Abstract
Background
Despite broad agreement on the necessity to improve quality of diabetic care through implementation of clinical guidelines, in Italy many people with diabetes still lack adequate care in general practice. In addition there is little evidence to support the choice of implementation strategies, especially in the Lazio region (central Italy), where comparative studies among general practitioners (GPs) are uncommon. The primary objective of the study is to assess the effectiveness of different strategies for the implementation of an evidence-based guideline for the management of non-complicated type 2 diabetes mellitus (DM) among GPs of the Lazio region.
Methods/Design
Three-arm cluster-randomised trial (C-RCT). 252 GPs were randomised either to arm 1 (comprising a training module and administration of the guideline), or to arm 2 (administration of guideline without training), or to arm 3 (control arm), continuing current practice. Arm 1 participants attended a two-day course with CME credits. Data collection will be performed using current information systems. Patients' health data was also collected to describe diabetic populations cared for by GP participants. Process outcomes will be measured at the patient level and at the cluster level one year after the intervention. We will assess GPs' adherence to guideline recommendations for diabetes management relative to: 1) pharmacological management of diabetes; 2) pharmacological management of cardiovascular risk factors (hypertension and dislypidaemia); 3) measurement of glycosilated haemoglobin as the principal indicator of glycaemic control; 4) micro- and macrovascular complications assessment tests. Outcomes will be expressed as proportions of patients cared for by GPs who will have prescriptions of drugs, requests for tests and for outpatient appointment visits. To estimate the efficiency of resource use associated with the intervention a cost-effectiveness analysis will be carried out. The design of the study is based on three Cochrane and one Health Technology Assessment systematic reviews of guideline dissemination and implementation strategies.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
In Italy diabetes mellitus (DM) is a major health problem with a prevalence of 3–4% [1, 2]. Considerable resources are committed to addressing important clinical problems connected with the treatment of micro- and macrovascular complications [3].
In Italy, patients with type 2 non-complicated DM are mainly treated in generale practice, whereas patients with type 1 DM usually receive care from specialists.
Although clinical guidelines are available from traditional means, data from an unpublished survey, conducted in a local health district of Lazio, show that the overall quality of DM care is generally poor and that the management of DM often fails to achieve the established standards.
Outcome measures
The most efficacious guidelines implementation strategies for changing general practitioner's (GP) behaviour are a topic for debate. A systematic rewiew by Grimshaw et al [4] concluded that there is an imperfect evidence base to support decisions on which strategy is likely to be efficient, due to methodological weakness of the majority of the studies included in the review.
Further uncertainties concern knowledge on service organisation and delivery of diabetic care and the lack of comparative studies on the effects of guideline implementation strategies in Italy [4].
We report on the design of a cluster-randomised trial (C-RCT) to assess the effects of two different strategies of introducing evidence-based guidelines for the treatment of type 2 DM in primary care. The C-RCT design (randomisation at the level of professional practice or health care organization) represents the optimal design when evaluating dissemination and implementation strategies [5].
As systematic reviews can inform and contribute to the correct design of randomised controlled trials, our protocol is based on three Cochrane and one Health Technology Assessment systematic reviews of guideline dissemination and implementation strategies. All reviews were prepared by members of the Cochrane EPOC group.
Objectives
The primary objective of the study is to assess the effectiveness of different strategies for the implementation of an evidence-based guideline for the management of non-complicated type 2 DM among GPs of the Lazio region of central Italy. Our null hypothesis is that a structured intervention will be no more effective than a passive dissemination of the guideline or a no-intervention strategy.
The secondary objective is to estimate the efficiency of resource use associated with the intervention through a cost-effectiveness analysis.
The third objective is to try to generalise our trial's findings to the rest of the GP population, mostly identifying the existence, the tipology and the direction of barriers to the implementation of the guideline in the primary care setting of Lazio region.
Methods
The choice of diabetes guideline
As a first step, we conducted a systematic review of the existing guidelines on diabetes care. Secondly, we used the quality assessment criteria and the quality assurance scores of the National Guideline Program of the Italian Institute of Health [6].
Thirdly, we performed a qualitative assessment of guidelines applicability to our regional context.
We chose the following guideline: Stratégie de prise en charge du patient diabétique de type 2 à l'exclusion de la prise en charge des complications. ANAES(Agence Nationale d'Accréditation et d'Evaluation en Santé) [7]. The guideline has been translated, updated and adapted for Italian GPs.
Study design
We chose a three-arm C-RCT. Single GPs are the unit of randomization and allocation is based on clusters.
Arm 1 includes GPs who underwent a two-day training module and consequent administration of the guideline.
Arm 2 includes GPs who received the guideline without any training but with a written request to implement the guideline.
Arm 3 includes GPs who continue current practice (control group).
Data on the care process for DM are being collected during the 12 months of duration of the study and main outcomes on GPs' prescribing behaviour will be constructed.
Methodological basis
The study follows the methodological recommendations and is based on the findings of systematic reviews performed by the Cochrane EPOC Group [8–10].
Grimshaw et al's review reported poor methodological quality of the majority of the included studies and, in particular, identified three recurring errors in C-RCTs:
Unit of analysis errors, when the unit of randomisation is the GP but the unit of analysis is the patient analysed independently of the cluster.
Baseline imbalance, when small arm denominators may cause imbalance between arms in important prognostic factors (e.g. baseline performance).
Within groups comparisons, when investigators undertake within groups analysis, rather than between groups.
Study setting and selection of participants
The study is carried out in the primary care setting of Italian National Health Service in the Lazio region. Eligible study participants are GPs taking part in an electronically-linked disease surveillance network and receiving a specific fee for computer-made prescriptions. Recruitment was performed by an invitation letter written in a standardized format, giving information about the study project. Five hundred GPs accepted to participate.
Sample size considerations
GPs were randomly selected from those who accepted to participate and randomised. As the observations on individuals in the same cluster tend to be correlated, C-RCTs require more participants than individually randomised RCTs, to obtain equivalent statistical power. Therefore, the sample size calculation took into account the estimate of the average number of diabetic patients per GP and the need to adjust for intracluster correlation factor, that, on the basis of the unpublished survey, was estimated to be 0.1 [11].
To demonstrate a 10% difference in effect, with a power of 90% (1- beta = 0.90) and a statistical significance level of 5% in outcome measures between the control and intervention groups, we estimated a need for a sample of 252 GPs [12]. Allocation was based on clusters. Random selection and randomization were performed by "REXSCO" [13] software.
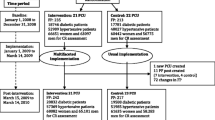
A flow-chart of participants' progress in the trial is at Figure 1.
Data collection
The main study outcome measures will be rates of performance of process of care for diabetic patients identified by recruited GPs. In this design, GPs are "units of measure" of the process of diabetic care delivery, as they manage laboratory and preventive care for most patients with type 2 DM.
Individual patients' data were collected and two different data sources were considered. Patient's anagraphic and health status data (baseline information) were transmitted through an on-line compilation. GP participants were given a login and a password to access personal web pages. The aim of collecting personal data is to describe accurately the diabetic population on which physician's behaviour may have an impact.
The second data set is based on drug prescriptions, requests for tests and for outpatient appointment visits. These data will be extracted by current information systems, which routinely assemble data for reimbursement purposes. Thus, data from administrative databases may be used to perform a cheap and exhaustive assessment of GP's performance and of the effectiveness of methods for influencing diabetes care process and outcome.
Similar baseline data (for the 12 months prior to the intervention) will be collected for all outcomes.
Details of intervention
In our trial the intervention pertains to the GP (cluster) level.
Although Grimshaw et al's systematic review has suggested that different combinations of multi-faceted interventions appear to be more effective than single interventions, we have decided to give priority to cheaper and more feasible interventions compared to potentially more effective, but more expensive and complex, interventions.
Therefore, we structured the intervention through a process of identifying barriers to implementation of recommendations and factors that may facilitate changing professional behaviour, together with an estimation of the resources available within the governance budget. It is often difficult to bring clinical practice in line with the scientific evidence by "passively disseminating" guidelines alone.
In brief, we developed the intervention in accordance with the following criteria:
-
to be preferably a single and not a multi-faceted intervention
-
to be easy to carry out
-
to be reproducible in the implementation of other guidelines
The main components of the intervention were:
-
a two-day training course
-
CME credits
The training course was organized as parallel sessions of teaching modules together with interactive and group work sessions with discussion of the content of the guideline. An entry questionnaire was given to participants to obtain a picture of the current care provided to diabetic patients.
Generalisability
An important issue of our C-RCT is the generalisability (external validity) of the trial's findings at the cluster (GP) level. The previous cited reviews [8–10] reported possible not specific effects on the control arms of C-RCTs which could hinder generalisability of results.
Commonly, Lazio region's GPs have difficulty in being involved in institutional programs for improving the quality of care. Numerous barriers can be identified. "Internal" barriers include time constraints, possibly inadequate reimbursement and disagreement with innovative programs. "External" barriers include individual patient needs, limited systems to support chronic disease management and poor patient adherence to treatment. Furthermore, a large part of GPs may not be accustomed to web-based data loading. Finally, Lazio GPs may not be accustomed to taking part in trials. On the basis of these observations we hypothesize that the performance of the control group (current practice arm) could be different from "true" current practice, and that it could unconsciously improve from the trial's beginning, with the consequent underestimation of the effects. This may have an impact on the applicability of the results on large scale.
Our intention is to test the presence and direction of aspecific effects on arm 3, by means of a controlled before-and-after (CBA) analysis. A CBA is made possible by our use of routine data collection systems.
Principles of data analysis
We will carry out comparisons between intervention and control groups.
All analysis will be "intention to treat" and performed at the individual level. Absolute risks (RA) and relative risks (RR) with confidence intervals will be calculated. A multivariate analysis will be performed to assess the role of physician-associated factors (age, sex, median number of patients and geographical area of the surgery). One interim analysis at 6 months and one final analysis will be carried out.
We will carry out a CBA analysis using cluster-adjusted chi-square test [14].
Outcome measures
Policy makers need to have information about the likely benefits and costs of different guideline dissemination and implementation strategies, as they must make informed decisions about the opportunity of introducing guidelines in common practice. Nevertheless, as Grimshaw's review show (4) and other reviews report [15], the methodological quality of economic evaluations of guideline dissemination and implementation strategies is generally poor and data reporting on costs and effects are insufficient to be useful for decision-makers planning guideline implementation in their own setting.
A cost-effectiveness analysis will be performed, from the perspective of the policy decision maker and reported in accordance with the BMJ criteria [16]. We will limit the evaluation to the estimation and interpretation of marginal costs and benefits of changing practice. We will calculate cost-effectiveness ratios (CER), measuring the incremental costs of arms 1 and 2 compared to those in the control arm.
Outcomes measures
Patient's health outcomes will be not registered. Process of care variables are considered study outcomes, all aimed at assessing physician-changing behaviour for the 12 months following the intervention. We will assess GPs' adherence to guideline recommendations for DM management relatively to the following issues:
-
pharmacological management of diabetes
-
pharmacological management of cardiovascular risk factors (hypertension, dislypidaemia)
-
measurement of glycosilated haemoglobin as the principal indicator of glycaemic control
-
tests to assess possible micro- and macrovascular risks and/or complications of DM
Outcomes will be expressed as proportions of patients with drug prescriptions, requests for tests and for outpatient appointment visits. For pharmacological indicators, patients are considered as previously untreated if they have no medication prescriptions recorded in the 12 months prior to the beginning of the trial.
We will also investigate the following areas:
-
differences in health care resources consumption
-
differences in comparing the costs of the study with the benefits resulting by the intervention (see Economic evaluation paragraph)
A list of categories of the study outcomes, with the main outcome of each category, is reported in table 1.
Presentation of results
Study results will be summarised in the following tables.
Table 1. Study participants' flow
Table 2. Before and after comparison of training course test results
Table 3. Description of characteristics of GPs
Table 4. Baseline data of diabetic patients cared for by participant GPs
Table 5. Glycaemic control evaluation outcomes
Table 6. Micro- and macrovascular complications assessment tests
Table 7. Indicators of pharmacological management of diabetes
Table 8. Indicators of pharmacological management of cardiovascular risk factors
Table 9. Economic evaluation outcomes
Ethical aspects
The project is exempt from ethical clearance according to the Italian Ministry of Health law number (ex art. 12bis, Dlgs 229/1999). The Italian Data Protection act will be followed in the handling of patient data.
Conflicts of interest
None declared.
Funding
The study is funded by the Italian Ministry of Health ("Special Programs" art. 12 bis D.lgs 229/99) and the Lazio Region. Agency of Public Health of Lazio region provided the computer instruments for data collection and the resources for planning and organizational support.
Note
The text of this protocol has been revised on the basis of the recommendations of the CONSORT statement extension to C-RCTs [17].
References
Garancini MP: L'epidemiologia del diabete non insulinodipendente e la ridotta tolleranza glucidica. In: Il diabete in Italia. Edited by: Vaccaio O, Bonora E, Bruno G, Garancini MP, Muntoni S. 1996, SID, Editrice Kurtis, Milano, 17-30.
Garancini MP, Sergi A, Lazzari P, Gallus G: Epidemiology of known diabetes in Lombardy, North Italy. Clinical charateristics and metodological aspects. Acta Diabetol. 1995, 32 (4): 268-72.
Lucioni C, Garancini MP, Massi Benedetti M, Mazzi M, Serra G, per conto dell'Advisory Board italiano dello studio Code-2: Il costo sociale del diabete di tipo 2 in Italia: lo studio Code-2. Pharmacoeconomics, Italian Research Articles. 2000, 1 (2): 1-21.
Grimshaw JM, Thomas RE, Maclennan G, Fraser C, Ramsay CR, Vale R, Whitty P, Eccles MP, Matowe L, Shirran L, Wensing M, Dikstra R, Donaldson C, Hutchinson A: Effectiveness and Efficiency of Guideline Dissemination and Implementation Strategies. Health Technol Assess. 2004, 8 (6:iii–iv): 1-72.
Grimshaw J, Campbell MK, Eccles M, Steen I: Experimental and quasi-experimental designs for evaluating guideline implementation strategies. Fam Pract. 2000, 17: S11-S18. 10.1093/fampra/17.suppl_1.S11.
National Guideline Program of the Italian Institute of Health. [http://www.pnlg.it]
Agence Nationale d'Accréditation et d'Evaluation en Santé. Stratégie de prise en charge du patient diabétique de type 2 à l'exclusion de la prise en charge des complications. 2000, [http://www.anaes.fr]
Thomson O'Brien MA, Freemantle N, Oxman AD, Wolf F, Davis DA, Herrin J: Continuing education meetings and workshops: effects on professional practice and health care outcomes (Cochrane Review). In: The Cochrane Library. 2004, Chichester, UK: John Wiley & Sons Ltd, 1
Thomson O'Brien MA, Oxman AD, Davis DA, et al: Educational outreach visits: effects on professional practice and health care outcomes (Cochrane Review). In: The Cochrane Library. 2004, Chichester, UK: John Wiley & Sons Ltd, 1
Thomas L, Cullum N, McColl E, et al: Guidelines in professions allied to medicine (Cochrane Review). In: The Cochrane Library. 2004, Chichester, UK: John Wiley & Sons Ltd, 1
Empirical estimates of ICCs from changing professional practices studies. University of Aberdeen. [http://www.abdn.ac.uk/hsru/epp/cluster.shtml]
Cluster Randomisation Sample Size Calculator ver 1.0.2, Health Services Research Unit, Aberdeen University.
A Delphi INTELx86 WIN32 platform software developed for internal use only by Agency of Public Health.
Dos Santos Silva I: Cancer Epidemiology: Principles and Methods. International Agency for Research on Cancer. WHO.
Gosden T, Forland F, Kristiansen IS, Sutton M, Leese B, Giuffrida A, Sergison M, Pedersen L: Capitation, salary, fee-for-service and mixed systems of payment: effects on the behaviour of primary care physicians (Cochrane Review). In: The Cochrane Library. 2004, Chichester, UK: John Wiley & Sons Ltd, 1
Drummond MF, Jefferson TO, for the BMJ Working Party on guidelines for authors and peer-reviewers of economic submissions to the British Medical Journal: Guidelines for authors and peer-reviewers of economic submissions to the British Medical Journal. BMJ. 1996, 313: 275-83.
Campbell MK, Elblourne DR, Altmen DG, for the CONSORT Group: CONSORT statement: extension to cluster randomised trials. BMJ. 2004, 328: 702-708. 10.1136/bmj.328.7441.702.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-6963/4/13/prepub
Acknowledgement
Members of the IMPLEMEG Study Group are:
Pierluigi Bartoletti MD (Lazio Region Family Practice Training School)
Paolo Billi MD (Lazio Region Public Health Agency)
Virgilio Calzini MD (Lazio Region Family Practice Training School)
Maurizio D'Amato statistician (Lazio Region Public Health Agency)
Alfonso Fiorillo MD (Lazio Region Family Practice Training School)
Gabriella Guasticchi MD (Lazio Region Public Health Agency)
Carmelina Guerrera MD (Lazio Region Public Health Agency)
Giuseppe Grasso MD (Lazio Region Family Practice Training School)
Tom Jefferson MD (Cochrane Collaboration)
Sergio Leotta MD (Diabetes Centre, Sandro Pertini Hospital, Rome)
Donatella Mandolini statistician (Lazio Region Public Health Agency)
Walter Marrocco MD (Lazio Region Family Practice Training School)
Mazzieri Scheggi (patient representative)
Amina Pasquarella MD (Lazio Region Public Health Agency)
Carla Perria MD (Lazio Region Public Health Agency)
Concetta Suraci MD (Diabetes Centre, S. Eugenio Hospital, Rome)
The scientific Committee thanks Professor Jeremy Grimshaw for the helpful advice given.
Author information
Authors and Affiliations
Consortia
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Perria, C., the IMPLEMEG Study Group. Strategies for the introduction and implementation of a guideline for the treatment of type 2 diabetics by general practitioners (GPs) of the Lazio region of Italy (IMPLEMEG study): Protocol for a cluster randomised controlled trial [ISRCTN80116232]. BMC Health Serv Res 4, 13 (2004). https://doi.org/10.1186/1472-6963-4-13
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-6963-4-13