol levels, QUICKI, Bennett, McAuley (1) and McAuley (2) indexes, and it was negatively correlated with BMI, waist, WHR, HOMA-IR index, serum triglyceride, insulin, resistin, plasma glucose and whole blood HbA1C levels (Table 3 and Table 4). The strongest correlation of serum adiponectin levels was with the insulin resistance indexes, serum HDL cholesterol, triglyceride and insulin levels (Table 3 and Table 4). Taking these findings together, it showed that adiponectin plays an important role in the modulation of lipid homeostasis (e.g. fatty acids oxidation) and insulin sensitivity. Adiponectin may also involve in the mediation of glucose homeostasis.
Serum resistin levels
Hyperresistinemia was strongly associated (df = 3; F = 49.165; P = 3.52 × 10-29) with increased risk of type 2 diabetes (T2DM) and metabolic syndrome (MS) in Malaysian men (Figure 2). Serum resistin levels were significantly higher in T2DM subjects who do not yet manifest MS as compared to the healthy subjects (Figure 2). However, there was no significant difference in serum resistin levels between the healthy and MS subjects who do not yet manifest T2DM (Figure 2). Hyperresistinemia was more severe in subjects presenting with both T2DM and MS (Figure 2). These findings were consistent with previous reports on resistin in most epidemiological studies [6, 11].
Association of fasting serum resistin levels with T2DM and MS susceptibility (n = 809). Data are expressed as mean (95% confident interval). ANCOVA test was used, followed by pairwise comparison using 1,000 stratified bootstrap samples with bias corrected and accelerated (BCa) 95% CI for multiple testing bias corrections due to deviation of normality. The p-value was also adjusted for covariate ages with stratified ethnicity. The location of statistically significant differences are displayed as the double arrow. Significant levels: P* < 0.05, P** < 0.01, P*** < 0.001. Note: T2DM = type 2 diabetes; MS = metabolic syndrome.
Serum resistin levels were positively correlated with BMI, waist, WHR, HOMA-IR index, serum insulin, plasma glucose and whole blood HbA1C levels, and it was negatively correlated with serum HDL cholesterol and adiponectin levels, QUICKI, Bennett, McAuley (1) and McAuley (2) indexes (Table 3 and Table 4). Serum resistin levels had the strongest correlation with the insulin resistance indexes, plasma glucose and whole blood HbA1C levels (Table 3 and Table 4). Taking these findings together, it showed that resistin plays an important role in the regulation of glucose homeostasis (e.g. gluconeogenesis) and insulin sensitivity.
Adiponectin-resistin interaction
The interaction effect of adiponectin and resistin was more strongly associated (P≤2.32x10-34) with increased risk of type 2 diabetes (T2DM) and metabolic syndrome (MS) compared to hypoadiponectinemia (P = 7.65 × 10-9) and hyperresistinemia (P = 3.52 × 10-29) alone (Table 5, Figure 1 and Figure 2). The condition of hypoadiponectinemia and hyperresistinemia tend to concurrent in subjects presenting with both T2DM and MS (Figure 1 and Figure 2). Also, serum adiponectin levels were negatively correlated (P = 0.0027) with serum resistin levels (Table 3). Given the opposite effects of adiponectin and resistin on the insulin sensitivity, it speculates that relative proportion of adiponectin-to-resistin might potentially influence the risk of T2DM and MS (Table 3 and Table 4). Taking these findings together, it may be speculated that adiponectin and resistin interact to modulate metabolic homeostasis.
Formulation of the adiponectin-resistin (AR) index
Taking the findings together, it may be speculated that the integration of adiponectin and resistin in a novel unified index would be better reflected metabolic homeostasis and metabolic disorders. Adiponectin (A0) and resistin (R0) levels having diametrically opposed physiological effects in the present study (Table 3, Table 4, Figure 1 and Figure 2). Thus, A0 and R0 are unified by multiplicative inverse as follows
Then (1) is logarithmically transformed for normalization,
Lastly, a numerical constant 1 is added to (2) to get a positive integer of the AR index
Note: R0 = fasting serum total resistin levels in ng/mL;
A0 = fasting serum total adiponectin levels in μg/mL.
Evaluation of the adiponectin-resistin (AR) index
The adiponectin-resistin (AR) index was more strongly associated (df = 3; F = 70.494; P = 1.77 × 10-40) with increased risk of type 2 diabetes (T2DM) and metabolic syndrome (MS) than adiponectin (df = 3; F = 13.900; P = 7.65 × 10-9) and resistin (df = 3; F = 49.165; P = 3.52 × 10-29) levels alone (Figure 1, Figure 2 and Figure 3). The AR index was the lowest in controls, followed by the subjects with MS and T2DM (Figure 3). The AR index was the highest in subjects presenting with both T2DM and MS (Figure 3).
Association of the adiponectin-resistin (AR) index with T2DM and MS susceptibility (n = 809). Data are expressed as mean (95% confident interval). ANCOVA test was used, followed by pairwise comparison using 1,000 stratified bootstrap samples with bias corrected and accelerated (BCa) 95% CI for multiple testing bias corrections due to deviation of normality. The p-value was also adjusted for covariate ages with stratified ethnicity. The location of statistically significant differences are displayed as the double arrow. Significant levels: P* < 0.05, P** < 0.01, P*** < 0.001. Note: T2DM = type 2 diabetes; MS = metabolic syndrome.
The normal reference range of the AR index for healthy individuals was between 1.120 and 1.206 (Figure 3). The minimum cut-off values of the novel AR index for diagnosis of T2DM and MS in Malaysian men were between 1.206 and 1.244 (Figure 3). An individual whose AR index is 1.244 or greater (indicator for developing MS) is defined as being in a metabolic syndrome or pre-diabetic state (Figure 3). When the AR index is 1.379 or greater (indicator for developing T2DM), the individual is diagnosed as having type 2 diabetes (Figure 3). When the AR index is 1.559 or greater (indicator for developing of diabetic complications), the individual is diagnosed as having both type 2 diabetes and metabolic syndrome (Figure 3). These predictive values of the AR index were only applicable to Malaysian men with 95% confident interval (Figure 3).
The AR index was correlated with a higher number of MS components than adiponectin and resistin levels alone (Table 3). The AR index was also more strongly correlated with the insulin resistance indexes and other risk factors including serum insulin, plasma glucose and whole blood HbA1C levels than adiponectin and resistin levels alone (Table 3 and Table 4). Thus, the AR index may play a greater role in reflecting circulating metabolite levels and insulin sensitivity than adiponectin and resistin levels alone.
Formulation of the insulin resistance (IRAR) index
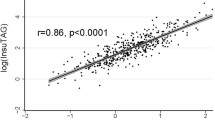
Among the existing insulin resistance indexes, quantitative insulin sensitivity check index (QUICKI) had the stongest correlation (P = 3.71 × 10-25) with the adiponectin-resistin (AR) index (Table 4). The QUICKI and AR indexes are formulated as follows
The QUICKI was negatively correlated with the AR index (Table 4). Therefore, (3) and (4) are unified by multiplicative inverse as follows
Lastly, (5) is simplify to become a finalized IRAR index as follows
Note: I0 = fasting serum insulin levels in μU/mL;
G0 = fasting plasma glucose levels in mg/dL;
R0 = fasting serum total resistin levels in ng/mL;
A0 = fasting serum total adiponectin levels in μg/mL.
Evaluation of the insulin resistance (IRAR) index
The insulin resistance (IRAR) index (df = 3; F = 117.190; P = 5.84 × 10-63) may better predict insulin resistance than classical surrogate indexes including the QUICKI (df = 3; F = 103.892; P = 7.62 × 10-57) (Table 6). The IRAR index was higher in the MS subjects who do not yet manifest T2DM as compared to the healthy subjects (Figure 4). The IRAR index was also higher in the T2DM subjects who do not yet manifest MS as compared to the healthy subjects (Figure 4). The IRAR index was the highest in subjects presenting with both T2DM and MS (Figure 4). In addition, the IRAR index had high precision, consistency and reproducibility in the assessment of the insulin resistance (Figure 4).
Evaluation of insulin sensitivity with the insulin resistance (IR AR ) index (n = 809). Data are expressed as mean (95% confident interval). ANCOVA test was used, followed by pairwise comparison using 1,000 stratified bootstrap samples with bias corrected and accelerated (BCa) 95% CI for multiple testing bias corrections due to deviation of normality. The p-value was also adjusted for covariate ages with stratified ethnicity. The location of statistically significant differences are displayed as the double arrow. Significant levels: P* < 0.05, P** < 0.01, P*** < 0.001. Note: T2DM = type 2 diabetes; MS = metabolic syndrome.
The normal reference range of the IRAR index for insulin sensitive individuals was between 3.265 and 3.538 (Figure 4). The minimum cut-off values of the novel IRAR index for insulin resistance assessment in Malaysian men were between 3.538 and 3.955 (Figure 4). An individual whose IRAR index is between 3.955 and 4.305 (indicator for developing MS) is defined as being in a mild insulin resistance state (Figure 4). An individual whose IRAR index is between 4.403 and 4.791 (indicator for developing T2DM) is defined as being in a moderate insulin resistance state (Figure 4). An individual whose IRAR index is between 5.305 and 5.612 (indicator for developing diabetic complications) is defined as being in a severe insulin resistance state (Figure 4). These predictive values of the IRAR index were only applicable to Malaysian men with 95% confident interval (Figure 4).
Discussion
Hypoadiponectinemia
Circulating levels of adiponectin are highly heritable, with more than 30-70% of its variability explained by genetics factors [17, 18]. A recent comprehensive linkage disequilibrium mapping revealed many SNPs in the adiponectin gene (ADIPOQ) were strongly associated with circulating adiponectin levels [18]. In addition, an extensive bioinformatics analysis revealed the ADIPOQ region might be a high copy number variable region which potentially influences circulating adiponectin levels [18]. Furthermore, ADIPOQ is a major gene influencing circulating adiponectin levels from the genome-wide perspective [18, 19]. The area of chromosome 3q27 where the ADIPOQ gene is located has been identified by genome-wide linkage studies (GWLSs) to be a susceptibility locus for risk for the type 2 diabetes (T2DM) [20], metabolic syndrome (MS) [21] and cardiovascular disease [22, 23]. However, this was not shown in recently published meta-analyses of genome-wide association studies (GWASs) [24–27]. The ADIPOQ gene is located nearby the IGF2BP2 (insulin-like growth factor 2 mRNA-binding protein 2) and AOMS1 (abdominal obesity-metabolic syndrome QTL 1) genes on chromosome 3q27 (based on Ensembl database). The other T2DM and MS susceptibility genes including HDLCQ5 (high density lipoprotein cholesterol level QTL 5) (on chromosome 3q24-q26), AGTR1 (angiotension receptor 1) (on chromosome 3q24), FGQTL6 (fasting plasma glucose level QTL 6) (on chromosome 3q21), RETNLB (resistin-like beta) (on chromosome 3q13.13), HYT7 (hypertension, essential, susceptibility to, 7) (on chromosome 3p14.1-q12.3), PPARG (peroxisome proliferator activated receptor gamma) (on chromosome 3p25.2) and ABHD5 (abhydrolase domain containing 5) (on chromosome 3p25.3-p24.3) may also in linkage disequilibrium with the ADIPOQ gene (based on Ensembl database). Thus, these genes may influence the functional mechanism and expression of the ADIPOQ gene including adiponectin levels.
Adiponectin has already been identified as a potential target for therapeutics to treat T2DM [28] and MS [29–31] in a series of clinical trials. Many existing drugs have been found to increase adiponectin levels, including statins (e.g. pravastatin, simvastatin, rosuvastatin and atorvastatin), angiotensin converting enzyme inhibitors and angiotensin receptor blockers (e.g. ramipril, quinapril, telmisartan, irbesartan and candesartan), β-adrenergic agonists, and thiazolidinediones (e.g. pioglitazone and rosiglitazone) [32]. Other drugs that increase serum adiponectin levels were including non-statin anti-hyperlipidemic drugs (e.g. fenofibrate), non-TZD anti-diabetic drugs (e.g. acarbose and sulfonylurea glimepiride) and androgen blockers [32]. A meta-analysis including 19 prospective studies had pointed out an increase in serum adiponectin levels in subjects undergoing treatment with thiazolidinediones (TZD) [33]. Moreover, a systematic review including 33 clinical trials showed that exercise of varying prescription was able to increase serum adiponectin levels [34]. However, a recent survey revealed the paradoxical findings regarding the role of adiponectin in human disease [35]. According to the concept of the reversal epidemiology in the adiponectin physiology, adiponectin would behave as an insulin sensitizing and cardioprotective factor in the health state and as a wasting marker in the advanced states of disease [35].
Hyperresistinemia
Genetic variants in RETN (the resistin gene) have been examined by many groups, and it was estimated that up to 70% of the variation in circulating resistin levels could be explained by genetic factors [36]. Moreover, recent fine-mapping of SNP studies which covering the full RETN gene revealed several SNPs of the RETN gene account for the high variablity of resistin levels [37]. In the San Antonio Family Heart Study, the maximum linkage signal for the RETN expression was found on chromosome 19p13 (location of the RETN gene) [38]. In addition, the RETN gene is located nearby the INSR (insulin receptor) and LDLR (low density lipoprotein receptor) genes on chromosome 19p13.2 (based on Ensembl database). This suggests that RETN expression may be cis-regulated, meaning there are variants in or near the RETN gene that influence the abundance of its mRNA [38]. The other T2DM and MS susceptibility genes including ATHS (atherosclerosis susceptibility, lipoprotein associated) (on chromosome 19p13.3-p13.2), AKT2 (murine thymoma viral homolog-2) (on chromosome 19q13.2), FFAR 1 (free fatty acids receptor 1) (on chromosome 19q13.12), FFAR 2 (on chromosome 19q13.12) and FFAR 3 (on chromosome 19q13.12) may also in linkage disequilibrium with the RETN gene (based on Ensembl database). In the meta-analyses of genome-wide linkage studies (GWLSs), suggestive evidence of linkage was observed for LDL cholesterol [39, 40], apolipoprotein B [39], total cholesterol [40], and HDL cholesterol [40] on chromosome 19p13. These provide compelling evidence that the region of chromosome 19p13 harbor important determinants of lipid levels in individuals with T2DM [40]. However, the RETN gene was not detected as a susceptibility locus for risk for the T2DM, MS and cardiovascular disease in recently published meta-analyses of genome-wide association studies (GWASs) [24–27]. Thus, whether the RETN gene modulates metabolic homeostasis independently or functions in concert with other causative genes in a haplotype block remains to be elucidated.
Compared to adiponectin, the effects of drugs treatment on resistin levels in patients with T2DM and MS is less described. However, a few clinical trials showed that the anti-diabetic (e.g. rosiglitazone) [41], anti-hypertensive (e.g. amlodipine) [31] and anti-dyslipidemic (e.g. pitavastatin) [42] drugs were able to reduce circulating resistin levels and may contribute to improving insulin action in patients with T2DM and MS. Recently, Koh et al. reported that amlodipine (a calcium channel blocker) therapies significantly decreased resistin levels greater than ramipril (an angiotensin-converting enzyme inhibitor) or candesartan (an angiotensin II receptor antagonist) therapies in patients with hypertension [31]. In addition, resistin concentration decreased after long-term exercise training in overweight adolescents [43]. A recent long-term follow-up study revealed elevated serum resistin levels were associated with higher rates of mortality and hospitalization for heart failure [11]. However, serum resistin levels do not add prognostic information among high-risk persons with established coronary heart disease [11].
Adiponectin-resistin interaction
In consistent with our study, a significant inverse correlation between serum adiponectin and resistin levels has also been reported in the literatures [12, 13]. It has been reported that those with highest increases of adiponectin also displayed a trend towards a decline in resistin levels [13]. Tuttolomondo et al. demonstrated that diabetic subjects with diabetic foot had higher resistin levels and lower adiponectin levels compared to diabetics without diabetic foot [44]. Both hypoadiponectinemia and hyperresistinemia were also positively correlated with diabetes duration, hypertension, dyslipidemia, retinopathy, previous cerebrovascular disease (TIA/ischemic stroke), neuropathy, and diabetic foot grade [44]. Furthermore, both hypoadiponectinemia and hyperresistinemia were associated with out-of-clinic hypertension [45] and may have prognostic significance for future cardiovascular events in patients with masked hypertension [46]. Elevated resistin opposed to adiponectin plasma levels was proposed to be a strong predictive factor for the occurrence of major adverse cardiac events in patients with stable multivessel coronary artery disease over 1-year follow-up [47]. A recent prospective longitudinal pilot trial revealed systemic therapy ameliorates endothelial cell function by increase adiponectin and decrease resistin levels in patients with plaque-type psoriasis [48]. In addition, analysis with (18)F-fluorodeoxyglucose positron emission tomography revealed both adiponectin and resistin may be useful as biomarkers to reflect vascular inflammation [49]. Thus, the balance of the opposite effects of adiponectin and resistin at the level of the endothelial cell may be an important determinant of endothelial dysfunction, and in turn the progress of atherosclerosis.
Several studies have illustrated the interaction between adipokines (including adiponectin and resistin) and adenosine 5' monophosphate-activated protein kinase (AMPK), and highlighted AMPK as a potential target for the development of tissue-specific AMPK modulators in the treatment of T2DM and MS [50]. In patients with metabolic syndrome, thiazolidinediones (TZDs) including pioglitazone [51] and rosiglitazone [52] treatment markedly increased adiponectin and decreased resistin levels. These treatment effects on both adiponectin and resistin may further contribute to the AMPK activation exerted by TZDs [52]. A recent randomized double blind clinical trial demonstrated that short-term treatment with losartan (an angiotensin II receptor antagonist drug) improved both adiponectin and resistin levels in hypertensive subjects [53]. Furthermore, fenofibrate therapy improved both adiponectin and resistin levels, and may directly contribute to improving insulin sensitivity in hypertriglyceridemic patients [54]. These may in turn exert detrimental and beneficial effects on glucose and lipid metabolism.
Crosstalk of adipokines including adiponectin and resistin at the expression level and/or sites of brain's central action may eventually lead to the development and perpetuation of T2DM and MS [55]. The intricate interactions between adiponectin and resistin with catecholamines may play an integral role in metabolism [56]. In addition, adipocyte-derived microvesicles mediated transport of adiponectin and resistin gene transcripts into macrophages and might play a role as a novel intercellular communication tool by transporting RNA in paracrine and possibly endocrine manners [57]. Miyamoto et al. found that resistin may increase the susceptibility of metabolic syndrome by modulating adiponectin secretion from adipocytes [58]. Resistin may enhance hepatic gluconeogenesis, presumably by antagonizing adiponectin, which inhibits enzymes involved in gluconeogenesis through AMPK activation [58]. Furthermore, SNP-420C > G of the resistin gene was associated with lower circulating adiponectin levels in a Japanese cohort study [58]. Thus, the transcriptional activity of the resistin gene may also influence circulating adiponectin levels.
Adiponectin [7] and resistin [10] hormones are considered significant root factors for the regulation of energy, glucose, and lipid homeostasis as well as insulin signalling pathway. Moreover, it has been reported that the overall structure of multimeric assembly of the resistin is similar to that of adiponectin [14]. Both have been characterized as coiled-coil trimers that formed tail-to-tail hexamers through disulfide bonds near their amino termini [14]. Furthermore, both of these hormones circulate in serum in two distinct assembly states [14]. The comparable domain architecture of these two adipocyte-specific hormones, despite having diametrically opposed physiological effects, suggested a common regulatory mechanism in metabolic homeostasis [14].
The adiponectin-resistin (AR) index
It has been reported that the adiponectin-resistin ratio might be potentially useful in prediction of the future cardiovascular risk in women with the polycystic ovary syndrome [13]. Moreover, changes of the relative proportion of adiponectin to resistin might play a more important role in hormonal disturbances in polycystic ovary syndrome than the absolute concentrations of these adipokines [59]. In addition, mice under chronic variable stress and fed with a high-fat diet showed impaired glucose tolerance associated with low plasma adiponectin-resistin ratios [60]. It seems that changes of circulating adiponectin and resistin levels may be the effect of their mutual interaction in adipose tissue. Thus, the AR index that included information on both serum adiponectin and resistin levels may has a more integrated and concentrated explanation than single measure of serum adiponectin and resistin levels in the present study.
Taking these studies together, adiponectin and resistin may be useful markers for insulin resistance and the variables that can integrate the abnormalities of the metabolic syndrome and cardiometabolic function. For this reason we attempted to estimate a threshold for the AR index for the identification of T2DM and MS. Although further studies may be necessary to confirm the efficacy of periodically measuring AR index in the management of insulin resistance, MS and T2DM, our study certainly highlights the potential for the AR index to move one step closer to becoming an established biomarker of the metabolic status. The evaluation of intervention strategies can be facilitated and strengthened by the use of the AR index that measure biological parameters of disease progression and therapeutic response. Routine assessment of the AR index may allow for a better understanding of the underlying disease conditions and optimization of anti-diabetic therapy targeting beyond simple glycemic control. Thus, the AR index has a potential for routinely available in general clinical practice and make a meaningful contribution to patient care.
The insulin resistance (IRAR) index
Given the complicated nature of the euglycemic hyperinsulinemic clamp technique and the potential dangers of hypoglycemia in some patients, alternatives have been sought to simplify the measurement of insulin resistance. In recent years, several markers have been proposed for the screening, diagnosis, and therapeutic monitoring of insulin resistance. However, all have problems that limit their use to research studies. None succeeds in integrating the global assessment of the metabolic abnormalities that may increase risk for developing type 2 diabetes (T2DM) and metabolic syndrome (MS).
Compared to other classical insulin resistance indexes, quantitative insulin sensitivity check index (QUICKI) was reported to have the advantage of being applicable to wider ranges of insulin sensitivity and more reproducible [61]. It has been showed that QUICKI was among the most accurate and useful surrogate indexes for determining insulin sensitivity in humans [62]. However, QUICKI and insulin action do not correlate highly, particularly in individuals with mildly insulin resistance, impaired glucose tolerance or elderly patients with poorly controlled T2DM [63]. Moreover, QUICKI is less robust for early diagnosis of insulin resistance in persons without T2DM or MS [63]. QUICKI has low sensitivity for detecting insulin resistance in lean individuals with beta cell dysfunction [63]. QUICKI use fasting glucose levels in their calculations and fasting glucose levels are steady-state levels that are not a reflection of glucose utilization after a glucose load [62]. Also, QUICKI reflects hepatic insulin resistance only, not insulin resistance at peripheral tissues [63].
The recently developed HOMA-AD was a more accurate indicator for assessing insulin resistance than the HOMA-IR [64]. HOMA-AD is a modified version of homeostasis model assessment of insulin resistance (HOMA-IR) index which calculated from the product of serum insulin and plasma glucose levels divided by serum adiponectin levels [64]. Modification of HOMA-IR with adiponectin levels resulted in an index exhibiting a good correlation with M-values even in diabetic patients with moderate hyperglycemia [64]. In addition, Zaletel et al. showed that the adiponectin derived index correlated best with the euglycemic hyperinsulinemic clamp derived sensitivity index compared to other surrogate measures of insulin resistance including HOMA-IR, QUICKI, fasting glucose/insulin ratio or McAuley index [65]. A recent electron spin resonance study revealed adiponectin might has a close correlation with rheological behavior and microcirculation in hypertension [66]. Moreover, adiponectin may be a marker for global metabolic status including insulin resistance and metabolic syndrome [67].
It is well-established that insulin resistance in adipose tissue will lead to elevated serum resistin levels and reduced serum adiponectin levels [10]. Lipolysis plays a role in the developing of insulin resistance in healthy subjects, with an estimated overall contribution of approximately 39% [68]. Increased lipolysis in adipose tissues was associated with elevation of systemic free fatty acids and insulin resistance [69]. Adiponectin in physiological concentrations inhibits spontaneous as well as catecholamine-induced lipolysis [70]. Resistin induces lipolysis and re-esterification of triacylglycerol stores, and increases cholesteryl ester deposition, in human macrophages [71]. Therefore, inclusion of adiponectin and resistin into the QUICKI formula can be beneficial and can increase its detection power by including those subjects with peripheral insulin resistance, especially in view of the following: increased fasting resistin levels [71] and reduced fasting adiponectin levels [70] could reflect insulin resistance earlier than hyperglycemia since lipolysis was more sensitive to insulin than glucose utilization; a small increase in resistin [72] and decrease in adiponectin levels [73] in healthy individuals were reported to induce insulin resistance; insulin resistance of lipolysis induced by adiponectin and resistin were suggested as explaining a large variation in insulin sensitivity of glucose disposal in lean individuals [69].
It has been well-reported that adiponectin [67] and resistin [6] are promising biomarkers of insulin resistance. A recent clinical trial revealed pioglitazone plus vildagliptin treatment improved both adiponectin and resistin levels and might effective in preserving beta-cell function, and in reducing insulin resistance and inflammatory state parameters in subjects with poorly controlled T2DM [74]. Moreover, the imbalance in deleterious and protective adipokines including adiponectin and resistin plays pivotal roles in the development and progression of pancreatic beta-cell dysfunction under insulin-resistant conditions [75]. Adiponectin and resistin levels were also strongly correlated with the key metabolic endpoints of T2DM and MS as well as insulin sensitivity in the present study (Table 3 and Table 4). Therefore, we generated a modified version of QUICKI, the novel IRAR index by taking account into adiponectin and resistin levels. The IRAR index clearly had the narrowest and most favourable distribution of residuals among the surrogate indexes (Table 6 and Figure 4). The IRAR index may has higher precision, consistency, reproducibility and robustness than classical surrogate indexes (Table 6 and Figure 4). Thus, we hypothesized that the IRAR index may be a more appropriate model of insulin sensitivity than other surrogate indexes in Malaysian men.
The reported values for the definition of insulin resistance vary widely. A World Health Organization (WHO) consensus group concluded that the insulin sensitivity index of the lowest 25% of a general population can be considered as an insulin resistance state [76]. The European Group for the Study of Insulin Resistance take a more restricted view, defining insulin resistance as the insulin sensitivity index of the lowest 10% of a nonobese, nondiabetic, normotensive white population [77]. Therefore, further investigation or replicate studies are required to validate the suggestive reference range or minimum cut-off values of insulin sensitivity for the IRAR index in Malaysian men. In addition, long-term prospective studies are required to determine the actual reference range or minimum cut-off values of the novel IRAR index for insulin resistance assessment in the general population. The novel IRAR indexes give an opportunity to implement and use of this index in the daily clinical practice for screening persons with increased risk of future development of T2DM and MS due to insulin resistance. It is also very useful for monitoring the diseases progression and therapeutic response. Furthermore, it will allow early treatment or delay the onset of long-term severe complications such as cardiovascular risk.
Strengths and limitations
This represent a first attempt to study the interaction effect of adiponectin and resistin in the modulation of the key metabolic endpoints of T2DM and MS. Our samples comprised of Malay, Chinese and Indian subjects from Malaysia, which represented a major segment of the Asian population. The covariates in term of ages and ethnicity were in homogeneity and were matched with the case-control groups (Table 1). Most potential confounders were carefully controlled for, which limits the possibility of residual confounding effect. Given the well-established difference in circulating adiponectin [8, 67] and resistin [6] levels between men and women, our samples were only comprised male subjects to avoid the confounding effect of gender. Clinical measurements were taken under standardized protocol and biomarkers were measured using assays with good precision (Additional file 1, Figure S1 and Additional file 1, Supplementary Methods). Although it has been reported that various definitions of MS hold different predictive powers in detecting pathological levels of key adipocytokines such as hypoadiponectinemia and hyperresistinemia, the International Diabetes Federation (IDF) definition is quantitatively more powerful than its counterparts in terms of prevalence [4, 78]. The IDF definition incorporates ethnicity by providing different criteria for the MS in different ethnic groups [4]. Moreover, the IDF definition is the most updated and globally accepted definition for MS [4].
Nevertheless, this study had limitations. The findings apply mainly to Malaysian men and may not be widely generalizable because of the homogeneity of the study population. Since this is not a prospective study, this study may have reverse causation due to possible effects of T2DM and MS on adiponectin and resistin levels. The results were based on single measurements of the adipokines and therefore may not reflect long-term exposure to these hormones. Although adjusted for known confounding factors, residual confounders imperfectly measured or unmeasured cannot be excluded.
We measured total adiponectin and not the high molecular weight fraction, which has been proposed to have substantially more potent effects on hepatic insulin sensitivity compared with total adiponectin [79]. However, a recent study showed that total and high-molecular-weight (HMW) adiponectin have similar utility for the identification of insulin resistance and metabolic disturbances [67]. This suggested that total adiponectin levels may provide clinical information of the same diagnostic value as HMW adiponectin [67]. It has been reported that the low molecular weight form of resistin displays significantly increased bioactivity [14]. However, the high molecular weight hexamer of resistin is predominant in the human circulation [14]. Moreover, the measurement of total adiponectin and resistin are better standardized, cheaper and more accessible than the high-molecular-weight adiponectin and the low-molecular-weight resistin. Thus, our findings may stimulate the use of adiponectin and resistin in clinical and epidemiological settings.
The euglycemic hyperinsulinemic clamp technique must be used as a gold standard index of insulin resistance to validate the robustness of the IRAR index before their widespread use. Further studies are required to investigate whether the superior predictive power of the AR and IRAR indexes demonstrated in the present study translates into a significant clinical benefit. The AR and IRAR indexes are unlikely to be cost-effective for short-time administration of these indexes. However, the AR and IRAR indexes are good indicator for long-term metabolic status. Thus, it will be very cost-effective for long-time administration since the frequency of monitoring the disease progression and therapeutic response periodically will be greatly reduced. A long-term prospective research is needed to reveal the predictive value of the AR and IRAR indexes for insulin resistance in association with T2DM and MS, and to find their optimal cutoff values for future risk assessment and disease prevention. Moreover, the normal AR and IRAR indexes range need to be established for each laboratory with an appropriate control group because of significant inter-laboratory variations in insulin and adipokines (including adiponectin and resistin) determinations and/or possible differences in various populations.
Conclusions
The novel AR and IRAR indexes are cost-effective, precise, reproducible and reliable integrated diagnostic biomarkers of insulin sensitivity for screening subjects with increased risk of future development of T2DM and MS. These surrogate indexes are useful for early diagnosis of insulin resistance, T2DM and MS in the daily clinical practice and for large-scale clinical investigation.
References
Shaw JE, Sicree RA, Zimmet PZ: Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010, 87: 4-14. 10.1016/j.diabres.2009.10.007.
Mohamud WN, Ismail AA, Sharifuddin A, Ismail IS, Musa KI, Kadir KA, Kamaruddin NA, Yaacob NA, Mustafa N, Ali O, Harnida S, Bebakar WM: Prevalence of metabolic syndrome and its risk factors in adult Malaysians: Results of a nationwide survey. Diabetes Res Clin Pract. 2010.
Utzschneider KM, Van de Lagemaat A, Faulenbach MV, Goedecke JH, Carr DB, Boyko EJ, Fujimoto WY, Kahn SE: Insulin resistance is the best predictor of the metabolic syndrome in subjects with a first-degree relative with type 2 diabetes. Obesity (Silver Spring). 2010, 18: 1781-1787. 10.1038/oby.2010.77.
Alberti KG, Zimmet P, Shaw J: The metabolic syndrome--a new worldwide definition. Lancet. 2005, 366: 1059-1062. 10.1016/S0140-6736(05)67402-8.
Kusminski CM, Scherer PE: The road from discovery to clinic: adiponectin as a biomarker of metabolic status. Nature. 2009, 86: 592-595.
Chen BH, Song Y, Ding EL, Roberts CK, Manson JE, Rifai N, Buring JE, Gaziano JM, Liu S: Circulating levels of resistin and risk of type 2 diabetes in men and women: results from two prospective cohorts. Diabetes Care. 2009, 32: 329-334. 10.2337/dc08-1625.
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T: Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2010, 8: 1288-1295. 10.1038/nm788.
Zhuo Q, Wang ZQ, Fu P, Piao JH, Tian Y, Xu J, Yang XG: Association between adiponectin and metabolic syndrome in older adults from major cities of China. Biomedical and Environmental Sciences. 2010, 23: 53-61. 10.1016/S0895-3988(10)60032-3.
Li S, Shin HJ, Ding EL, van Dam RM: Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009, 302: 179-188. 10.1001/jama.2009.976.
Galic S, Oakhill JS, Steinberg GR: Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010, 316: 129-139. 10.1016/j.mce.2009.08.018.
Zhang MH, Na B, Schiller NB, Whooley MA: Association of resistin with heart failure and mortality in patients with stable coronary heart disease: data from the heart and soul study. J Card Fail. 2011, 17: 24-30. 10.1016/j.cardfail.2010.08.007.
Wasim H, Al-Daghri NM, Chetty R, McTernan PG, Barnett AH, Kumar S: Relationship of serum adiponectin and resistin to glucose intolerance and fat topography in South-Asians. Cardiovascular Diabetology. 2006, 5: 1-5. 10.1186/1475-2840-5-10.
Lewandowski KC, Szosland K, O'Callaghan C, Tan BK, Randeva HS, Lewinski A: Adiponectin and resistin serum levels in women with polycystic ovary syndrome during oral glucose tolerance test: a significant reciprocal correlation between adiponectin and resistin independent of insulin resistance indices. Mol Genet Metab. 2005, 85: 61-69. 10.1016/j.ymgme.2004.12.014.
Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L: Disulfide-dependent multimeric assembly of resistin family hormones. Science. 2004, 304: 1154-1158. 10.1126/science.1093466.
American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008, 31 (Suppl 1): S55-60.
Koh SB, Park JK, Yoon JH, Chang SJ, Oh SS, Kim JY, Ryu SY, Kim KS, Lee TY, You JS: Preliminary report: a serious link between adiponectin levels and metabolic syndrome in a Korean nondiabetic population. Metabolism. 2010, 59: 333-337. 10.1016/j.metabol.2009.07.031.
Henneman P, Aulchenko YS, Frants RR, Zorkoltseva IV, Zillikens MC, Frolich M, Oostra BA, van Dijk KW, van Duijn CM: Genetic architecture of plasma adiponectin overlaps with the genetics of metabolic syndrome-related traits. Diabetes Care. 2010, 33: 908-913. 10.2337/dc09-1385.
Heid IM, Henneman P, Hicks A, Coassin S, Winkler T, Aulchenko YS, Fuchsberger C, Song K, Hivert MF, Waterworth DM, Timpson NJ, Richards JB, Perry JR, Tanaka T, Amin N, Kollerits B, Pichler I, Oostra BA, Thorand B, Frants RR, Illig T, Dupuis J, Glaser B, Spector T, Guralnik J, Egan JM, Florez JC, Evans DM, Soranzo N, Bandinelli S, Carlson OD, Frayling TM, Burling K, Smith GD, Mooser V, Ferrucci L, Meigs JB, Vollenweider P, Dijk KW, Pramstaller P, Kronenberg F, van Duijn CM: Clear detection of ADIPOQ locus as the major gene for plasma adiponectin: results of genome-wide association analyses including 4659 European individuals. Atherosclerosis. 2010, 208: 412-420. 10.1016/j.atherosclerosis.2009.11.035.
Ling H, Waterworth DM, Stirnadel HA, Pollin TI, Barter PJ, Kesaniemi YA, Mahley RW, McPherson R, Waeber G, Bersot TP, Cohen JC, Grundy SM, Mooser VE, Mitchell BD: Genome-wide linkage and association analyses to identify genes influencing adiponectin levels: The GEMS Study. Obesity. 2009, 17: 737-744. 10.1038/oby.2008.625.
Lillioja S, Wilton A: Agreement among type 2 diabetes linkage studies but a poor correlation with results from genome-wide association studies. Diabetologia. 2009, 52: 1061-1074. 10.1007/s00125-009-1324-9.
Edwards KL, Hutter CM, Wan JY, Kim H, Monks SA: Genome-wide linkage scan for the metabolic syndrome: the GENNID study. Obesity (Silver Spring). 2008, 16: 1596-1601. 10.1038/oby.2008.236.
Bowden DW, Rudock M, Ziegler J, Lehtinen AB, Xu J, Wagenknecht LE, Herrington D, Rich SS, Freedman BI, Carr JJ, Langefeld CD: Coincident linkage of type 2 diabetes, metabolic syndrome, and measures of cardiovascular disease in a genome scan of the diabetes heart study. Diabetes. 2006, 55: 1985-1994. 10.2337/db06-0003.
Chiodini BD, Lewis CM: Meta-analysis of 4 coronary heart disease genome-wide linkage studies confirms a susceptibility locus on chromosome 3q. Arterioscler Thromb Vasc Biol. 2003, 23: 1863-1868. 10.1161/01.ATV.0000093281.10213.DB.
Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, McCulloch LJ, Ferreira T, Grallert H, Amin N, Wu G, Willer CJ, Raychaudhuri S, McCarroll SA, Langenberg C, Hofmann OM, Dupuis J, Qi L, Segre AV, van Hoek M, Navarro P, Ardlie K, Balkau B, Benediktsson R, Bennett AJ, Blagieva R, Boerwinkle E, Bonnycastle LL, Bengtsson Bostrom K, Bravenboer B, Bumpstead S, Burtt NP, Charpentier G, Chines PS, Cornelis M, Couper DJ, Crawford G, Doney AS, Elliott KS, Elliott AL, Erdos MR, Fox CS, Franklin CS, Ganser M, Gieger C, Grarup N, Green T, Griffin S, Groves CJ, Guiducci C, Hadjadj S, Hassanali N, Herder C, Isomaa B, Jackson AU, Johnson PR, Jorgensen T, Kao WH, Klopp N, Kong A, Kraft P, Kuusisto J, Lauritzen T, Li M, Lieverse A, Lindgren CM, Lyssenko V, Marre M, Meitinger T, Midthjell K, Morken MA, Narisu N, Nilsson P, Owen KR, Payne F, Perry JR, Petersen AK, Platou C, Proenca C, Prokopenko I, Rathmann W, Rayner NW, Robertson NR, Rocheleau G, Roden M, Sampson MJ, Saxena R, Shields BM, Shrader P, Sigurdsson G, Sparso T, Strassburger K, Stringham HM, Sun Q, Swift AJ, Thorand B, Tichet J, Tuomi T, van Dam RM, van Haeften TW, van Herpt T, van Vliet-Ostaptchouk JV, Bragi Walters G, Weedon MN, Wijmenga C, Witteman J, Bergman RN, Cauchi S, Collins FS, Gloyn AL, Gyllensten U, Hansen T, Hide WA, Hitman GA, Hofman A, Hunter DJ, Hveem K, Laakso M, Mohlke KL, Morris AD, Palmer CN, Pramstaller PP, Rudan I, Sijbrands E, Stein LD, Tuomilehto J, Uitterlinden A, Walker M, Wareham NJ, Watanabe RM, Abecasis GR, Boehm BO, Campbell H, Daly MJ, Hattersley AT, Hu FB, Meigs JB, Pankow JS, Pedersen O, Wichmann HE, Barroso I, Florez JC, Frayling TM, Groop L, Sladek R, Thorsteinsdottir U, Wilson JF, Illig T, Froguel P, van Duijn CM, Stefansson K, Altshuler D, Boehnke M, McCarthy MI: Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010, 42: 579-589. 10.1038/ng.609.
Franklin CS, Aulchenko YS, Huffman JE, Vitart V, Hayward C, Polasek O, Knott S, Zgaga L, Zemunik T, Rudan I, Campbell H, Wright AF, Wild SH, Wilson JF: The TCF7L2 diabetes risk variant is associated with HbA(C) levels: a genome-wide association meta-analysis. Ann Hum Genet. 2010, 74: 471-478. 10.1111/j.1469-1809.2010.00607.x.
Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Magi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparso T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proenca C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O'Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Bottcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jorgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martinez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orru M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvanen AC, Tanaka T, Thorand B, Tichet J, Tonjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Rios M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF, Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I: New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010, 42: 105-116. 10.1038/ng.520.
Preuss M, Konig IR, Thompson JR, Erdmann J, Absher D, Assimes TL, Blankenberg S, Boerwinkle E, Chen L, Cupples LA, Hall AS, Halperin E, Hengstenberg C, Holm H, Laaksonen R, Li M, Marz W, McPherson R, Musunuru K, Nelson CP, Burnett MS, Epstein SE, O'Donnell CJ, Quertermous T, Rader DJ, Roberts R, Schillert A, Stefansson K, Stewart AF, Thorleifsson G, Voight BF, Wells GA, Ziegler A, Kathiresan S, Reilly MP, Samani NJ, Schunkert H: Design of the Coronary ARtery DIsease Genome-Wide Replication And Meta-Analysis (CARDIoGRAM) Study: A Genome-wide association meta-analysis involving more than 22 000 cases and 60 000 controls. Circ Cardiovasc Genet. 2010, 3: 475-483. 10.1161/CIRCGENETICS.109.899443.
Ochiai H, Ooka H, Shida C, Ishikawa T, Inoue D, Okazaki R: Acarbose treatment increases serum total adiponectin levels in patients with type 2 diabetes. Endocr J. 2008, 55: 549-556. 10.1507/endocrj.K07E-107.
Nezu U, Tsunoda S, Yoshimura H, Kuwabara T, Tomura S, Seki Y, Kaneshiro M, Kamiyama H, Harada Y, Shigematsu E, Aoki K, Yamakawa T, Ohshige K, Natsumeda Y, Terauchi Y: Pravastatin potentiates increases in serum adiponectin concentration in dyslipidemic patients receiving thiazolidinedione: the DOLPHIN study. J Atheroscler Thromb. 2010, 17: 1063-1069.
Fuke Y, Fujita T, Satomura A, Wada Y, Matsumoto K: Alterations of insulin resistance and the serum adiponectin level in patients with type 2 diabetes mellitus under the usual antihypertensive dosage of telmisartan treatment. Diabetes Technol Ther. 2010, 12: 393-398. 10.1089/dia.2009.0126.
Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Koh Y, Shin EK: Distinct vascular and metabolic effects of different classes of anti-hypertensive drugs. Int J Cardiol. 2010, 140: 73-81. 10.1016/j.ijcard.2008.11.017.
Phillips SA, Kung JT: Mechanisms of adiponectin regulation and use as a pharmacological target. Curr Opin Pharmacol. 2010, 10: 1-8. 10.1016/j.coph.2010.08.002.
Riera-Guardia N, Rothenbacher D: The effect of thiazolidinediones on adiponectin serum level: a meta-analysis. Diabetes Obes Metab. 2008, 10: 367-375. 10.1111/j.1463-1326.2007.00755.x.
Simpson KA, Singh MA: Effects of exercise on adiponectin: a systematic review. Obesity (Silver Spring). 2008, 16: 241-256. 10.1038/oby.2007.53.
Diez JJ, Iglesias P: The role of the novel adipocyte-derived protein adiponectin in human disease: an update. Mini Rev Med Chem. 2010, 10: 856-869. 10.2174/138955710791608325.
Menzaghi C, Coco A, Salvemini L, Thompson R, De Cosmo S, Doria A, Trischitta V: Heritability of serum resistin and its genetic correlation with insulin resistance-related features in nondiabetic Caucasians. J Clin Endocrinol Metab. 2006, 91: 2792-2795. 10.1210/jc.2005-2715.
Asano H, Izawa H, Nagata K, Nakatochi M, Kobayashi M, Hirashiki A, Shintani S, Nishizawa T, Tanimura D, Naruse K, Matsubara T, Murohara T, Yokota M: Plasma resistin concentration determined by common variants in the resistin gene and associated with metabolic traits in an aged Japanese population. Diabetologia. 2010, 53: 234-246. 10.1007/s00125-009-1517-2.
Tejero ME, Voruganti VS, Proffitt JM, Curran JE, Goring HH, Johnson MP, Dyer TD, Jowett JB, Collier GR, Moses EK, MacCluer JW, Mahaney MC, Blangero J, Comuzzie AG, Cole SA: Cross-species replication of a resistin mRNA QTL, but not QTLs for circulating levels of resistin, in human and baboon. Heredity. 2008, 101: 60-66. 10.1038/hdy.2008.28.
Heijmans BT, Beekman M, Putter H, Lakenberg N, van der Wijk HJ, Whitfield JB, Posthuma D, Pedersen NL, Martin NG, Boomsma DI, Slagboom PE: Meta-analysis of four new genome scans for lipid parameters and analysis of positional candidates in positive linkage regions. Eur J Hum Genet. 2005, 13: 1143-1153. 10.1038/sj.ejhg.5201466.
Malhotra A, Elbein SC, Ng MC, Duggirala R, Arya R, Imperatore G, Adeyemo A, Pollin TI, Hsueh WC, Chan JC, Rotimi C, Hanson RL, Hasstedt SJ, Wolford JK: Meta-analysis of genome-wide linkage studies of quantitative lipid traits in families ascertained for type 2 diabetes. Diabetes. 2007, 56: 890-896. 10.2337/db06-1057.
Jung HS, Youn BS, Cho YM, Yu KY, Park HJ, Shin CS, Kim SY, Lee HK, Park KS: The effects of rosiglitazone and metformin on the plasma concentrations of resistin in patients with type 2 diabetes mellitus. Metabolism. 2005, 54: 314-320. 10.1016/j.metabol.2004.05.019.
Ohbayashi H: Pitavastatin improves serum resistin levels in patients with hypercholesterolemia. J Atheroscler Thromb. 2008, 15: 87-93.
Jones TE, Basilio JL, Brophy PM, McCammon MR, Hickner RC: Long-term exercise training in overweight adolescents improves plasma peptide YY and resistin. Obesity (Silver Spring). 2009, 17: 1189-1195.
Tuttolomondo A, Placa SA, Raimondo DD, Bellia C, Caruso A, Sasso BL, Guercio G, Diana G, Ciaccio M, Licata G, Pinto A: Adiponectin, resistin and IL-6 plasma levels in subjects with diabetic foot and possible correlations with clinical variables and cardiovascular co-morbidity. Cardiovascular Diabetology. 2010, 9: 50-10.1186/1475-2840-9-50.
Thomopoulos C, Daskalaki M, Papazachou O, Rodolakis N, Bratsas A, Papadopoulos DP, Papavasileiou MV, Perrea D, Makris T: Association of resistin and adiponectin with different clinical blood pressure phenotypes. J Hum Hypertens. 2011, 25: 38-46. 10.1038/jhh.2010.22.
Papadopoulos DP, Perrea D, Thomopoulos C, Sanidas E, Daskalaki M, Papazachou U, Votteas V, Makris T: Masked hypertension and atherogenesis: the impact on adiponectin and resistin plasma levels. J Clin Hypertens (Greenwich). 2009, 11: 61-65. 10.1111/j.1751-7176.2008.00070.x.
Krecki R, Krzeminska-Pakula M, Peruga JZ, Szczesniak P, Lipiec P, Wierzbowska-Drabik K, Orszulak-Michalak D, Kasprzak JD: Elevated resistin opposed to adiponectin or angiogenin plasma levels as a strong, independent predictive factor for the occurrence of major adverse cardiac and cerebrovascular events in patients with stable multivessel coronary artery disease over 1-year follow-up. Med Sci Monit. 2011, 17: CR26-32.
Boehncke S, Fichtlscherer S, Salgo R, Garbaraviciene J, Beschmann H, Diehl S, Hardt K, Thaci D, Boehncke WH: Systemic therapy of plaque-type psoriasis ameliorates endothelial cell function: results of a prospective longitudinal pilot trial. Arch Dermatol Res. 2010.
Choi HY, Kim S, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM: Association of Adiponectin, Resistin, and Vascular Inflammation: Analysis With 18F-Fluorodeoxyglucose Positron Emission Tomography. Arterioscler Thromb Vasc Biol. 2011.
Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M: Expanding role of AMPK in endocrinology. Trends Endocrinol Metab. 2006, 17: 205-215. 10.1016/j.tem.2006.05.006.
Barac A, Campia U, Matuskey LA, Lu L, Panza JA: Effects of peroxisome proliferator-activated receptor-gamma activation with pioglitazone on plasma adipokines in nondiabetic patients with either hypercholesterolemia or hypertension. Am J Cardiol. 2008, 101: 980-985. 10.1016/j.amjcard.2007.11.058.
Samaha FF, Szapary PO, Iqbal N, Williams MM, Bloedon LT, Kochar A, Wolfe ML, Rader DJ: Effects of rosiglitazone on lipids, adipokines, and inflammatory markers in nondiabetic patients with low high-density lipoprotein cholesterol and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006, 26: 624-630. 10.1161/01.ATV.0000200136.56716.30.
Derosa G, Maffioli P, Ferrari I, Palumbo I, Randazzo S, Fogari E, D'Angelo A, Cicero AF: Different actions of losartan and ramipril on adipose tissue activity and vascular remodeling biomarkers in hypertensive patients. Hypertens Res. 2011, 34: 145-151. 10.1038/hr.2010.205.
Koh KK, Quon MJ, Lim S, Lee Y, Sakuma I, Lee YH, Han SH, Shin EK: Effects of fenofibrate therapy on circulating adipocytokines in patients with primary hypertriglyceridemia. Atherosclerosis. 2011, 214: 144-147. 10.1016/j.atherosclerosis.2010.10.023.
Schulz C, Paulus K, Lehnert H: Adipocyte-brain: crosstalk. Results Probl Cell Differ. 2010, 52: 189-201. full_text.
Than A, Ye F, Xue R, Ong JW, Poh CL, Chen P: The crosstalks between adipokines and catecholamines. Mol Cell Endocrinol. 2011, 332: 261-270. 10.1016/j.mce.2010.11.002.
Ogawa R, Tanaka C, Sato M, Nagasaki H, Sugimura K, Okumura K, Nakagawa Y, Aoki N: Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem Biophys Res Commun. 2010, 398: 723-729. 10.1016/j.bbrc.2010.07.008.
Miyamoto Y, Morisaki H, Kokubo Y, Yamanaka I, Tomoike H, Okayama A, Yoshimasa Y, Morisaki T: Resistin gene variations are associated with the metabolic syndrome in Japanese men. Obesity Research & Clinical Practice. 2009, 3: 65-74.
Olszanecka-Glinianowicz M, Kuglin D, Dabkowska-Huc A, Skalba P: Serum adiponectin and resistin in relation to insulin resistance and markers of hyperandrogenism in lean and obese women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2010.
Castaneda TR, Nogueiras R, Muller TD, Krishna R, Grant E, Jones A, Ottaway N, Ananthakrishnan G, Pfluger PT, Chaudhary N, Solomon MB, Woods SC, Herman JP, Tschop MH: Decreased glucose tolerance and plasma adiponectin:resistin ratio in a mouse model of post-traumatic stress disorder. Diabetologia. 2010.
Sarafidis PA, Lasaridis AN, Nilsson PM, Pikilidou MI, Stafilas PC, Kanaki A, Kazakos K, Yovos J, Bakris GL: Validity and reproducibility of HOMA-IR, 1/HOMA-IR, QUICKI and McAuley's indices in patients with hypertension and type II diabetes. J Hum Hypertens. 2007, 21: 709-716. 10.1038/sj.jhh.1002201.
Chen H, Sullivan G, Quon MJ: Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes. 2005, 54: 1914-1925. 10.2337/diabetes.54.7.1914.
Bahijri SM, Alissa EM, Akbar DH, Ghabrah TM: Estimation of insulin resistance in non-diabetic normotensive Saudi adults by QUICKI, HOMA-IR and modified QUICKI: a comparative study. Ann Saudi Med. 2010, 30: 257-264. 10.4103/0256-4947.65252.
Matsuhisa M, Yamasaki Y, Emoto M, Shimabukuro M, Ueda S, Funahashi T, Matsuzawa Y: A novel index of insulin resistance determined from the homeostasis model assessment index and adiponectin levels in Japanese subjects. Diabetes Res Clin Pract. 2007, 77: 151-154. 10.1016/j.diabres.2006.10.005.
Zaletel J, Barlovic DP, Prezelj J: Adiponectin-leptin ratio: a useful estimate of insulin resistance in patients with Type 2 diabetes. J Endocrinol Invest. 2010, 33: 514-518.
Tsuda K: Roles of adiponectin and oxidative stress in the regulation of membrane microviscosity of red blood cells in hypertensive men-an electron spin resonance study. J Obes. 2011.
Almeda-Valdes P, Cuevas-Ramos D, Mehta R, Gomez-Perez FJ, Cruz-Bautista I, Arellano-Campos O, Navarrete-Lopez M, Aguilar-Salinas CA: Total and high molecular weight adiponectin have similar utility for the identification of insulin resistance. Cardiovasc Diabetol. 2010, 9: 26-10.1186/1475-2840-9-26.
Lucidi P, Rossetti P, Porcellati F, Pampanelli S, Candeloro P, Andreoli AM, Perriello G, Bolli GB, Fanelli CG: Mechanisms of insulin resistance after insulin-induced hypoglycemia in humans: the role of lipolysis. Diabetes. 2010, 59: 1349-1357. 10.2337/db09-0745.
Zhai W, Xu C, Ling Y, Liu S, Deng J, Qi Y, Londos C, Xu G: Increased lipolysis in adipose tissues is associated with elevation of systemic free fatty acids and insulin resistance in perilipin null mice. Horm Metab Res. 2010, 42: 247-253. 10.1055/s-0029-1243599.
Hnevkovska Z, Dietrich J, Siklova-Vitkova M, Kolostova K, Kovacikova M, Duskova M, Broz J, Vedral T, Stich V, Polak J: Adiponectin inhibits spontaneous and catecholamine-induced lipolysis in human adipocytes of non-obese subjects through AMPK-dependent mechanisms. Physiol Res. 2010.
Rae C, Robertson SA, Taylor JM, Graham A: Resistin induces lipolysis and re-esterification of triacylglycerol stores, and increases cholesteryl ester deposition, in human macrophages. FEBS Lett. 2007, 581: 4877-4883. 10.1016/j.febslet.2007.09.014.
Li FP, Wang F, Nie FR, Li ZZ, Huang YQ, Zhang JG, Li F, Xue SN, Yan L: The effects of glucose fluctuation on resistin. Zhonghua Nei Ke Za Zhi. 2010, 49: 484-487.
Ziemke F, Mantzoros CS: Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. 2010, 91: 258S-261S. 10.3945/ajcn.2009.28449C.
Derosa G, Maffioli P, Ferrari I, Mereu R, Ragonesi PD, Querci F, Franzetti IG, Gadaleta G, Ciccarelli L, Piccinni MN, D'Angelo A, Salvadeo SA: Effects of one year treatment of vildagliptin added to pioglitazone or glimepiride in poorly controlled type 2 diabetic patients. Horm Metab Res. 2010, 42: 663-669. 10.1055/s-0030-1255036.
Wang C, Guan Y, Yang J: Cytokines in the Progression of Pancreatic beta-Cell Dysfunction. Int J Endocrinol. 2010, 2010: 515136.
Alberti KG, Zimmet PZ: Definition, diagnosis, and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine. 1998, 15: 539-553. 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S.
Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G: Insulin Resistance and Hypersecretion in Obesity. Journal of Clinical Investigation. 1997, 100: 1166-1173. 10.1172/JCI119628.
Al-Daghri NM, Al-Attas OS, Al-Rubeaan K, Sallam R: Adipocytokine profile of type 2 diabetics in metabolic syndrome as defined by various criteria. Diabetes Metab Res Rev. 2008, 24: 52-58. 10.1002/dmrr.763.
Simpson F, Whitehead JP: Adiponectin-it's all about the modifications. The International Journal of Biochemistry & Cell Biology. 2010, 42: 785-788.
Acknowledgements
This work was supported by University of Malaya postgraduate research grants (PS102-2009A and PS201-2010A), short-term research university grant (FS232-2008C), and e-science fund grant (12-02-03-2044). We thank all the participants in the project. We are grateful to our lab members for support and helpful discussion throughout the project, and to the nurses of University Malaya Medical Centre (UMMC) particulary Madam Farahwahidah for helping in blood sample collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CHL carried out the laboratory works, collected the data and samples, performed the statistical analysis, and wrote the manuscript. SM participated in the design and coordination of the study and helped to edit the manuscript. All authors read and approved the final manuscript.
Cia-Hin Lau contributed equally to this work.
Electronic supplementary material
12933_2010_320_MOESM1_ESM.PDF
Additional file 1: Supplementary Information. 1. Supplementary Methods: Subjects Determination of the anthropometric clinical and metabolic parameters 2. Supplementary Table Table S1. Mathematical equations for each insulin resistance (IR) index 3. Supplementary Figure and Figure Legend Figure S1. Standard curve for ELISA adiponectin and resistin (PDF 252 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Lau, CH., Muniandy, S. Novel adiponectin-resistin (AR) and insulin resistance (IRAR) indexes are useful integrated diagnostic biomarkers for insulin resistance, type 2 diabetes and metabolic syndrome: a case control study. Cardiovasc Diabetol 10, 8 (2011). https://doi.org/10.1186/1475-2840-10-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2840-10-8