Abstract
Background
The aim of this study was to determine factors associated with the severity of cancer related fatigue (CRF) and predictors of improvement of CRF at the first follow-up visit in patients with advanced cancer referred to outpatient palliative care clinic (OPC).
Methods
We reviewed the records of consecutive patients with advanced cancer presenting to OPC. Edmonton Symptom Assessment System (ESAS) scores were obtained at the initial and subsequent visits between January 2003 and December 2008. All patients received interdisciplinary care led by palliative medicine specialists following an institutional protocol. Fatigue improvement was defined as a reduction of ≥2 points in ESAS score relative to the baseline. Descriptive statistics were used to summarize patient characterstics. Univariate analyses were performed and only significant variables were included in multivariate regression analysis to determine factors associated with severity and improvement in CRF.
Results
A total of 1778 evaluable patients were analyzed (median age, 59 years; 52% male). The median time between visits was 15 days. Median fatigue scores on the ESAS were 6 at baseline and 5 at follow-up. Severity of all ESAS items and low serum albumin were associated with fatigue at baseline (p < 0.0001). The improvement of fatigue was observed in 586 patients (33%). The hierarchical model showed that fatigue improved over time (b = −0.009; p = 0.0009). low appetite (odds ratio [OR] = 1.09 per point; p = 0.0113) and genitourinary cancer (OR = 1.74 per point; p = 0.0458) were significantly associated with improvement of fatigue.
Conclusions
CRF is strongly associated with physical and emotional symptoms. Genitourinary cancer and low appetite at baseline were associated with successful improvement of fatigue.
Similar content being viewed by others
Background
Previous research has shown that patients with advanced cancer develop severe physical and psychosocial symptoms as a result of cancer and treatments [1, 2]. Among cancer-related symptoms, cancer-related fatigue (CRF) is the most common chronic and distressing [2], with a frequency of 60-90% [1]. Since CRF is more severe in advanced stages than in early stages of disease, CRF can prevent patients with advanced cancer from receiving effective cancer therapy [2]. The National Comprehensive Cancer Network defines CRF as a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and that interferes with usual functioning [3]. Despite CRF’s prevalence, severity, and effects on quality of life in patients with advanced cancer, available treatment options are limited [4].
Most referrals to outpatient palliative care clinics (OPC) were made late in the trajectory of the disease [5, 6]. Thus patients had only one or two follow-up visits due to late referral and logistics of receiving cancer care in a comprehensive center away from home. Hence it is vitally important to obtain prompt control of CRF in a short period of time. However there limited studies regarding factors associated with the severity of fatigue at the initial visit and predictors of improvement in patients with advanced cancer seen in outpatient palliative care clinics at first follow-up visit. Such data would enable researchers to develop treatment strategies to manage CRF in ambulatory advanced cancer patients. The aim of this study was to determine factors associated with the severity of fatigue and predictors of improvement of fatigue at the first follow-up visit in patients with advanced cancer referred to outpatient palliative care clinic.
Methods
Patient eligibility and assessments
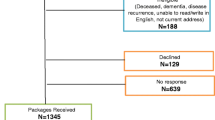
We reviewed the charts of 2071 consecutive advanced cancer patients (defined as locally advanced or metastatic) who had received care at the outpatient palliative care clinic (OPC) at The University of Texas MD Anderson Cancer Center and had prospectively completed the Edmonton Symptom Assessment System (ESAS) questionnaire including the ESAS fatigue score at the initial visit and subsequent follow-upvisits between January 2003 and December 2008. These patients had at least one follow-up visit after the initial consultation. Only patients who had prospectively completed an ESAS questionnaire at the initial visit and at any follow-up visits within 7–30 days of the initial visit were eligible for inclusion. Of the 2071 patients screened, a total of 1788 met the eligibility criteria. The patient characterstics and symptoms of patients with follow-up and those who did not were compared to determine if the study sample truly represents the OPC advanced cancer population.
To test the impact of palliative consultation on fatigue between the initial visit and the subsequent follow-up visit, we defined improvement of fatigue as a reduction of ≥2 points from the baseline, which is a threshold based on previous quality of life studies [7, 8].
Process of palliative service and interventions performed at the outpatient palliative care clinic
Care for all patients in the palliative care clinic was provided by an interdisciplinary palliative care team led by board-certified palliative care specialists. All 10 specialists work as a team and provide mutual coverage in cases of absence or illness to avoid clinic cancellations, which helps the team maintain a homogeneous approach to assessment, management, and communication with patients and their families. The other team members include a registered nurse trained specifically in palliative care, a pharmacist, a nutritionist, a chaplain, a social worker, and an advanced nurse practitioner trained in palliative care and psychiatry who provides counseling service. Other specialists in services such as wound management, speech therapy, occupational therapy, and physical therapy are consulted when needed.
The care of all patients followed a standardized management plan [9]. Patients and their families were initially assessed by the registered nurse using ESAS, Memorial Delirium Assessment Scale (MDAS), CAGE and constipation and family support questionnaires. The findings were discussed with a palliative care specialist, who then interviewed the patients and their families and performed a physical examination. The physician and nurse asked appropriate members of the interdisciplinary team to participate depending on the individual needs of patients and their families. Most patients referred to the outpatient palliative care clinic receive active cancer treatment including targeted therapy. In all patients fatigue was treated as a multidimensional construct irrespective of whether it was due to disease, cancer treatment or comorbidities. These interventions and care provided by the interdisciplinary team complied with palliative care guidelines established by the National Comprehensive Cancer Network and National Consensus Project and have been outlined elsewhere [10]. Specifically for the management of fatigue, the clinic coordinates a comprehensive assessment and management of all associated cancer-related symptoms such as pain, anorexia, anxiety, depression, sedation, shortness of breath, and sleep disturbance. The clinic also provides expressive supportive counseling and cognitive interventions so that patients and their families understand the treatment goals. Special emphasis is placed on medication review and any plans to discontinue medications associated with fatigue and sedation such as muscle relaxants, benzodiazepines, and antihistamines. In addition, the clinic team emphasizes exercise, light therapy, and methods to maintain and enhance socialization.
Edmonton symptom assessment system
The ESAS is a simple, validated, and reliable multi-item instrument developed to measure various symptoms in patients with advanced cancer [11, 12]. The ESAS questionnaire, visualized graphically as a numerical rating scale (0–10), was used to evaluate nine items (pain, fatigue, nausea, depression, anxiety, drowsiness, appetite, well-being, and shortness of breath) and one patient-specific symptom; the symptom distress score is calculated by adding the scores for the nine ESAS items. The patients were asked to rate the average intensity of these symptoms in the previous 24 hours, with higher scores indicating higher symptom intensity. In the outpatient settings the vast majority of patients are able to complete ESAS by themselves with minimal assistance. The ESAS fatigue score was used to measure changes in fatigue.
The memorial delirium assessment scale
The MDAS is a structured, 10-item clinician-rated scale, with each item scored as 0–3 and a possible total score of 0–30, designed to quantify the severity of delirium in medically ill patients. This tool was originally tested in a heterogeneous population of cancer patients and patients without cancer. The MDAS has been validated and used for the diagnosis of delirium in cancer patients [13]. Delirium was defined as an MDAS score of ≥7. MDAS is administered routinely in all patients presenting to the OPC as advanced patient are at risk for delirium.
CAGE questionnaire
The Cut Down, Annoyed, Guilty, and Eye Opener (CAGE) questionnaire is a simple, four-item screening survey for alcoholism. Previous studies by our group showed that patients who scored positively for alcoholism, with positive responses to ≥2 of the four items, had higher symptom expression than those who scored negatively [14]. CAGE questionnaire is administered routinely in all patients presenting to the OPC as advanced patients have severe symptom distress including significant pain and are on opioids and psychostimulants.
We received University of Texas MD Anderson Cancer Center Institutional Review Board approval for the study (protocol DR01-0710).
Statistical analyses
Descriptive statistics (means, medians, frequencies, and percentages) were used to summarize the patients’ symptoms measured by ESAS, CAGE and MDAS and their demographic characteristics. Comparison of patient characteristics and symptoms of patients with initial consult and follow-up visit and patients with no follow-up visit were compared using t-test, and Fisher exact t test. Statistical significance was set at p-value ≤0.05. To determine the association between baseline fatigue and gender, race, cancer site, anemia status, albumin level, and alcoholism status, we calculated summary statistics for ESAS for each of these variables and used a Kruskal-Wallis test to determine whether the distribution of baseline fatigue was the same for each. We also calculated correlation coefficients to determine the association between age and other baseline ESAS items.
To determine the predictive factors associated with severity of fatigue at baseline a linear regression model was created with the baseline fatigue score as the dependent variable and all the other baseline ESAS scores, MDAS score, alcoholism, low albumin (<3.5 g/dl anemia (hemoglobin <10 g/dl), and primary cancer type as the independent variables. All variables that were statistically significant in the univariate analyses (p ≤ 0.10) were included in the linear regression model.
Finally, a logistic regression model was created to determine whether ESAS score, MDAS score, anemia, low albumin, alcoholism, or primary cancer type at baseline was related to improvement in fatigue, with improvement of fatigue defined as a decrease of ≥2 in the ESAS fatigue score [7]. In these analyses, a p value of <0.05 was considered significant.
Results
Table 1 summarizes the demographic and clinical characteristics of the study population. The 1778 evaluable patients had a median age of 59 years; 52% were male. The most common primary cancer types were head and neck cancer and lung cancer (27%). The median time between visits was 15 days. The mean (standard deviation) fatigue score at baseline was 6 (2.39). 1489 patients (80%) reported moderate or severe fatigue (≥4/10).
Table 2 summarizes the ESAS item scores at the initial presentation and the first follow-up visit. At the initial visit, the median MDAS score was 2 (normal range, 0–7 of a possible 30; n = 793), the median blood hemoglobin level was 11.3 g/dl (normal range, 12–16 g/dl; n = 1426), and the median serum albumin level was 3.7 g/dl (normal range, 3.5-4.7 g/dl; n = 1178). Of the 793 patients with MDAS data, 31 (1.74%) had delirium according to MDAS (≥7 of 30). Of 1426 patients, 314 (17.6%) had anemia (≤10 g/dl), and of the 1178 patients with albumin data, 192 (10.7%) had low albumin levels (<3.5 g/dl).
Table 3 shows baseline data for 1788 patients who met the eligibility criteria and for 283 who had no follow-up and therefore were not evaluable. The results show that the patients who were not evaluable had similar characteristics to those evaluated but had lower severity of ESAS scores.
We found no univariate associations between fatigue and age (p = 0.06), gender (p = 0.07), race (p = 0.11), type of cancer (p = 0.32), anemia (p = 0.09) or alcoholism (p = 0.18) (Table 4). There were correlations between fatigue and severity levels of pain (r = 0.23), nausea (r = 0.31), anxiety (r = 0.33), depression (r = 0.33), drowsiness (r = 0.29), appetite (r = 0.41), sleep disturbance (r = 0.25), shortness of breath (r = 0.33), and well-being (r = 0.36) and the total ESAS symptom distress score (r = 0.54), with p < 0.0001 for each. According to the linear regression model, factors associated with fatigue were levels of pain (p < 0.0001), depression (p = 0.0017), appetite (p < 0.0001), drowsiness (p < 0.0001), well-being (p < 0.0001), shortness of breath (p < 0.0001), albumin level (p < 0.0001), and nausea status (p = 0.0001) (Table 5). In this predictive model, adjusted r2 = 0.33.
The hierarchical model showed that fatigue did improve at the first follow-up visit (b = −0.009, p = 0.0009), and the logistic regression model showed that low appetite, genitourinary (GU) cancer, high nausea, and low shortness of breath were predictors of this improvement of fatigue by outpatient palliative care consultation. Baseline low appetite was the strongest predictor of improvement in the reduced model (odds ratio [OR] = 1.09 per point; p = 0.0113). Additionally, patients with GU cancer were more likely to improve in fatigue in the reduced model (OR = 1.74 per point; p = 0.0458) (Table 6). The improvement of fatigue was observed in 586 patients (33%). Baseline nausea was the strongest predictor of improvement in fatigue with higher baseline nausea scores predictive of improvement (OR = 1.07 per point; p = 0.0442). In patients with severe fatigue (score 8–10), those with less shortness of breath at baseline were more likely to improve in fatigue (OR = 0.88 per point, p = 0.0068).
Changes in fatigue were positively associated with changes in the other cancer related symptoms as assessed by ESAS (Table 7).
Discussion
Our findings provide preliminary evidence that palliative care consultation for fatigue for advanced cancer patients seen at an outpatient palliative care clinic in a comprehensive cancer center was associated with improvement of fatigue at the time of the first follow-up visit.
Moderate to severe fatigue was common (84%) in the patients we evaluated. Severity of fatigue at the time of consultation significantly correlated with all ESAS items, with pain and appetite having the strongest associations. These findings are consistent with those from previous studies [15–23].
One of the strengths of this study, despite its retrospective design, is that the fatigue assessment was performed prospectively at both the initial and subsequent follow-up visits using validated tools in a dedicated outpatient palliative care clinic. The fatigue assessment was completed by the patients under the supervision of nurses trained specifically in palliative care, and standardized management was provided by a specialist-led palliative care team in accordance with our institutional protocol based on the findings from previous fatigue assessments [9]. Moreover, this study differed from earlier studies in its relatively large population sample size of over 1700 patients and its focus on factors associated with improvement in fatigue after an outpatient palliative care consultation. Thus, our findings provide preliminary reference data about the effectiveness of outpatient palliative care when standard palliative care is applied to patients with fatigue.
We found that the severity levels of pain, depression, appetite, nausea, drowsiness, well-being, and shortness of breath and albumin level were predictive of the severity of fatigue at the time of the initial consultation. This information emphasizes the importance of thorough serial assessment of all cancer-related symptoms for optimal management of fatigue. Future prospective trials are needed, and fatigue interventions should incorporate a multimodal interdisciplinary approach with targets including the treatment of fatigue-related symptoms such as pain, low appetite, and depression in addition to specific pharmacological interventions for fatigue. These results are consistent with prior studies by Hwang et al [20], who found that shortness of breath, pain, lack of appetite, drowsiness, sadness, and irritability predicted fatigue. However, female gender and low hemoglobin levels, which prior studies have found to be predictive of severity of fatigue [24–26], were not associated with fatigue in our study. This result could be due to the overwhelmingly high symptom burden in our patient population, which may reduce the role of factors such as anemia [27–29].
We also found that severe anorexia at the initial visit to the palliative care clinic was associated with improvement of fatigue. This may be due to a strong association of fatigue with anorexia and cachexia, as both of which may be caused by the same pathophysiologic process, namely inflammation [30–32].
We also found that patients with GU cancers (prostate, renal, and transitional cell cancers) were more likely to improve in fatigue (OR = 1.74 per point, p = 0.045). In patients with prostate cancer, anorexia cachexia is less important contributor to fatigue [31]. The authors speculate that fatigue as a result of androgen deprivation in prostate cancer patients may be more amenable to palliative interventions such as exercise [33, 34]. However, further studies are needed to understand this result.
The results of his study suggest that the changes in the fatigue scores were positively associated with the changes in the severity of other ESAS symptoms; these finding would strongly imply a causal relation between symptom load and fatigue.
Though the results of this study show preliminary evidence that palliative care consultation was successful in reducing the severity of fatigue, our model did not capture all the factors associated with improvement of fatigue, as indicated by adjusted r2 of 0.33. Further studies are needed.
The results show that the severity of fatigue and other ESAS symptoms in patients who did not have a follow-up visit (excluded patients) were milder than in those who had at least one follow-up visit (the study sample) (Table 3). This analysis validates that the results are a good representation of the patients seen in outpatient palliative care.
Our study has several limitations, the most important of which was the retrospective design and its lack of patients in the setting other than an outpatient palliative care clinic. Another limitation is the use of single item measure to assess physical and emotional symptoms using ESAS. However prior studies have shown that ESAS items and other single item questionnaires correlate well with multi-item symptom assessment tools [22, 35–38]. Although in the vast majority cases patient complete the ESAS by themselves in outpatient setting there is a possibility that in the most fatigued patients the nurse or caregiver could have introduced the bias by assisting the patient. More research is necessary to address this possibility. This study also did not assess other well-known factors that may contribute to fatigue such as inflammatory biomarkers, which play an important role in fatigue causation [32, 39, 40]. Some of the statistically significant associations in this study may be as a result of multiple analysis. Future prospective studies are needed to confirm these findings.
Conclusion
Our findings suggest that fatigue is the most severe symptom in patients with advanced cancer. Severity levels of pain, depression, appetite, nausea, drowsiness, well-being, and shortness of breath and the albumin level were predictive of the severity of fatigue at the time of the initial consultation, with pain and low appetite being the most significant predictors. Genitourinary cancer and low appetite at baseline were associated with successful improvement of fatigue.
References
Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J: Evidence Report on the Occurrence, Assessment, and Treatment of Fatigue in Cancer Patients. JNCI Monographs. 2004, 32: 40-50.
Yennurajalingam S, Bruera E: Palliative Management of Fatigue at the Close of Life. JAMA. 2007, 297: 295-304. 10.1001/jama.297.3.295.
Piper BF, Cella D: Cancer-Related Fatigue: Definitions and Clinical Subtypes. J Natl Compr Canc Netw. 2010, 8 (8): 958-966.
Minton O, Richardson A, Sharpe M, Hotopf M, Stone P: A Systematic Review and Meta-Analysis of the Pharmacological Treatment of Cancer-Related Fatigue. J Natl Cancer Inst. 2008, 100: 1155-1166. 10.1093/jnci/djn250.
Osta BE, Palmer JL, Paraskevopoulos T, Pei B-L, Roberts LE, Poulter VA, Chacko R, Bruera E: Interval between First Palliative Care Consult and Death in Patients Diagnosed with Advanced Cancer at a Comprehensive Cancer Center. J Palliat Med. 2008, 11: 51-57. 10.1089/jpm.2007.0103.
Dalal S, Palla S, Hui D, Nguyen L, Chacko R, Li Z, Fadul N, Scott C, Thornton V, Coldman B, Amin Y, Bruera E: Association Between a Name Change from Palliative to Supportive Care and the Timing of Patient Referrals at a Comprehensive Cancer Center. Oncologist. 2011, 16: 105-111. 10.1634/theoncologist.2010-0161.
Osoba D, Rodrigues G, Myles J, Zee B, Pater J: Interpreting the significance of changes in health-related quality-of- life scores. J Clin Oncol. 1998, 16: 139-144.
Joly F, Vardy J, Pintilie M, Tannock IF: Quality of life and/or symptom control in randomized clinical trials for patients with advanced cancer. Ann Oncol. 2007, 18: 1935-1942. 10.1093/annonc/mdm121.
Elsayem A, Buera E: The M.D. Anderson symptom control and palliative care handbook. Houston, Printing Services. 2008, USA: University of Health Science Center at Houston
National Consensus Panel. http://www.nationalconsensusproject.org.
Bruera E: KN, Miller MJ, Selmser P, Macmillan K:The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991, 7: 6-9.
Chang VT, Hwang SS, Feuerman M: Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000, 88: 2164-2171. 10.1002/(SICI)1097-0142(20000501)88:9<2164::AID-CNCR24>3.0.CO;2-5.
Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S: The memorial delirium assessment scale. J Pain Symptom Manage. 1997, 13: 128-137. 10.1016/S0885-3924(96)00316-8.
Bush B, Shaw S, Cleary P, Delbanco TL, Aronson MD: Screening for alcohol abuse using the cage questionnaire. Am J Med. 1987, 82: 231-235. 10.1016/0002-9343(87)90061-1.
Akechi T, Kugaya A, Okamura H, Yamawaki S, Uchitomi Y: Fatigue and Its Associated Factors in Ambulatory Cancer Patients: A Preliminary Study. J Pain Symptom Manage. 1999, 17: 42-48. 10.1016/S0885-3924(98)00105-5.
Zeng L, Koo K, Zhang L, Jon F, Dennis K, Holden L, Nguyen J, Tsao M, Barnes E, Danjoux C, Sahgal A, Chow E: Fatigue in advanced cancer patients attending an outpatient palliative radiotherapy clinic as screened by the Edmonton Symptom Assessment System. Support Care Cancer. 2012, 30: 1037-1042.
Okuyama T, Akechi T, Shima Y, Sugahara Y, Okamura H, Hosaka T, Furukawa TA, Uchitomi Y: Factors Correlated with Fatigue in Terminally Ill Cancer Patients: A Longitudinal Study. J Pain Symptom Manage. 2008, 35: 515-523. 10.1016/j.jpainsymman.2007.06.014.
Respini D, Jacobsen PB, Thors C, Tralongo P, Balducci L: The prevalence and correlates of fatigue in older cancer patients. Crit Rev Oncol Hematol. 2003, 47 (3): 273-279. 10.1016/s1040-8428(02)00176-2.
Broeckel JA, Jacobsen PB, Norton J, Balducci L, Lyman GH: Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J Clin Oncol. 1998, 16: 1689-1696.
Hwang SS, Chang VT, Rue M, Kasimis B: Multidimensional independent predictors of cancer-related fatigue. J Pain Symptom Manage. 2003, 26: 604-614. 10.1016/S0885-3924(03)00218-5.
Seo Y, Oh H, Seo W: Causal relationships among factors associated with cancer-related fatigue. Eur J Oncol Nurs. 2010, 14: 380-386. 10.1016/j.ejon.2009.09.008.
Yennurajalingam S, Palmer J, Zhang T, Poulter V, Bruera E: Association between fatigue and other cancer-related symptoms in patients with advanced cancer. Support Care Cancer. 2008, 16: 1125-1130. 10.1007/s00520-008-0466-5.
Stone P, Hardy J, Broadley K, Tookman AJ, Kurowska A, A'Hern R: Fatigue in advanced cancer: a prospective controlled cross-sectional study. Br J Cancer. 1999, 79: 1479-1486. 10.1038/sj.bjc.6690236.
Jacobsen PB, Garland LL, Booth-Jones M, Donovan KA, Thors CL, Winters E, Grendys E: Relationship of hemoglobin levels to fatigue and cognitive functioning among cancer patients receiving chemotherapy. J Pain Symptom Manage. 2004, 28: 7-18. 10.1016/j.jpainsymman.2003.11.002.
Lind M, Vernon C, Cruickshank D, Wilkinson P, Littlewood T, Stuart N, Jenkinson C, Grey-Amante P, Doll H, Wild D: The level of haemoglobin in anaemic cancer patients correlates positively with quality of life. Br J Cancer. 2002, 86: 1243-1249. 10.1038/sj.bjc.6600247.
Cella D, Lai J-s, Chang C-H, Peterman A, Slavin M: Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002, 94: 528-538. 10.1002/cncr.10245.
Munch TN, Zhang T, Willey J, Palmer JL, Bruera E: The Association between Anemia and Fatigue in Patients with Advanced Cancer Receiving Palliative Care. J Palliat Med. 2005, 8: 1144-1149. 10.1089/jpm.2005.8.1144.
Stone P, Richards M, A'Hern R, Hardy J: A study to investigate the prevalence, severity and correlates of fatigue among patients with cancer in comparison with a control group of volunteers without cancer. Ann Oncol. 2000, 11: 561-567. 10.1023/A:1008331230608.
Conill C, Verger E, Henríquez I, Saiz N, Espier M, Lugo F, Garrigos A: Symptom prevalence in the last week of life. J Pain Symptom Manage. 1997, 14: 328-331. 10.1016/S0885-3924(97)00263-7.
Gupta SC, Kim JH, Kannappan R, Reuter S, Dougherty PM, Aggarwal BB: Role of nuclear factor-κB-mediated inflammatory pathways in cancer-related symptoms and their regulation by nutritional agents. Exp Biol Med. 2011, 236: 658-671. 10.1258/ebm.2011.011028.
Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, Steiner MS: Muscle Wasting in Cancer Cachexia: Clinical Implications, Diagnosis, and Emerging Treatment Strategies. Annu Rev Med. 2011, 62: 265-279. 10.1146/annurev-med-061509-131248.
Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR: Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008, 26: 971-982. 10.1200/JCO.2007.10.7805.
Bourke L, Doll H, Crank H, Daley A, Rosario D, Saxton JM: Lifestyle Intervention in Men with Advanced Prostate Cancer Receiving Androgen Suppression Therapy: A Feasibility Study. Cancer Epidemiol Biomarkers Prev. 2011, 20: 647-657. 10.1158/1055-9965.EPI-10-1143.
Segal RJ, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud'Homme DG, Malone SC, Wells GA, Scott CG, Slovinec D'Angelo ME: Randomized Controlled Trial of Resistance or Aerobic Exercise in Men Receiving Radiation Therapy for Prostate Cancer. J Clin Oncol. 2009, 27: 344-350.
Vignaroli EPE, Willey J, Palmer JL, Zhang T, Bruera E: The Edmonton Symptom Assessment System as a screening tool for depression and anxiety. J Palliat Med. 2006, 9 (2): 296-303. 10.1089/jpm.2006.9.296.
Butt Z, Wagner LI, Beaumont JL, Paice JA, Peterman AH, Shevrin D, Von Roenn JH, Carro G, Straus JL, Muir JC, Cella D: Use of a Single-Item Screening Tool to Detect Clinically Significant Fatigue, Pain, Distress, and Anorexia in Ambulatory Cancer Practice. J Pain Symptom Manage. 2008, 35: 20-30. 10.1016/j.jpainsymman.2007.02.040.
Bernhard J, Sullivan M, Hürny C, Coates AS, Rudenstam CM: Clinical relevance of single item quality of life indicators in cancer clinical trials. Br J Cancer. 2001, 84: 1156-1165. 10.1054/bjoc.2001.1785.
Locke DEC, Decker PA, Sloan JA, Brown PD, Malec JF, Clark MM, Rummans TA, Ballman KV, Schaefer PL, Buckner JC: Validation of Single-Item Linear Analog Scale Assessment of Quality of Life in Neuro-Oncology Patients. J Pain Symptom Manage. 2007, 34: 628-638. 10.1016/j.jpainsymman.2007.01.016.
Orre IJ, Reinertsen KV, Aukrust P, Dahl AA, Fosså SD, Ueland T: Murison R Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J Psychosom Res. 2011, 71: 136-141. 10.1016/j.jpsychores.2011.04.003.
Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW: Inflammation and Behavioral Symptoms After Breast Cancer Treatment: Do Fatigue, Depression, and Sleep Disturbance Share a Common Underlying Mechanism?. J Clin Oncol. 2011, 29: 3517-3522. 10.1200/JCO.2011.36.1154.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-684X/11/16/prepub
Acknowledgments
Preparation of this manuscript is supported in part by the. MD Anderson Cancer Center support grant CA 016672; American Cancer Society (RSG-11-170-01-PCSM)[S.Y] and National Institutes of Health grants R01NR010162-01A1, R01CA1222292.01, and R01CA124481-01E.B].
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors’ contributions
SY and EB were involved in the study concept, design, assembly, analysis, interpretation of the data and drafting the manuscript. DLU was involved in the design, assembly, analysis, interpretation of the data and drafting of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yennu, S., Urbauer, D.L. & Bruera, E. Factors associated with the severity and improvement of fatigue in patients with advanced cancer presenting to an outpatient palliative care clinic. BMC Palliat Care 11, 16 (2012). https://doi.org/10.1186/1472-684X-11-16
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-684X-11-16