Abstract
Background
Multidrug resistance (MDR) is a major hurdle for cancer treatment worldwide and accounts for chemotherapy failure in over 90% of patients with metastatic cancer. Evidence of the cytotoxicity of Cameroonian plants against cancer cell lines including MDR phenotypes is been intensively and progressively provided. The present work was therefore designed to evaluate the cytotoxicity of the methanol extracts of twenty-two Cameroonian medicinal plants against sensitive and MDR cancer cell lines.
Methods
The methanol maceration was used to obtain the crude plant extracts whilst the cytotoxicity of the studied extracts was determined using a resazurin reduction assay.
Results
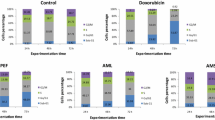
A preliminary assay on leukemia CCRF-CEM cells at 40 μg/mL shows that six of the twenty plant extract were able to enhance less than 50% of the growth proliferation of CCRF-CEM cells. These include Crinum zeylanicum (32.22%), Entada abyssinica (34.67%), Elaoephorbia drupifera (35.05%), Dioscorea bulbifera (45.88%), Eremomastax speciosa (46.07%) and Polistigma thonningii (45.11%). Among these six plants, E. drupifera showed the best activity with IC50 values below or around 30 μg/mL against the nine tested cancer cell lines. The lowest IC50 value of 8.40 μg/mL was recorded with the extract of E. drupifera against MDA-MB231 breast cancer cell line. The IC50 values below 10 μg/mL were recorded with the extracts of E. drupifera against MDA-MB231 breast cancer cells, C. zeylanicum against HCT116 p53+/+ and HCT116p53-/- colon cancer cells and E. abyssinica against HCT116 p53+/+ cells.
Conclusion
The results of the present study provide evidence of the cytotoxic potential of some Cameroonian medicinal plants and a baseline information for the potential use of Elaoephorbia drupifera in the treatment of sensitive and drug-resistant cancer cell lines.
Similar content being viewed by others
Background
The escape of cancer cells from chemotherapy by multidrug resistance (MDR) mechanisms is until now a major reason for systemic cancer treatment failure. So far, limited progress has been made in the fight against MDR cancer, and even the use of combination chemotherapy cannot solve the problem [1, 2]. Medicinal plants and alternative medicine are undeniable sources of new exploitable active principles to manage infectious and degenerative diseases. The structural diversity of chemicals from the medicinal plants makes them valuable tools in the search for potentially active drugs on sensitive and resistant phenotypes. It is estimated that more than 60% of the approved anticancer drugs in the United States of America (from 1983 to 1994) were from natural origin [3, 4]. In Cameroon, medicinal plants are traditionally used to manage infectious diseases and different types of cancers [5]. Evidence of the cytotoxicity of these plants against cancer cell lines has been provided [6–11]. In a recent research program, we started to investigate the cytotoxicity of Cameroonian plants against drug-resistant cancer cell lines. The idea is to identify plants able to kill drug-resistant cancer cells with similar efficacy as their drug-sensitive counterparts. Some of the plants identified so far include Echinops giganteus, Imperata cylindrica, Piper capense and Xylopia aethiopica which displayed considerable activities against the P-glycoprotein-expressing adriamycin-resistant cell line, CEM/ADR5000 [7, 12]. This encourage us to move forward to search for new cytotoxic agents from Cameroonian medicinal plants, with emphasis on MDR phenotypes with different mechanism of action. The present work was therefore designed to evaluate the cytotoxicity of twenty-two Cameroonian plants against both sensitive and drug-resistant cancer cell lines.
Methods
Plant material
All medicinal plants used in the present work were collected at various locations of Dschang, West-Region of Cameroon, between January and April 2012. The plants were identified at the National Herbarium (Yaounde, Cameroon), where voucher specimens were deposited under the reference numbers indicated in Table 1. The air-dried and powdered plant material was soaked in methanol for 48 h, at room temperature. The methanol extract was concentrated under reduced pressure to give the crude extract. This extract was then conserved at 4°C until further use.
Extraction
The air-dried and powdered plant samples (1 kg) were soaked in methanol (3 L) for 48 h, at room temperature. The methanol extract was concentrated under vacuum to give the crude extract. This extract was then conserved at 4°C until use.
Chemicals
Doxorubicin, vinblastine and daunorubicin were provided by the University Medical Center of the Johannes Gutenberg University (Mainz, Germany) and dissolved in PBS (Invitrogen, Eggenstein, Germany) at a concentration of 10 mM. Geneticin was purchased from Sigma-Aldrich (Munich, Germany) at a concentration of 50 mg/mL in sterile-filtered H2O.
Cell cultures
Drug-sensitive CCRF-CEM and multidrug-resistant CEM/ADR5000 leukemia cells were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal calf serum in a humidified 5% CO2 atmosphere at 37°C. Sensitive and resistant cells were kindly provided by Dr. Axel Sauerbrey (Department of Pediatrics, University of Jena, Jena, Germany). The generation of the resistant subline was previously described [52]. The specific overexpression of P-glycorprotein, but not other ABC transporters has been reported [53, 54]. Breast cancer cells, transduced with control vector (MDA-MB-231-pcDNA3) or with cDNA for the breast cancer resistance protein BCRP (MDA-MB-231-BCRP clone 23), were maintained under standard conditions as described above for CCRF-CEM cells. Human wild-type HCT116 (p53 +/+) colon cancer cells as well as knockout clones HCT116 (p53 -/-) derived by homologous recombination were a generous gift from Dr. B. Vogelstein and H. Hermeking (Howard Hughes Medical Institute, Baltimore, MD). Human glioblastoma multiforme U87MG cells (non-transduced) and U87MG cell line transduced with an expression vector harboring an epidermal growth factor receptor (EGFR) gene with a genomic deletion of exons 2 through 7 (U87MG.ΔEGFR) were kindly provided by Dr. W. K. Cavenee (Ludwig Institute for Cancer Research, San Diego, CA) [55]. MDA-MB-231-BCRP, U87MG.ΔEGFR and HCT116 (p53 -/- ) were maintained in DMEM medium containing 10% FBS (Invitrogen) and 1% penicillin (100 U/mL)-streptomycin (100 μg/mL) (Invitrogen) and were continuously treated with 800 ng/mL and 400 μg/mL geneticin, respectively. Human HepG2 hepatocellular carcinoma cells and normal AML12 heptocytes were obtained from the American Type Culture Collection (ATCC, USA). The above medium without geneticin was used to maintain MDA-MB-231, U87MG, HCT116 (p53 +/+), HepG2 and AML12 cell lines. The cells were passaged twice weekly. All experiments were performed with cells in the logarithmic growth phase.
Resazurin reduction assay
Resazurin reduction assay [56] was performed to assess the cytotoxicity of the studied samples toward various sensitive and resistant cancer cell lines. The assay is based on the reduction of resazurin, to the highly fluorescent resorufin by viable cells. Non-viable cells rapidly lose the metabolic capacity to reduce resazurin and thus produce no fluorescent signal. Briefly, adherent cells were detached by treatment with 0.25% trypsin/EDTA (Invitrogen, Darmstadt, Germany) and an aliquot of 1 × 104 cells was placed in each well of a 96-well cell culture plate (Thermo Scientific, Langenselbold, Germany) in a total volume of 200 μL. Cells were allowed to attach overnight and then treated with different concentrations of the studied sample. For suspension cells, aliquots of 2 × 104 cells per well were seeded in 96-well-plates in a total volume of 100 μL. The studied sample was immediately added in varying concentrations in an additional 100 μL of culture medium to obtain a total volume of 200 μL/well. After 24 h or 48 h, 20 μL resazurin (Sigma-Aldrich, Schnelldorf, Germany) 0.01% w/v in double-distilled water (ddH2O) were added to each well and the plates incubated at 37°C for 4 h. Fluorescence was measured on an Infinite M2000 Pro™ plate reader (Tecan, Crailsheim, Germany) using an excitation wavelength of 544 nm and an emission wavelength of 590 nm. Each assay was done at least two times, with six replicate each. The viability was evaluated based on a comparison with untreated cells. IC50 values represent the sample’s concentrations required to inhibit 50% of cell proliferation and were calculated from a calibration curve by linear regression using Microsoft Excel.
Results and discussion
In a prescreening of twenty-two plants, we tested a single concentration of 40 μg/mL for each sample against the sensitive CCRF-CEM leukemia cell line. The results depicted in Figure 1 indicate that six of the twenty-two plant extracts were able to display less than 50% growth proliferation of CCRF-CEM cells. These include Crinum zeylanicum (32.22%), Entada abyssinica (34.67%), Elaoephorbia drupifera (35.05%), Dioscorea bulbifera (45.88%), Eremomastax speciosa (46.07%) and Polistigma thonningii (45.11%). The IC50 values of these samples were then determined on a panel of cancer cell lines, including both sensitive and MDR phenotypes. The results are shown in Table 2. Only the Elaoephorbia drupifera extract as well as the control drug doxorubucin inhibited the proliferation of the nine studied cancer cell lines, with IC50 values below 40 μg/mL. Other extracts showed selective activities, the IC50 values being obtained on 6/10 tested cells lines for Crinum zeylanicum, 4/10 for Dioscorea bulbifera and Entada abyssinica, 3/10 for Eremomastax speciosa and Polistigma thonningii (Table 2). According to the criteria of the American National Cancer Institute, 30 μg/mL is the upper IC50 limit considered promising for purification of a crude extract [57]. Consequently, the highest concentration tested (40 μg/mL) in our screening was slightly above this limit. Considering this cutoff point, the IC50 values below or around 30 μg/mL were recorded with only the E. drupifera extract against the nine tested cancer cell lines (Table 2). However, other extract also displayed activities with IC50 values below 30 μg/mL on at least one of the cancer cell line tested.
MDR is a major hurdle for cancer treatment worldwide and accounts for chemotherapy failure in over 90% of patients with metastatic cancer [1, 58]. In the present work, we investigated both sensitive and MDR cell lines. The degrees of resistance were calculated by dividing the IC50 value of the resistant cell line by the corresponding parental sensitive cell line. We tested cell models overexpressing two ATP-binding cassette transporters, i.e. P-glycoprotein (ABCB1/MDR1) or breast cancer resistance protein (ABCG2/BCRP). Furthermore, we tested a p53 knockout cell line and a transfectant cell line harboring a mutation-activated EGFR gene (ΔEGFR) as examples for resistance-inducing tumor suppressors and oncogenes. Finally, we investigated HepG2 liver cancer cells and AML12 normal hepatocytes to compare carcinoma cells with normal cells. The degree of resistance on the tested cell line toward the control drug doxorubicin was generally high, showing that the studied cell lines can obviously be considered as suitable cell models to study drug resistance. For the most active extract E. drupifera, it can be observed that the degrees of resistance were in all cases lower than those of doxorubicin, suggesting that this sample can be exploited in a possible fight against cancer diseases involving MDR phenotypes. In addition, collateral sensitivity (sample more active on resistant cells than on sensitive cells) was observed with the extract of E. drupifera against U87MG.ΔEGFR, highlighting its good antiproliferative activity.
To the best of our knowledge, the cytotoxicity of the six most active extracts (C. zeylanicum, D. bulbifera, E. drupifera, E. abyssinica, E. speciosa and P. thonningii) is being reported for the first time. Nevertheless, compounds with activities against malignant cells such as crinine, 6-hydroxybuphanidrine and 6-ethoxybuphanidrine were isolated from C. zeylanicum[19]. Also, lupeol [27, 28] a moderately active cytotoxic compound [59] was identified in E. drupifera, the plant that displayed the best activity as observed in this study. The presence of such compounds could probably explain their antiproliferative activity.
Conclusion
In conclusion, the results of the present study provide evidence of the cytotoxic potential of some Cameroonian medicinal plants and highlight the good activity of Elaoephorbia drupifera on sensitive and drug-resistant cancer cell lines. This plant is a potential cytotoxic source, that could be explored in more details in the future to develop novel anticancer drugs against sensitive and resistant phenotypes.
References
Liu J, Zhao Y, Guo Q, Wang Z, Wang H, Yang Y, Huang Y: TAT-modified nanosilver for combating multidrug-resistant cancer. Biomaterials. 2012, 33 (26): 6155-6161. 10.1016/j.biomaterials.2012.05.035.
Broxterman H, Gotink K, Verheul H: Understanding the causes of multidrug resistance in cancer: a comparison of doxorubicin and sunitinib. Drug Resist Update. 2009, 12: 114-126. 10.1016/j.drup.2009.07.001.
Newman DJ, Cragg GM: Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007, 70 (3): 461-477. 10.1021/np068054v.
Stevigny C, Bailly C, Quetin-Leclercq J: Cytotoxic and antitumor potentialities of aporphinoid alkaloids. Curr Med Chem Anticancer Agents. 2005, 5 (2): 173-182. 10.2174/1568011053174864.
Adjanohoun J, Aboubakar N, Dramane K, Ebot M, Ekpere J, Enow-Orock E, Focho D, Gbile Z, Kamanyi A, Kamsu-Kom J: Traditional medicine and pharmacopoeia: contribution to ethnobotanical and floristic studies in Cameroon. 1996, Lagos-Nigeria: Technical and Research Commission of Organisation of African Unity (OAU/STRC)
Kuete V, Mbaveng AT, Tsaffack M, Beng VP, Etoa FX, Nkengfack AE, Meyer JJM, Lall N: Antitumor, antioxidant and antimicrobial activities of Bersama engleriana (Melianthaceae). J Ethnopharmacol. 2007, 115 (3): 494-501.
Kuete V, Krusche B, Youns M, Voukeng I, Fankam AG, Tankeo S, Lacmata S, Efferth T: Cytotoxicity of some Cameroonian spices and selected medicinal plant extracts. J Ethnopharmacol. 2011, 134 (3): 803-812. 10.1016/j.jep.2011.01.035.
Dzoyem JP, Nkuete AH, Kuete V, Tala MF, Wabo HK, Guru SK, Rajput VS, Sharma A, Tane P, Khan IA: Cytotoxicity and antimicrobial activity of the methanol extract and compounds from Polygonum limbatum. Planta Med. 2012, 78 (8): 787-792.
Choumessi AT, Danel M, Chassaing S, Truchet I, Penlap VB, Pieme AC, Asonganyi T, Ducommun B, Valette A: Characterization of the antiproliferative activity of Xylopia aethiopica. Cell Div. 2012, 7 (1): 8-10.1186/1747-1028-7-8.
Dzoyem J, Guru S, Pieme C, Kuete V, Sharma A, Khan I, Saxena A, Vishwakarma R: Cytotoxic and antimicrobial activity of selected Cameroonian edible plants. BMC Complement Altern Med. 2013, 13 (1): 78-10.1186/1472-6882-13-78.
Tamokou Jde D, Chouna J, Fischer-Fodor E, Chereches G, Barbos O, Damian G, Benedec D, Duma M, Efouet A, Wabo H: Anticancer and antimicrobial activities of some antioxidant-rich cameroonian medicinal plants. PLoS One. 2013, 8 (2): e55880-10.1371/journal.pone.0055880.
Kuete V, Sandjo L, Wiench B, Efferth T: Cytotoxicity and modes of action of four Cameroonian dietary spices ethno-medically used to treat Cancers: Echinops giganteus, Xylopia aethiopica, Imperata cylindrica and Piper capense. J Ethnopharmacol. 2013, doi. 10.1016/j.jep.2013.06.029
Okunade AL: Ageratum conyzoides L. (Asteraceae). Fitoterapia. 2002, 73 (1): 1-16. 10.1016/S0367-326X(01)00364-1.
Rukunga GM, Waterman PG: A new oleanane glycoside from the stem bark of Albizia gummifera. Fitoterapia. 2001, 72 (2): 140-145. 10.1016/S0367-326X(00)00276-8.
Rukunga GM, Muregi FW, Tolo FM, Omar SA, Mwitari P, Muthaura CN, Omlin F, Lwande W, Hassanali A, Githure J, Iraqi FW, Mungai GM, Kraus W, Kofi-Tsekpo : The antiplasmodial activity of spermine alkaloids isolated from Albizia gummifera. Fitoterapia. 2007, 78 (7–8): 455-459.
Boudreau MD, Beland FA: An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), Aloe vera. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2006, 24 (1): 103-154. 10.1080/10590500600614303.
Jainu M, Devi S: In vitro and in vivo evaluation of free radical scavenging potential of Cissus quadrangularis. Afr J Biomed Res. 2005, 8: 95-99.
Jainu M, Devi CS: Gastroprotective action of Cissus quadrangularis extract against NSAID induced gastric ulcer: role of proinflammatory cytokines and oxidative damage. Chem Biol Interact. 2006, 161 (3): 262-270. 10.1016/j.cbi.2006.04.011.
Berkov S, Romani S, Herrera M, Viladomat F, Codina C, Momekov G, Ionkova I, Bastida J: Antiproliferative alkaloids from Crinum zeylanicum. Phytother Res. 2011, 25 (11): 1686-1692. 10.1002/ptr.3468.
Kapingu C, Guillaume D, Mbwambo H, Moshi J, Uliso C, Mahunnah A: Diterpenoids from the roots of Croton macrostachys. Phytochemistry. 2000, 54: 767-770. 10.1016/S0031-9422(00)00166-7.
Gao H, Kuroyanagi M, Wu L, Kawahara N, Yasuno T, Nakamura Y: Antitumor-promoting constituents from Dioscorea bulbifera L. in JB6 mouse epidermal cells. Biol Pharm Bull. 2002, 25 (9): 1241-1243. 10.1248/bpb.25.1241.
Kuete V, Betrandteponno R, Mbaveng AT, Tapondjou LA, Meyer JJ, Barboni L, Lall N: Antibacterial activities of the extracts, fractions and compounds from Dioscorea bulbifera. BMC Complement Altern Med. 2012, 12: 228-10.1186/1472-6882-12-228.
Mbiantcha M, Kamanyi A, Teponno RB, Tapondjou AL, Watcho P, Nguelefack TB: Analgesic and anti-inflammatory properties of extracts from the bulbils of Dioscorea bulbifera L. var sativa (Dioscoreaceae) in mice and rats. Evid Based Complement Alternat Med. 2011, 2011: 912935-
Corley G, Tempesta S: Convulsant alkaloids from Dioscorea dumetorum. Tetrahedron Lett. 1985, 26 (13): 1615-1618. 10.1016/S0040-4039(00)98566-6.
Adesanya S, Ogundana S, Roberts M: Dihydrostilbene phytoalexins from Dioscorea bulbifera and D. dumentorum. Phytochemistry. 1989, 28 (3): 773-774. 10.1016/0031-9422(89)80113-X.
Eno A, Azah N: Effect of ethanolic extract from Elaeophorbia drupifera leaves on the gastrointestinal smooth muscle of the rabbit. Niger J Physiol Sci. 2004, 19 (1–2): 60-68.
Kinghorn A, Evans F: Occurrence of ingenol in Elaeophorbia species. Planta Med. 1974, 26: 150-154. 10.1055/s-0028-1097982.
Ahiahonu PW, Goodenowe DB: Triterpenoids from leaves of Elaeophorbia drupifera. Fitoterapia. 2007, 78 (5): 337-341. 10.1016/j.fitote.2007.02.002.
Ayisi N, Nyadedzor C: Comparative in vitro effects of AZT and extract of Ocimum gratissimum, Ficus polita, Clausena anisata, Alchornea cordifolia, and Elaeophorbia drupifera against HIV-1 and HIV-2 infections. Antivir Res. 2003, 58: 25-33. 10.1016/S0166-3542(02)00166-3.
Eno AE, Owo OI: Cardiovascular effects of an extract from the roots of a shrub Elaeophorbia drupifera. Phytother Res. 1999, 13 (7): 549-554. 10.1002/(SICI)1099-1573(199911)13:7<549::AID-PTR464>3.0.CO;2-Q.
Olajide OA, Alada AR: Studies on the anti-inflammatory properties of Entada abyssinica. Fitoterapia. 2001, 72 (5): 492-496. 10.1016/S0367-326X(01)00273-8.
Oben J, Assi S, Agbor G, Musoro D: Effect of Eremomastax speciosa on experimantal diarrhoea. Afr J Trad Complement Altern Med. 2006, 3 (1): 95-100.
Essien E, Aboaba O, Ogunwande A: Constituents and antimicrobial properties of the leaf essential oil of Gossypium barbadense (Linn.). J Med Plant Res. 2011, 5: 702-705.
Stipanovic D, Bell A, Mace E, Howell R: Antimicrobial terpenoids of Gossypium: 6-methoxygossypol and 6,6′-dimethoxygossypol. Phytochemistry. 1975, 14: 1077-1081. 10.1016/0031-9422(75)85190-9.
Bell A, Stipanovic D, Howell R, Fryxell A: Antimicrobial terpenoids of Gossypium: Hemigossypol, 6-methoxyhemigossypol and 6-deoxyhemigossypol. Phytochemistry. 1975, 14: 225-231. 10.1016/0031-9422(75)85044-8.
Saini S, Kaur H, Verma B, Ripudaman , Singh K: Kigelia africana (Lam.) Benth: - an overview. Nat Prod Rad. 2009, 8 (2): 190-197.
Aladesanmi A, Iwalewa E, Adebajo A, Akinkunmi E, Taiwo B, Olorunmola F, Lamikanra A: Antimicrobial and antioxidant activities of some Nigerian medicinal plants. Afr J Trad Complement Altern Med. 2007, 4 (2): 173-184.
Tantangmo F, Lenta BN, Boyom FF, Ngouela S, Kaiser M, Tsamo E, Weniger B, Rosenthal PJ, Vonthron-Senecheau C: Antiprotozoal activities of some constituents of Markhamia tomentosa (Bignoniaceae). Ann Trop Med Parasitol. 2010, 104 (5): 391-398. 10.1179/136485910X12743554760180.
Okpekon T, Yolou S, Gleye C, Roblot F, Loiseau P, Bories C, Grellier P, Frappier F, Laurens A, Hocquemiller R: Antiparasitic activities of medicinal plants used in Ivory Coast. J Ethnopharmacol. 2004, 90 (1): 91-97. 10.1016/j.jep.2003.09.029.
Miemanang R, Krohn K, Hussain H, Dongo E: Paullinoside a and paullinomide a: a new cerebroside and a new ceramide from leaves of Paullinia pinnata. Z Naturforsch. 2006, 61b: 1123-1127.
Akinpelu DA, Obuotor EM: Antibacterial activity of Piliostigma thonningii stem bark. Fitoterapia. 2000, 71 (4): 442-443. 10.1016/S0367-326X(00)00136-2.
Ibewuike JC, Ogundaini AO, Ogungbamila FO, Martin M-T, Gallard J-F, Bohlin L, Païs M: Piliostigmin, a 2-phenoxychromone, and C-methylflavonols from Piliostigma thonningii. Phytochemistry. 1996, 43 (3): 687-690. 10.1016/0031-9422(96)00367-6.
Pianaro A, Pinto P, Ferreira T, Ishikawa K, Braz-Filho R: Iridoid glucoside and antifungal phenolic compounds from Spathodea campanulata roots. Semina: Ciências Agrárias, Londrina. 2007, 28 (2): 251-256.
Simbo D: An ethnobotanical survey of medicinal plants in Babungo, Northwest Region. Cameroon. Ethnobiol Ethnomed. 2010, 6: 8-10.1186/1746-4269-6-8.
Ekor M, Ashorobi R, Ibitoye SF, Kasimi L: Acute toxicity, analgesic potential and preliminary antimocrobial studies of the aqueous plant extract of Spilanthes filicaulis. Niger J Health Biomed Sci. 2005, 4 (1): 30-34.
Tan V, Njimi K, Ayafor F: Screening of some African Medicinal plants for antiulcerogenic activity: part 1. Phytother res. 1997, 11: 45-47. 10.1002/(SICI)1099-1573(199702)11:1<45::AID-PTR945>3.0.CO;2-I.
Ramsay KS, Wafo P, Ali Z, Khan A, Oluyemisi OO, Marasini BP, Khan IA, Bonaventure NT, Choudhary MI, Atta ur R: Chemical constituents of Stereospermum acuminatissimum and their urease and alpha-chymotrypsin inhibitions. Fitoterapia. 2012, 83 (1): 204-208. 10.1016/j.fitote.2011.10.014.
Sob SV, Wabo HK, Tang CP, Tane P, Ngadjui BT, Ye Y: Phenol esters and other constituents from the stem barks of Stereospermum acuminatissimum. J Asian Nat Prod Res. 2011, 13 (12): 1128-1134. 10.1080/10286020.2011.619182.
Ndjakou LB, Vonthron-Sénécheau C, Fongang Soh R, Tantangmo F, Ngouela KM, Tsamo E, Anton R, Weniger B: In vitro antiprotozoal activities and cytotoxicity of some selected Cameroonian medicinal plants. J Ethnopharmacol. 2007, 111 (1): 8-12. 10.1016/j.jep.2006.10.036.
Atta-ur-Rahman , Zareen S, Choudhary I, Akhtar N, Shujaat S, Ngounou N: Some chemical constituents of Terminalia glaucescens and their enzymes inhibition activity. Z Naturforsch. 2005, 60b: 347-350.
Adebayo E, Ishola O: Phytochemical and antimicrobial screening of crude extracts from the root, stem bark, and leaves of Terminalia glaucescens. Afr J Pharm Pharmacol. 2009, 3 (5): 217-221.
Kimmig A, Gekeler V, Neumann M, Frese G, Handgretinger R, Kardos G, Diddens H, Niethammer D: Susceptibility of multidrug-resistant human leukemia cell lines to human interleukin 2-activated killer cells. Cancer Res. 1990, 50 (21): 6793-6799.
Efferth T, Sauerbrey A, Olbrich A, Gebhart E, Rauch P, Weber HO, Hengstler JG, Halatsch ME, Volm M, Tew KD, Ross DD, Funk JO: Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol. 2003, 64 (2): 382-394. 10.1124/mol.64.2.382.
Gillet J, Efferth T, Steinbach D, Hamels J, de Longueville F, Bertholet V, Remacle J: Microarray-based detection of multidrug resistance in human tumor cells by expression profiling of ATP-binding cassette transporter genes. Cancer Res. 2004, 64 (24): 8987-8993. 10.1158/0008-5472.CAN-04-1978.
Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD, Huang CM, Gill GN, Wiley HS, Cavenee WK: The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997, 272 (5): 2927-2935. 10.1074/jbc.272.5.2927.
O’Brien J, Wilson I, Orton T, Pognan F: Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000, 267 (17): 5421-5426. 10.1046/j.1432-1327.2000.01606.x.
Suffness M, Pezzuto JM: Assays related to cancer drug discovery. Methods in Plant Biochemistry: Assays for Bioactivity. Edited by: Hostettmann K. 1990, London: Academic Press, 6-
Longley D, Johnston P: Molecular mechanisms of drug resistance. J Pathol. 2005, 205: 275-292. 10.1002/path.1706.
Kuete V, Efferth T: Pharmacogenomics of Cameroonian traditional herbal medicine for cancer therapy. J Ethnopharmacol. 2011, 137 (1): 752-766. 10.1016/j.jep.2011.06.035.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-6882/13/250/prepub
Acknowledgments
VK is very grateful to the Alexander von Humboldt foundation for an 18 months’ fellowship in Germany through the “Georg Foster Research Fellowship for Experienced Researcher” program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
VK, IKV, ATM, RT, BW, and VPB carried out the study; VK and TE designed the experiments. VK wrote the manuscript; VK and TE supervised the work. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kuete, V., Voukeng, I.K., Tsobou, R. et al. Cytotoxicity of Elaoephorbia drupifera and other Cameroonian medicinal plants against drug sensitive and multidrug resistant cancer cells. BMC Complement Altern Med 13, 250 (2013). https://doi.org/10.1186/1472-6882-13-250
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-6882-13-250