Abstract
Vaccines have made a major contribution to public health, including the eradication of one deadly disease, small pox, and the near eradication of another, poliomyelitis.Through the introduction of new vaccines, such as those against rotavirus and pneumococcal diseases, and with further improvements in coverage, vaccination can significantly contribute to the achievement of the health-related United Nations Millennium Development Goals.The Global Immunization Vision and Strategy (GIVS) was developed by WHO and UNICEF as a framework for strengthening national immunization programmes and protect as many people as possible against more diseases by expanding the reach of immunization, including new vaccines, to every eligible person.This paper briefly reviews global progress and challenges with respect to public vaccination programmes.
The most striking recent achievement has been that of reduction of global measles mortality from an estimated 750,000 deaths in 2000 down to 197,000 in 2007. Global vaccination coverage trends continued to be positive. In 2007 most regions reached more than 80% of their target populations with three doses of DPT containing vaccines. However, the coverage remains well short of the 2010 goal on 90% coverage, particularly in the WHO region of Africa (estimated coverage 74%), and South-East Asia, (estimated coverage 69%). Elements that have contributed to the gain in immunization coverage include national multi-year planning, district-level planning and monitoring, re-establishment of outreach services and the establishment of national budget lines for immunization services strengthening.
Remaining challenges include the need to: develop and implement strategies for reaching the difficult to reach; support evidence-based decisions to prioritize new vaccines for introduction; strengthening immunization systems to deliver new vaccines; expand vaccination to include older age groups; scale up vaccine preventable disease surveillance; improve quality of immunization coverage monitoring and use the data to improve programme performance; and explore financing options for reaching the GIVS goals, particularly in lower-middle income countries.
Although introduction of new vaccines is important,this should not be at the expense of sustaining existing immunization activities. Instead the introduction of new vaccine introduction should be viewed as an opportunity to strengthen immunization systems, increase vaccine coverage and reduce inequities of access to immunization services.
Similar content being viewed by others
Introduction
Vaccination has made enormous contributions to public health, including the eradication of one dreaded disease, small pox, and elimination of poliomyelitis from all but a handful of countries. It is estimated that between two and three million child deaths are averted annually through vaccination against diphtheria, tetanus, pertussis and measles and many more future deaths averted in older groups (e.g. 600,000 future deaths prevented annually through hepatitis B vaccination). However, vaccine-preventable diseases are still responsible for about 25% of the 10 million deaths occurring annually among children under five years of age [1]. This is partly related to the fact that an increasing number of infectious diseases can now be classified as vaccine-preventable. With the availability of new vaccines, such as those against rotavirus and pneumococcal diseases, and further improvements in vaccination coverage, a much larger proportion of children can now be protected against a broader range of infectious diseases. Thus vaccines have the potential to make a significant contribution to the achievement of the health-related United Nations Millennium Development Goals (MDG), especially MDG4 [2] that calls for a two third reduction in the under-five mortality rate by 2015 compared to 1990 levels. However, if the trend in mortality reduction observed between 1990 and 2005 continues, the goal will not be achieved [3]. The cost of such a failure would be close to 40 million children deaths.
In 2005, the 58th World Health Assembly, recognizing the role that vaccines and immunization can play in reducing under-five mortality, welcomed the Global Immunization Vision and Strategy (GIVS) 2006-2015 developed by WHO and UNICEF as a framework for strengthening national immunization programmes [4, 5]. Its goal is to protect as many people as possible against more diseases by expanding the reach of immunization to every eligible person and ensuring that immunization is high on every health agenda. The strategy aims to increase, or at least sustain, very high levels of vaccine coverage, not just for infants but for all age groups, introduce new vaccines and link immunization with the delivery of other health interventions. This strategy was drawn up against a background of increasing demand for vaccines, rapid progress in developing new vaccines and technologies, continuing health-sector development, increasing vulnerability to pandemics and other health emergencies and more potential opportunities for partnerships.
The purpose of this paper is to briefly review global progress and challenges with respect to public vaccination programmes.
Progress
Success of measles mortality reduction efforts
In 2003, the World Health Assembly urged full implementation of the WHO-UNICEF strategic plan for measles mortality reduction 2001-2005 [6], and, at the end of 2005, the major public health goal of reducing global measles mortality by 50% compared with the 1999 level had been surpassed, with a reduction of 60% [7]. In 2005 the World Health Assembly endorsed a revised goal to reduce global measles deaths by 90% by 2010 (or earlier) compared with 2000 as one of the GIVS goals [4]. Global mortality due to measles was reduced by 74% from an estimated 750,000 deaths in 2000 to 197,000 in 2007 [8]. The largest percentage reduction in estimated measles mortality during this period occurred in the Eastern Mediterranean (90%) and African regions (89%), accounting for 79% of the global reduction in measles mortality. Immunization coverage estimates produced annually by WHO and UNICEF, based on official data reported by member states and other published data, showed that in 2007, global coverage with the scheduled dose of a measles-containing vaccine reached 82%, increasing from 72% in 2000 [9, 10]. The decrease in measles mortality was the result of both improved routine coverage and the implementation of mass vaccination campaigns. These public health accomplishments helped to prevent nearly 11 million measles deaths between 2000 and 2007, with vaccination campaigns in which more than 578 million children aged nine months to 15 years were vaccinated against measles between 2000 and 2007 in 47 highpriority countries accounting for 3.6 million of these deaths averted. [8] They were made possible by the enormous efforts made by the national governments of the targeted priority countries with the highest disease burden and the concentrated focus of immunization partners on the most effective strategies to control measles rapidly, supported by predictable financing of the programme.
Though there has been tremendous success in reducing measles mortality, deficiencies in routine immunization coverage are threatening to offset these gains with outbreaks occurring in regions with low routine immunization coverage e.g. in Democratic Republic of Congo, Nigeria, Tanzania and Uganda [8]. This also suggests that the achievements are still fragile.
All countries have implemented a measles mortality reduction strategy, except India. India's failure to implement a strategy would mean that the region may not achieve the 90% mortality reduction goal, thus also affecting the achievement of the global measles mortality reduction goal [8].
Progress with routine immunization
Less striking than the success with measles mortality reduction, but equally important, have been overall improvements in routine immunization coverage since 2000. These have been most marked in lowest-income countries, and particularly in sub-Saharan Africa; other regions, apart from South-East Asia, have continued to sustain high levels of immunization coverage. In 2007, out of the estimated 129 million annual surviving infants, a record 105 million children under one year of age were vaccinated worldwide with three doses of diphtheria, tetanus and pertussis (DTP3) vaccine, and the number of unvaccinated children decreased to 24.1 million (11.5 million of which in South East Asia and 7.3 million in Africa) compared with 33.6 million in 2000 [11]. An estimated 86% of the unimmunized children live in countries eligible for funding from the GAVI Alliance and 75% live in just 10 countries in Africa and Asia. These countries include India with close to 10 million unimmunized children, Nigeria, China, Indonesia, as well as Bangladesh, the Democratic Republic of the Congo, Ethiopia, Niger, Pakistan and Uganda. This is due to the large number of children born in these countries and/or low vaccination coverage.
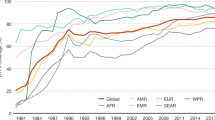
Trends related to global vaccination coverage (as measured by estimates of delivery of DTP3) continued to be positive in 2007, as shown in Figure 1 with most regions sustaining estimated levels of coverage in excess of 80%. The African region reached a record high vaccination coverage level of 74%, while estimates for South-East Asia indicate coverage increasing to 69% yet far away from the 80% mark [11]. In a few countries, however, interruption in immunization services resulted in an actual decline. A total of 117 (61%) countries reached 90% or more DTP3 coverage in 2007 while 156 (81%) reached a DTP3 coverage of 80% or more (Figure 2) [11]. It must be noted, however, that little progress has been achieved towards all countries ensuring at least 80% vaccination coverage in every district or equivalent administrative unit (i.e. one of the GIVS goals based on equity) and only 44 of the developing countries (28%) report DTP3 coverage ≥ 80% in all districts (Figure 3). Indeed, focusing only on average coverage at global or country levels may hide variability among countries and within countries among districts. Reaching unreached children remains one of the major challenges for many developing countries.
Immunization coverage with DTP3 vaccines in infants, 2007. Source:WHO/UNICEF coverage estimates 1980-2007, August 2008, 193 WHO Member States.The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. ©WHO 2008.All rights reserved.
Countries with % of districts achieving at least 80% DTP3 coverage, 2007. The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. ©WHO 2008.All rights reserved.
Elements that are believed to have contributed to the gain in immunization coverage include national multi-year planning, district-level planning and monitoring, and the establishment of national budget lines, funded with domestic and external resources, including those provided by the GAVI for immunization services strengthening. As a result, routine immunization coverage, seemingly in stagnation since the early 1990s, now shows an encouraging rising trend, particularly in several countries of sub-Saharan Africa.
The district planning and monitoring approach promoted by WHO is based on five key strategies that were initially repackaged in western Africa into a single strategy, which has since rapidly gained acceptance globally as the "reaching every district" (RED) strategy. As an example, with this strategy, vaccination coverage of children in Ethiopia with a third dose of DTP vaccine improved in 14 of the worst performing districts, from an average of 35% in 2002 to 71% in 2005. An evaluation of the RED strategy in nine countries in the region concluded that outreach services had contributed to increased coverage in districts where the strategy was implemented. However, lack of adequate transport facilities remained a limitation to sustaining outreach services [12]. The strategy has now been implemented to various degrees in 53 developing countries, mostly in Africa and south and south-east Asia [13].
The strategy of child health days, led by UNICEF, has also helped to promote routine immunization. Consistent with the emphasis of the GIVS on linking immunization with other health interventions, child health days are regular events designed to deliver an integrated package of preventive services such as immunization, vitamin A supplementation, deworming, growth monitoring and distribution of insecticide-treated bed nets. Of the 25 priority countries conducting measles supplementary immunization activities in 2006, 20 (80%) integrated at least one other child-survival intervention with measles. For example, in 2006 approximately 21 million insecticide-treated bednets were distributed during measles campaigns [14]. Child health days which became routine in many African countries, have achieved high coverage and have been shown to reduce inequalities in access to basic health services. They are usually conducted twice a year and the integrated package that they offer is defined according to epidemiological needs and local circumstances. Preliminary analysis of experience so far in Ethiopia, Uganda and the United Republic of Tanzania shows that child health days have helped to deliver multiple interventions effectively (including immunization), to improve routine immunization coverage, and to reduce operational costs per child reached. In contrast to other health programmes, successful implementation of immunization programmes in Africa has resulted in high rates of vaccination coverage in most countries.
Vaccination weeks to promote immunization coverage using new and existing vaccines are regularly organized in the Region of the Americas and the European Region. Endorsed by all Member States in the Region of the Americas in 2003, vaccination weeks have already reached more than 200 million children and adults in that Region, especially in difficult-to-reach populations, isolated communities and towns with low immunization coverage [15]. During the second European Immunization Week in April 2007, 25 Member States in the European Region were involved, underlining the importance of immunization through workshops, debates, training courses, exhibitions and media events [16].
New and underused vaccines
The 2003 State of the World's Vaccines and Immunization has emphasized the inequity in access to new vaccines that increased over the last decades as new life-saving vaccines became available at prices that most low-income countries were unable to afford [17]. Additional factors such as inadequacy of disease surveillance, inter alia, are also contributing to the North South divide with 12 childhood vaccines routinely used in established market countries and only six in most developing countries. Since that time, the introduction of new and underused vaccines has made and continues to make progress. By the end of 2007, 171 (of which two in part of the country) member states had introduced hepatitis B vaccine into their routine immunization programme compared to 31 Member States in 1992, the year of the World Health Assembly resolution recommending global vaccination against hepatitis B [18]. Global coverage with three doses of hepatitis B vaccine was then estimated at 65% (Figure 4) and is as high as 88% in the WHO Region of the Americas, in contrast to 69% in the African Region and 30% in the South-East Asian Region. This has to do with the lack or partial introduction of hepatitis B vaccination in large population countries. One hundred and seventy eight (92%) member states introduced hepatitis B vaccination by the end of 2008. However, whereas much progress has been made with the routine use of hepatitis B vaccine, this has taken 15 years since the World Health Assembly recommended its universal use. A similar time lag is unfortunately being experienced with Haemophilus influenzae type b (Hib) vaccine, for which global coverage remains low at 26% in 2007 (Figure 4). WHO recommends, that in view of their demonstrated safety and efficacy, conjugate Hib vaccines should be included in all routine infant immunization programmes [19]. A previous WHO recommendation encouraged vaccine use only in countries where burden was demonstrated and this may have limited vaccine introduction particularly in the Asian region where the burden was once debated. One hundred and thirty five states (70%) introduced Hib vaccination by the end of 2008 and a further nine countries are expected to introduce the vaccine before the end of 2009. Hib vaccine uptake is highest in the Americas (91% with three doses of Hib). This reflects, in great part, the support from the Pan American Health Organization (WHO Regional Office for the Americas) Revolving Fund [20]. This pooled procurement mechanism has helped supply nearly 40 countries with a range of affordable quality vaccines and syringes for over 30 years. Sri Lanka was the first country in the WHO South-East Asia region to introduce Hib vaccine as of January 2008. In 2007, a record number of countries applied to the GAVI for Hib vaccine introduction. Projections from GAVI applications suggest that by the end of 2009, more than 50% of children living in countries eligible for GAVI funding support will have access to Hib vaccines.
These developments are accompanied by member states increasing uptake of newly licensed vaccines against rotavirus diarrhoea and human papillomavirus infection and of the pneumococcal conjugate vaccine. The fast progress in introducing new vaccines has been facilitated by member states' growing recognition of the value of the protection conferred by vaccines and immunization. Such progress has also been made possible by the establishment of global financing mechanisms, including the GAVI, and the important role played by regional procurement mechanisms, for example the Revolving Fund for Vaccine Procurement in the Region of the Americas.
Rotavirus vaccine is now in use in 14 countries (two with partial introduction); five additional countries are expected to have introduced the vaccine by the end of 2009 with GAVI support (WHO/IVB database on new vaccine introduction, as of 31 May 2009). This marks the first time that the introduction of a vaccine has occurred simultaneously in both developed and developing countries.
By the end of 2008, pneumococcal conjugate vaccine was in universal use in 21 countries (four with partial introduction). Another 17 countries have plans to introduce the vaccine between 2009 and 2012. In March 2007, WHO published a position paper encouraging countries with high child mortality to consider introducing pneumococcal conjugate vaccines into their national immunization programmes (WHO/IVB database on new vaccine introduction, as of 31 May 2009) [21]. On April 24, 2009, Rwanda became the first GAVI-eligible country to introduce pneumococcal conjugate vaccine.
Further efforts needed and challenges
Indeed, both coverage expansion to reach the never/unreached with traditional Expanded Programme on Immunization vaccines and the addition of a number of new vaccines available by 2012 are critical elements of the GIVS. In spite of progress, however, much remains to be done if the full potential of immunization is to be exploited in achieving the health-related MDGs. About 1.1 million deaths of children under the age of five could be prevented through immunization with new vaccines against pneumococcal disease and rotavirus diarrhoea. In addition, vaccines against human papillomavirus infection could prevent nearly 250,000 annual deaths of women from cervical cancer.
More vaccines will soon become available on a large scale for use, among others, against meningococcal diseases, Japanese encephalitis and typhoid [22–24]. In addition, governments, multilateral agencies, foundations, and research institutions, among others, have substantially increased their investment in the development of new vaccines. As a result, various new vaccines are likely to be available for introduction in the next 10 years. These include, in particular, vaccines against dengue, tuberculosis and malaria. However, countries increasingly have to decide which of these life-saving tools they should finance and use on a routine basis.
The introduction of new vaccines poses challenges to the existing logistics and cold chain requirements due to their current presentations. In particular, the high volume of the prefilled glass syringe presentation of the 7-valent pneumococcal conjugate vaccine is exceeding the central cold chain storage capacity of some countries and the safe use and disposal of used glass syringes and needles poses a waste management challenge. These issues are being addressed through assistance to countries to improve vaccine and waste management and through interaction with industry to seek more suitable formulations and presentations of new vaccines. Many activities are also ongoing in the area of surveillance of diseases targeted by new vaccines including enhanced laboratory networks and centres of excellence.
WHO and its immunization partners have identified a set of activities to accelerate the introduction of new life-saving vaccines. WHO maintains a global new and under-utilized vaccines action plan, which provides a platform for coordinating the activities of global partners related to the introduction of vaccines in countries that need them most. Decisions on implementing new and underutilized vaccines require scientific evidence and data, a reliable supply of affordable vaccines, which are adapted to the country's immunization schedule, and an integrated disease monitoring and surveillance system. Work has begun on the implementation of this action plan, including the development of strategic options to support the introduction of more expensive new vaccines in low middle-income countries.
The GIVS provides the countries with the opportunities to also implement other strategies for expanding the benefits of vaccines to older age groups, either to complement disease control achieved by infant immunization (i.e. catch up vaccination of adolescents against hepatitis B or administration of booster doses of other vaccines to increase the duration of protection) or to target diseases that occur in older age groups, like human papillomavirus seasonal influenza, and typhoid.
In setting the future agenda two other points deserve attention. First is the need to develop integrated strategies, whereby immunization is implemented as one element of a comprehensive approach to disease control, be it meningitis/pneumonia control, diarrhoeal diseases control, cancer control or epidemic/pandemic prevention and control. Second, the delivery of routine immunization must be seen by all as the basis and the foundation of immunization programmes and must be given attention and dedicated resources. Indeed, reaping the full potential of immunization and including the full benefit of new vaccines can only occur with increasing overall protection and reducing coverage inequities.
Overall national vaccination coverage figures may mask local inequities and challenges. It is also essential that one looks at age-appropriate immunization for all antigens separately and not only at the proportion of children fully immunized by a certain age. Understanding the reasons for the lack of or delayed vaccination and finding innovative ways to reach the unreached and expand immunization to include older age groups to deliver new vaccines or booster doses is essential and operational research is needed to this effect. In this context, one can only applaud the multifaceted and geographic range of research efforts undertaken under the aegis of the Canadian International Immunization Initiative Phase 2 (CIII2) Operational research grants and reported in this supplemental issue [25–37]. These research projects have provided information on what needs to be better understood at a local level when health workers are trying to increase coverage in order to tailor their immunization programming: a) perception of childhood illness and what households see as appropriate time to vaccinate; b) gap in decision making at household level and vaccination process; c) vaccination procedures set by health services; d) who are those that are not completely vaccinated and why; e) role of religion in the decision to vaccinate or not and how often, etc. They also provided evidence on the impact of gender in relation to age-appropriate immunisation.
To be successful in the future, we must tackle the technical, logistics, political and social obstacles that are impeding progress. At global level, we learn that evidence-based policies and well-designed strategic direction are critical factors in guiding the choices of countries and their partners. Since 1974, WHO has facilitated global consensus, commitment and cooperation among several partners, on vaccines standards, immunization policies and strategic direction in support of developing countries.
Internationally, WHO provides recommendations via three main groups: (1) the Strategic Advisory Group of Experts (SAGE); (2) the Global Advisory Committee on Vaccine Safety (GACVS); and (3) the Expert Committee on Biological Standardization (ECBS) [38].
Since 1999, SAGE for immunization gathers some of the best world experts in the field of vaccines and immunization and provides policy and strategic advice to WHO. SAGE was restructured in 2005 to meet the needs of the GIVS and now reports to the WHO Director-General, reviews and approves all WHO policy recommendations, including the WHO position papers on vaccines. These are summaries of information about licensed vaccines of public health interest which are based on an extensive review and ranking of evidence by experts, and inputs from interested parties and industry. They are designed to be used by immunization and public health officials to make decisions about the public health value and use of specific vaccines in regions and countries. Recently SAGE helped to clarify WHO's position on the global use of Hib vaccine, thus facilitating the work of the GAVI Hib vaccine Initiative in support of country level decision-making. Over the past couple of years, SAGE made recommendations to WHO on the use of pneumococcal conjugate, rotavirus and typhoid vaccines, to mention a few, which were used to develop WHO related position papers [21, 24, 40].
Recommendations need to be adapted to each country. Their aim is not to prescribe rigid immunization schedules that all programmes must follow, but rather to offer a framework which countries can adapt to existing schedules and local epidemiological, economical and other circumstances and in the context of other health priorities. Supporting the establishment/strengthening of National Immunization Technical Advisory Committees that can convert global policy recommendations into a national policy is one of WHO's priorities. This is part of the process to ensure evidence-based decision at country level, which is particularly needed in view of the complexity of the immunization programs and cost of new vaccines [40].
The GACVS was established to respond promptly to vaccine safety issues of potential global importance. The committee does not directly determine immunization policies, but it does express its scientific opinion on vaccine safety, which could result in policy changes. The committee evaluates questions of vaccine safety by thoroughly reviewing the latest developments in basic science, epidemiology and clinical practice. All aspects of vaccine safety are covered, whether of national or international interest. The impartiality of the committee is essential and explains why its mandate is distinct from that of SAGE. The committee has on occasion found the alleged harmfulness of certain vaccines to be unsubstantiated, yet has also promptly recognized, when the need has arisen, the link between a given vaccine and adverse effects [41].
The WHO ECBS was established to set norms and standards for the manufacturing, licensing and control of biologicals. The committee provides guidelines on vaccine manufacturing, quality control, product labeling, transportation and storage, and makes recommendations on assays and other tests of vaccine quality, safety and immunogenicity [38].
Finally, the prequalification is the procedure that WHO has established to assess the acceptability, in principle, of vaccines for purchase by UN agencies. The pre-qualification process was originally codified in 1989, and was revised in 1996 and 2002 [42, 42]. In addition to UN agencies, many countries now use the list of WHO pre-qualified vaccines to select reliable and high quality vaccines.
Securing adequate and affordable vaccine supply as well as long-term predictable funding for vaccines and immunization is one of the top priorities of the global community in support of the world's poorest people.
For 117 middle and low-income countries, the related cost has been estimated to be $76 billion for vaccines and delivery systems for routine immunization, mass campaigns for accelerated disease control initiatives and new vaccines introduction [43]. The need for accelerated introduction of new vaccines in all high-burden countries must be matched by adequate financial support, including support for countries with lower-middle incomes. Such countries are not eligible for funding from the GAVI and support for them has heretofore been insufficient or lacking. As a result, these countries are starting to face increasing financial and technical challenges in order to maintain the same levels of access to newer technologies as low-income countries, which benefit from financial and technical assistance from sources such as the GAVI Fund. Limited access to international support is resulting in lower-middle income countries beginning to lag behind the poorest countries in protecting their populations from vaccine-preventable diseases using newer vaccines and combination vaccines. The current global financial crisis will unfortunately make the overall financing of the immunization programmes even more challenging.
To meet the above challenges and reach the immunization objectives already expressed in the United Nations General Assembly special session on children (2002) and further enunciated in the GIVS, strong disease surveillance and programme monitoring systems are required. WHO and its partners have developed a global framework for vaccine preventable disease surveillance and immunization programme monitoring [44]. This framework combines the use of countrywide active surveillance, passive aggregate disease reporting, sentinel site surveillance, and prospective, time-limited projects to generate the comprehensive epidemiological data required to guide immunization programmes. It also outlines strategies such as ongoing monitoring of vaccine management and vaccine safety, as well as cross-sectional programme reviews to assess the state of programmes at the district and health facility levels.
Continuous measurement of vaccination coverage is key to assessing programme performance and also taking timely corrective action. Ideally, coverage monitoring should be based on accurate and timely reporting of administrative data that is reported up from the most peripheral levels to the national, regional and global levels. The data should be used at the different administrative levels for timely corrective action when the data indicate gaps or failures in the programme. Population-based surveys and data quality audits serve to validate the coverage estimates. The WHO-UNICEF joint reporting format on national immunization performance collects annually information from 193 member states and has been an important source for global monitoring of immunization performance [45]. While in most countries, the coverage estimates based on administrative data reported by countries are validated by surveys, in some countries wide discrepancies between coverage estimates based on administrative data and coverage surveys still exists. Efforts are ongoing to provide such countries with support to determine the sources of error in the administrative coverage data and take corrective action [46, 47].
As has been demonstrated by the global poliomyelitis eradication initiative, efficient surveillance systems can be established, even in resource-poor settings, at quite low cost relative to the cost of the intervention itself. The poliomyelitis surveillance network provides a structure for rapidly detecting and responding to diseases of national and international importance. Where appropriate, this network should serve as the platform both for an integrated disease surveillance system that provides epidemiological data on other communicable diseases, and for detection and response to emerging infectious disease threats. Funding for disease surveillance is usually disease specific and time limited. In the presence of weak national systems, parallel systems tend to be established in order to generate data suited to the needs of specific programmes. These uncoordinated efforts may address short-term needs, but are unsustainable in the long term. The global framework provides an opportunity for immunization partners to coordinate their efforts to secure sustainable funding for surveillance and programme monitoring.
Developments in vaccines and immunization provide us with tremendous opportunities to impact the health of our populations, particularly the health of poor and marginalized communities who carry the disproportionate burden of disease. This opportunity comes with big challenges for weak health systems. GIVS provides a framework for maximizing the benefits of vaccination, but also creating efficiencies through an integrated and synergistic approach to health care delivery.
Abbreviations
- BCG:
-
Bacille Calmette-Guérin
- DTP:
-
Diphtheria, Tetanus, Pertussis
- GAVI:
-
GAVI Alliance
- GIVS:
-
Global Immunization Vision and Strategy
- MDG:
-
Millennium Development Goals
- RED:
-
Reaching Every District
- SAGE:
-
Strategic Advisory Group of Experts
- GACVS:
-
Global Advisory Committee on Vaccine Safety
- ECBS:
-
Expert Committee on Biological Standardization
- CIII2:
-
Canadian International Immunization Initiative Phase 2.
References
Challenges in global immunization and the Global Immunization Vision and Strategy 2006-2015. Weekly Epidemiol Rec. 2006, 81: 190-195. [http://www.who.int/wer/2006/wer8119.pdf]
Millennium Project. [http://www.unmillenniumproject.org/goals/index.htm]
United Nations Children's Fund (UNICEF) New York: UNICEF: The State of the World's Children 2006. 2005, [http://www.unicef.org/sowc06/]
World Health Organization and UNICEF. GIVS Global Immunization Vision and Strategy 2006-2015. 2005, World Health Organization, [http://www.who.int/vaccines-documents/DocsPDF05/GIVS_Final_EN.pdf]
Bilous J, Eggers R, Gasse F, Jarrett S, Lydon P, Magan A, Okwo-Bele JM, Salama P, Vandelaer J, Villeneuve P, Wolfson LJ: A new global immunisation vision and strategy. Lancet. 2006, 367: 1464-1466. 10.1016/S0140-6736(06)68625-X.
WHO-UNICEF Joint statement on strategies to reduce measles mortality worldwide. 2001, World Health Organization and UNICEF, [http://www.who.int/immunization_delivery/adc/measles/Joint%20Statement.pdf]
Wolfson LJ, Strebel PM, Gacic-Dobo M, Hoekstra EJ, McFarland JW, Hersh BS, Measles Initiative.: Has the 2005 measles mortality reduction goal been achieved? A natural history modelling study. Lancet. 2007, 369: 191-200. 10.1016/S0140-6736(07)60107-X.
Progress in global measles control and mortality reduction, 2000-2007. Weekly Epidemiol Rec. 2008, 83: 441-448.
WHO/UNICEF estimates of national immunization coverage. [http://www.who.int/immunization_monitoring/routine/immunization_coverage/en/index4.html]
Burton AH, Monasch R, Lautenbach B, Gacic-Dobo M, Neill M, Karimov R, Wolfson LJ, Jones G, Birmingham M: WHO and UNICEF estimates of national infant immunization coverage: methods and processes. Bull WHO. 2009,
Progress Towards Global Immunization Goals - 2007 Summary presentation of key indicators Updated September 2008. [http://www.who.int/immunization_monitoring/data/SlidesGlobalImmunization.pdf]
In-Depth Evaluation of the REACHING EVERY DISTRICT APPROACH in the African Region, 2007. [http://www.afro.who.int/ddc/vpd/routine/red-2007.pdf]
Vandelaer J, Bilous J, Nshimirimana D: Reaching Every District (RED) approach: a way to improve immunization performance. Bull WHO. 2008, 86 (3): A-B.
Progress in global measles control and mortality reduction, 2000-2006. Weekly Epidemiol Rec. 2007, 82: 417-424.
Vaccination Week in the Americas, 2008. [http://www.paho.org/English/DD/PIN/vw2008.htm]
European Immunization Week. [http://www.euro.who.int/eiw]
World Health Organization: States of the world's vaccines and Immunization 2003: revised edition. 2003, World Health Organization, 96-[http://www.who.int/vaccines-documents/DocsPDF04/wwwSOWV_E.pdf]
McGregor A: WHO: World Health Assembly. Lancet. 1992, 339: 1287-10.1016/0140-6736(92)91605-8.
WHO Position Paper on Haemophilus influenzae type b conjugate vaccines. Weekly Epidemiol Rec. 2006, 81: 445-452.
Andrus JK, Crouch AA, Fitzsimmons J, Vicari A, Tambini G: Immunization and the Millennium Development Goals: progress and challenges in Latin America and the Caribbean. Health Aff (Millwood). 2008, 27: 487-493. 10.1377/hlthaff.27.2.487.
Pneumococcal conjugate vaccine for childhood immunization - WHO Position Paper. Weekly Epidemiol Rec. 2007, 82: 93-104.
Meeting of the immunization Strategic Advisory Group of Experts, November 2008 -conclusions and recommendations. Weekly Epidemiol Rec. 2008, 83: 193-208.
Japanese encephalitis vaccines: WHO Position Paper. Weekly Epidemiol Rec. 2006, 81: 331-340.
Typhoid vaccines: WHO Position Paper. Weekly Epidemiol Rec. 2008, 83: 49-59.
Bicaba A, Haddad S, Kabore M, Taminy E, Feletto M, Fournier P: Monitoring the performance of the Expanded Program on Immunization: the case of Burkina Faso. BMC Int Health Hum Rights. 2009, 9 (Suppl 1): S12-10.1186/1472-698X-9-S1-S12.
Cockcroft A, Andersson N, Omer K, Ansari NM, Khan A, Chaudhry UU, Ansari U: One size does not fit all: local determinants of measles vaccination in four districts of Pakistan. BMC Int Health Hum Rights. 2009, 9 (Suppl 1): S4-10.1186/1472-698X-9-S1-S4.
Corsi DJ, Bassani DG, Kumar R, Awasthi S, Jotkar R, Kaur N, Jha P: Gender inequity and age-appropriate immunization coverage in India from 1992 to 2006. BMC Int Health Hum Rights. 2009, 9 (Suppl 1): S3-10.1186/1472-698X-9-S1-S3.
Djibuti M, Gotsadze G, Zoidze A, Mataradze G, Esmail LC, Kohler JC: The role of supportive supervision on immunization program outcome - a randomized field trial from Georgia. BMC Int Health Hum Rights. 2009, 9 (Suppl 1): S11-10.1186/1472-698X-9-S1-S11.
Dugas M, Dubé E, Kouyaté B, Sanou A, Bibeau G: Portrait of a lenghty vaccination trajectory in Burkina Faso: from cultural acceptance of vaccines to actual immunization. BMC Int Health Hum Rights. 2009, 9 (Suppl 1): S9-10.1186/1472-698X-9-S1-S9.
Ledogar RJ, Fleming J, Andersson N: Knowledge synthesis of benefits and adverse effects of measles vaccination: the Lasbela balance sheet. BMC Int Health Hum Rights. 2009, 9 (Suppl 1): S6-10.1186/1472-698X-9-S1-S6.
Mitchell S, Andersson N, Ansari NM, Omer K, Soberanis JL, Cockcroft A: Equity and vaccine uptake: a cross-sectional study of measles vaccination in Lasbela District, Pakistan. BMC Int Health Hum Rights. 2009, 9 (Suppl 1): S7-10.1186/1472-698X-9-S1-S7.
Shea B, Andersson N, Henry D: Increasing the demand for childhood vaccination in developing countries: a systematic review. BMC Int Health Hum Rights. 2009, 9 (Suppl 1): S5-10.1186/1472-698X-9-S1-S5.
Koumaré AK, Traore D, Haidara F, Sissoko F, Traoré I, Dramé S, Sangaré K, Diakité K, Coulibaly B, Togola B, MaÏga A: Evaluation of immunization coverage within the Expanded Program on Immunization in Kita Circle, Mali: a cross-sectional survey. BMC Int Health Hum Rights. 2009, 9 (Suppl 1): S13-10.1186/1472-698X-9-S1-S13.
Fourn L, Haddad S, Fournier P, Gansey R: Determinants of parents' reticence toward vaccination in urban areas in Benin (West Africa). BMC Int Health Hum Rights. 2009, 9 (Suppl 1): S14-10.1186/1472-698X-9-S1-S14.
Haddad S, Bicaba A, Feletto M, Taminy E, Kabore M, Ouedraogo B, Contreras G, Larocque R, Fournier P: System-level determinants of immunization coverage disparities among health districts in Burkina Faso:a multiple case study. BMC Int Health Hum Rights. 2009, 9 (Suppl 1): S15-10.1186/1472-698X-9-S1-S15.
Sanou A, Simboro S, Kouyaté B, Gugas M, Graham J, Bibeau G: Assessment of factors associated with complete immunization coverage in children aged 12-23 months: a cross-sectional study in Nouna district, Burkina Faso. BMC Int Health Hum Rights. 2009, 9 (Suppl 1): S10-10.1186/1472-698X-9-S1-S10.
Andersson N, Cockroft A, Ansari NM, Omer K, Baloch M, Foster AH, Shea B: Evidence-based discussion increases childhood vaccination uptake: a randomised cluster controlled trial of knowledge translation in Pakistan. BMC Int Health Hum Rights. 2009, 9 (Suppl 1): S8-10.1186/1472-698X-9-S1-S8.
Duclos P, Okwo-Bele JM: Recommandations et politiques vaccinales mondiales Le rôle de l'OMS. Médecine/Sciences. 2007, 23: 409-416.
Rotavirus vaccines: WHO Position Paper. Weekly Epidemiol Rec. 2007, 82: 285-295.
Meeting of the immunization Strategic Advisory Group of Experts, November 2008 -conclusions and recommendations. Weekly Epidemiol Rec. 2009, 84: 1-16.
Folb PI, Bernatowska E, Chen R, Clemens J, Dodoo AN, Ellenberg SS, Farrington CP, John TJ, Lambert PH, Macdonald NE, Miller E, Salisbury D, Schmitt HJ, Siegrist CA, Wimalaratne O: A global perspective on vaccine safety and public health: the global advisory committee on vaccine safety. Am J Pub Health. 2004, 94: 1926-1931. 10.2105/AJPH.94.11.1926.
Procedure for assessing the acceptability, in principle, of vaccines for purchase by the United Nations agencies. 2006, World Health Organization, [http://www.who.int/vaccines-documents/DocsPDF06/812.pdf]
Wolfson LJ, Gasse F, Wolfson LJ, Gasse F, Lee-Martin SP, Lydon P , Magan A, Tibouti A, Johns B, Hutubessy R, Salama P, Okwo-Bele JM: Estimating the cost of achieving the WHO-UNICEF Global Immunization Vision and Strategy, 2006-2015. Bull WHO. 2008, 86: 27-39.
Dabbagh A, Eggers R, Cochi S, Dietz V, Strebel P, Cherian T: A new global framework for immunization monitoring and surveillance. Bull WHO. 2007, 85: 901-980.
WHO/UNICEF Joint Reporting Process. [http://www.who.int/immunization_monitoring/routine/joint_reporting/en/index.html]
Lim SS, Stein DB, Charrow A, Murray CJ: Tracking progress towards universal childhood immunisation and the impact of global initiatives: a systematic analysis of three-dose diphtheria, tetanus, and pertussis immunisation coverage. Lancet. 2008, 372: 2031-2046. 10.1016/S0140-6736(08)61869-3.
Burton T, Neil M, Okwo-Bele JM, Salama P, Wardlaw T: Measurement of immunisation coverage. Lancet. 2009, 373: 210-211. 10.1016/S0140-6736(08)61895-4.
Acknowledgements
This article is published as part of BMC International Health and Human Rights Volume 9 Supplement 1, 2009: The fallacy of coverage: uncovering disparities to improve immunization rates through evidence.The Canadian International Immunization Initiative Phase 2 (CIII2) Operational Research Grants. The full contents of the supplement are available online at http://www.biomedcentral.com/1472-698X/9?issue=S1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Duclos, P., Okwo-Bele, JM., Gacic-Dobo, M. et al. Global immunization: status, progress, challenges and future. BMC Int Health Hum Rights 9 (Suppl 1), S2 (2009). https://doi.org/10.1186/1472-698X-9-S1-S2
Published:
DOI: https://doi.org/10.1186/1472-698X-9-S1-S2