Abstract
Background
Obesity is a multifactorial disorder influenced by genetic and environmental factors. Animal models of obesity are required to help us understand the signaling pathways underlying this condition. Zebrafish possess many structural and functional similarities with humans and have been used to model various human diseases, including a genetic model of obesity. The purpose of this study was to establish a zebrafish model of diet-induced obesity (DIO).
Results
Zebrafish were assigned into two dietary groups. One group of zebrafish was overfed with Artemia (60 mg dry weight/day/fish), a living prey consisting of a relatively high amount of fat. The other group of zebrafish was fed with Artemia sufficient to meet their energy requirements (5 mg dry weight/day/fish). Zebrafish were fed under these dietary protocols for 8 weeks. The zebrafish overfed with Artemia exhibited increased body mass index, which was calculated by dividing the body weight by the square of the body length, hypertriglyceridemia and hepatosteatosis, unlike the control zebrafish. Calorie restriction for 2 weeks was applied to zebrafish after the 8-week overfeeding period. The increased body weight and plasma triglyceride level were improved by calorie restriction. We also performed comparative transcriptome analysis of visceral adipose tissue from DIO zebrafish, DIO rats, DIO mice and obese humans. This analysis revealed that obese zebrafish and mammals share common pathophysiological pathways related to the coagulation cascade and lipid metabolism. Furthermore, several regulators were identified in zebrafish and mammals, including APOH, IL-6 and IL-1β in the coagulation cascade, and SREBF1, PPARα/γ, NR1H3 and LEP in lipid metabolism.
Conclusion
We established a zebrafish model of DIO that shared common pathophysiological pathways with mammalian obesity. The DIO zebrafish can be used to identify putative pharmacological targets and to test novel drugs for the treatment of human obesity.
Similar content being viewed by others
Background
According to the World Health Organization, an estimated 310 million people worldwide are obese [1]. Such estimates are particularly alarming given the strong association between obesity and various adverse health consequences, including atherosclerosis, hypertension, type 2 diabetes and certain types of cancer [1–3]. While the exact causes remain elusive, it is now accepted that obesity is a complex, multifactorial disease that develops from an interaction between the genotype and the environment [1, 2, 4].
A number of genes involved in monogenic, syndromic and polygenic obesity have been identified [2, 5]. In addition to genetic predisposition, environmental and behavioral factors resulting in increased physical inactivity and calorie intake also contribute to the development of obesity [6]. Numerous studies in rodents have attempted to characterize the functions of obesity-related genes and whole-animal responses to high-calorie and high-fat diets [7]. Such studies have generally shown that the obesity phenotype can differ based on the functions of knockout genes, genetic background, and dietary protocols [6–10]. This suggests the importance of developing and analyzing genetic and diet-induced models of obesity.
Although rodent models have greatly contributed to our understanding of human obesity [11], experiments using rodent models require considerable staff and infrastructural support, and are relatively expensive. Therefore, the development of simple and inexpensive animal models of obesity to complement the currently used rodent models has been anticipated. Recent studies on energy homeostasis in worms, fly and zebrafish have shown that these lower organisms can be used to unravel the metabolic processes underlying obesity [12, 13].
As vertebrates, zebrafish possess many structural similarities with humans that worms and flies do not [13] and have been used to model various human diseases [14–19]. For example, zebrafish digestive organs, adipose tissues (AT), and skeletal muscle are physically arranged in a manner similar to their human counterparts [13]. Neural and endocrine signals regulating food intake are also conserved in zebrafish, including agouti-related protein (AgRP) [15, 20], leptin [21] and adiponectin [22]. Although zebrafish larvae have been used in genetic and chemical screening experiments to identify novel genes involved in the regulation of energy homeostasis and potential therapeutic targets to treat obesity [13, 23], it remains unclear whether zebrafish can be used as a model for diet-induced obesity (DIO), similar to that observed in mammals.
We have developed a zebrafish model for DIO and validated the model by several methods, including biochemical and histological analyses, diet therapies and DNA microarray analysis of visceral AT with comparison to those of mammalian obesity. These studies revealed that DIO zebrafish and obese mammals share common pathophysiological pathways, suggesting that zebrafish can be used as an alternative model of DIO.
Results
Zebrafish overfed with Artemia become obese
To develop a zebrafish model of DIO, we overfed the fish with freshly hatched nauplii of brine shrimp Artemia, a common food source for aquaculture [24]. Given the hierarchy of macronutrient effects on the perception of hunger (i.e. fat is least satiating while protein is most satiating [4]), it is plausible that zebrafish would overfeed on Artemia and become obese. Indeed, increases in the amount of dietary fat are associated with the risk of developing obesity [1, 25].
We first assessed the consumption of Artemia by zebrafish. It was previously reported that the energy requirement for a Leopard Danio, a spotted color morph of zebrafish, is under 30 cal [26]. Therefore, we fed zebrafish at 3.5 months post-fertilization (mpf) with 5 or 60 mg of freshly hatched Artemia to meet their energy requirements (control) or to overfeed (OF), respectively. Zebrafish fed 5 or 60 mg of Artemia per day consumed about 80 or 50% of the provided Artemia, respectively (Methods). Because 1 mg of Artemia contains approximately 5 calories, it was estimated that a zebrafish would obtain 20 calories from 5 mg of Artemia at 80% consumption, and 150 calories from 60 mg of Artemia at 50% consumption. Zebrafish were fed under these dietary protocols for 8 weeks.
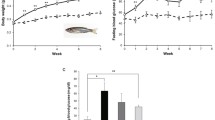
As shown in Figures 1A and 1B, significant (p < 0.05) increases in body mass index (BMI), which was calculated by dividing the body weight (g) by the square of the body length (cm), were evident in male (1.1-fold) and female (1.3-fold) zebrafish in the OF group compared with those in the control group within 1 week of the dietary protocol. This trend was maintained throughout the 8-week dietary protocol. As shown in Figures 1C and 1D, plasma TG levels were also significantly (p < 0.05) higher in male (2.4-fold) and female (1.8-fold) zebrafish in the OF group than in the control group at week 8, but not at week 1. Lipid-specific Oil Red O staining of frozen liver sections revealed abnormalities consistent with hepatosteatosis in both male (Figure 1G) and female (Figure 1H) zebrafish in the OF group at week 8, but not in the control group. These results indicate that zebrafish overfed with Artemia develop obesity in a manner similar to those observed in transgenic zebrafish overexpressing AgRP [15] and in mammalian models of DIO [8].
Assessment of BMI, plasma TG and hepatic steatosis in zebrafish overfed with Artemia. Changes in BMI (g/cm2) in male (A) and female (B) zebrafish in the control and OF groups. Values are means ± SEM. OF group: n = 17 males and 16 females. Control group: n = 14 males and 17 females. Changes in plasma TG levels in male (C) and female (D) zebrafish in the control and OF groups. Values are means ± SEM. OF group: n = 7 males and 8 females. Control group: n = 7 males and 9 females. Statistical analyses were performed using Student's t-test to compare the OF and control groups at each time-point. *P < 0.05, **P < 0.01. (E-H) Oil Red O staining of liver sections from a male (E) and female (F) in the control group and a male (G) and female (H) in the OF group.
Calorie restriction improved body weight and hypertriglyceridemia
Calorie restriction (CR) is the most frequently prescribed treatment for obesity, although the health benefits of CR based on hard endpoints such as cardiovascular morbidity and mortality remain to be elucidated [1]. To validate the effect of CR, we fed the previously OF zebrafish with 2.5 mg of freshly hatched Artemia per day (50% of the control level) for 2 weeks (CR2W) after the 8-week overfeeding period (OF8W). The zebrafish consumed almost all of the provided Artemia (Methods) and the body weight of male and female zebrafish decreased to 84% and 82%, respectively, at OF8W (Figures 2A and 2B). Plasma TG levels were significantly (p < 0.05) decreased to 69% 52% at OF8W in male and female, respectively (Figures 2C and 2D). These data indicate that CR is effective for the treatment of DIO in zebrafish, similar to mammalian DIO [6–8].
Effects of calorie restriction on body weight and plasma TG level. Body weight is significantly decreased by CR for 2 weeks in both male (A) and female (B) zebrafish. Values are means ± SEM. n = 7 for each group. The plasma TG level is significantly decreased by 2 weeks of CR in male (C) and female (D) zebrafish. Values are means ± SEM. n = 7 for each group. Statistical analyses were performed using Student's t-test to compare the OF8W and the OF8W+CR2W groups. *P < 0.05, **P < 0.01.
Gene expression profiling of visceral AT revealed common pathways in obesity
It has been reported that visceral AT plays an active role in the development of obesity-related complications such as cardiovascular diseases, insulin resistance and cancer [27]. Therefore, we performed DNA microarray analysis using Agilent Zebrafish DNA Oligoarrays of visceral AT from zebrafish overfed Artemia for 1 (OF1W) or 8 (OF8W) weeks, and treated the zebrafish with CR for 2 weeks (CR2W) after 8 weeks of overfeeding. The amount of visceral AT in the control group was so small that we could not extract total RNA. Therefore, we compared the genome-wide expression profiles of zebrafish at OF1W and OF8W to identify genes dysregulated by overfeeding using Rank Product Analysis (RankProd) [28]. RankProd analysis identified 120 and 48 genes as significantly (false discovery rate [FDR] <15%) increased and decreased, respectively, at OF8W [Additional file 1: Supplementary Table S1]. To identify the genes ameliorated by CR, we compared the genome-wide expression profiles of OF8W and CR2W. Among the 168 genes dysregulated by overfeeding, 97 genes (58%) were significantly (FDR <15%) ameliorated by CR [Additional file 2: Supplementary Table S2].
To compare the gene expression profiles of visceral AT of zebrafish and mammalian DIO, we downloaded multiple sets of microarray data from the Gene Expression Omnibus [29] (Table 1). GSE8700 was designed to analyze the differences in epididymal AT gene expression in rats fed a high-fat diet with those fed a control diet using Affymetrix Rat Genome Arrays [30]. GSE11790 was designed to analyze the differences in omental AT gene expression in mice fed a high-fat diet with those fed a control diet using custom arrays [31]. GSE15524 was designed to analyze the differences in omental AT gene expression in obese versus lean human individuals using CodeLink Uniset Human Bioarrays [32].
We analyzed the microarray data using RankProd and identified genes that were differentially expressed in mammalian obesity. RankProd identified 461, 364 and 358 genes as being significantly (FDR <15%) dysregulated in rat, mouse and human obesity, respectively [Additional files 3, 4 and 5: Supplemental Tables S3,5]. We compared these gene lists with the 168 genes that were significantly dysregulated in zebrafish DIO [Additional file 6: Supplemental Table S6]. One gene (fatty acid binding protein 1, FABP1) was dysregulated in all three models of mammalian obesity, but was not spotted in the zebrafish microarray. Quantitative PCR (qPCR) analysis of zebrafish fabp1a revealed a positive trend in OF8W [Additional file 7: Supplemental Figure S1]. Fourteen genes were dysregulated in two models of mammalian obesity, while 22 genes were dysregulated in zebrafish DIO and in one model of mammalian obesity [Additional file 6: Supplemental Table S6]. qPCR analysis confirmed the induction of hpx in OF8W compared to OF1W [Additional file 7: Supplemental Figure S1].
Because the microarray platform differed among these four datasets, it is plausible that the composition of the gene list differs among these four species. Therefore, we analyzed the microarray data using Gene Set Enrichment Analysis (GSEA) [33] to identify gene sets defined based on prior biological knowledge, such as the Gene Ontology category. GSEA can determine whether members of a gene set belonging to a same biological pathway tend to occur toward the top of a given gene list, such as the gene list established from microarray analysis. The GSEA revealed that genes related to blood coagulation were significantly dysregulated in zebrafish, rat and mouse DIO and in human obesity. Genes related to platelet activation, fatty acid metabolism, cholesterol efflux, and triglyceride metabolism were significantly dysregulated in zebrafish, rat and mouse DIO (Table 2).
We also performed Sub-Network Enriched Analysis (SNEA) [34] to identify key molecules regulating the expression of the genes in the coagulation cascade or lipid metabolism identified by GSEA. SNEA revealed apolipoprotein H (APOH), interleukin-6 (IL-6) and interleukin-1β (IL-1β) as regulatory molecules in the coagulation cascade in obese zebrafish, rats, mice and humans (Table 3). SNEA also revealed sterol regulatory element binding transcription factor 1 (SREBF1), peroxisome proliferator-activated receptor alpha (PPARA), nuclear receptor subfamily 1 group H member 3 (NR1H3), PPAR gamma (PPARG) and leptin (LEP) as regulatory molecules involved in lipid metabolism in obese zebrafish, rats, mice and humans (Table 3).
The interactions between these regulatory molecules and their target genes are shown in Figure 3 for the coagulation cascade and Figure 4 for lipid metabolism. The color of many genes, including fibrinogen-γ (FGG) and factor II (F2, also known as thrombin) in Figure 3 and apolipoprotein A-I (APOA1) in Figure 4, are red, suggesting that genes related to coagulation cascade and lipid metabolism are induced by obesity. By contrast, the color of these genes was turned green by CR, suggesting that CR ameliorates dysfunctions in the coagulation cascade and lipid metabolism (Figure 5). These results suggest that DIO zebrafish share common pathophysiological pathways with mammalian obesity.
Network for the coagulation cascade. The network shows direct interactions between the three key regulatory molecules (IL-6, IL-1β and APOH, shown in yellow) identified by SNEA (Table 3) and their target genes in the coagulation cascade (blood coagulation and platelet activation) identified by GSEA (Table 2). Red and green denote genes with increased and decreased expression, respectively, in obese (OF8W) compared with control (OF1W) zebrafish. Gray denotes genes that were not spotted in the microarray. To reduce the complexity, the rat, mouse and human networks were constructed using the three regulatory factors and their target genes that were spotted in Agilent Zebrafish Oligoarray. A. zebrafish DIO (GSE18566), B. rat DIO (GSE8700), C. mouse DIO (GSE11790), D. human obesity (GSE15524).
Network for lipid metabolic pathways. The network shows direct interactions between the five key regulatory molecules (SREBP1, PPARα/γ, NR3H1 and LEP, shown in yellow) identified by SNEA (Table 3) and their target genes involved in the lipid metabolic pathways (fatty acid metabolism, cholesterol efflux and triglyceride metabolism) identified by GSEA (Table 2). Red and green denote genes with increased and decreased expression, respectively, in obese (OF8W) compared with control (OF1W) zebrafish. Gray denotes genes that were not spotted in the microarray. To reduce the complexity, the rat, mouse and human networks were constructed using the five regulatory factors and their target genes that were spotted in the Agilent Zebrafish Oligoarray. A. zebrafish DIO (GSE18566), B. rat DIO (GSE8700), C. mouse DIO (GSE11790), D. human obesity (GSE15524).
Effects of CR on the coagulation cascade and lipid metabolism in DIO zebrafish. A. Network showing direct interactions between the three key regulatory molecules (IL-6, IL-1β and APOH, shown in yellow) identified by SNEA (Table 3) and their target genes in the coagulation cascade (blood coagulation and platelet activation) identified by GSEA (Table 2). B. Network showing direct interactions between the five key regulatory molecules (SREBP1, PPARα/γ, NR3H1 and LEP, shown in yellow) identified by SNEA (Table 3) and their target genes involved in the lipid metabolic pathways (fatty acid metabolism, cholesterol efflux and triglyceride metabolism) identified by GSEA (Table 2). Red and green denote genes with increased and decreased expression, respectively, at CR2W compared with OF1W. Gray denotes genes that were not spotted in the microarray.
Discussion
In this study, we developed a zebrafish model of DIO. DIO zebrafish exhibited increased BMI, hypertriglyceridemia and hepatosteatosis. Comparative transcriptome analysis using visceral AT revealed that the DIO zebrafish and obese mammals share common pathophysiological pathways. These findings suggest that the DIO zebrafish model can be used to identify putative pharmacological targets and to test novel drugs for the treatment of human obesity.
Zebrafish can be used as a useful animal model of DIO
The regulation of feeding behavior occurs via close interactions between peripheral regions and the brain [1–4, 35–38]. Peripheral endocrine and metabolic factors convey information regarding nutritional status to the brain. The peripheral signals include satiety signals, such as PYY [39], GLP-1 [40] and ghrelin [41], which originate from the gastrointestinal tract, as well as adiposity signals, such as adiponectin [22] and insulin [42], the blood levels of which are proportional to body nutrient stores [38]. The brain then processes this peripheral information and induces neuropeptide signaling (for example, via NPY [43], α-MSH [20], AgRP [20] and orexin [44]), mainly from the hypothalamus, to stimulate or inhibit feeding. These peripheral and central factors controlling food intake are well conserved in zebrafish [20, 22, 39–45].
Probably because of the low satiating effect of fat consumption [4], high dietary fat intake is associated with an increased risk of obesity [1, 25]. Because of the relatively high fat content in Artemia, as compared with flake foods, we fed the zebrafish a diet consisting solely of Artemia to induce obesity. We have demonstrated that zebrafish overfed with Artemia showed significant increases in BMI and plasma TG levels and hepatosteatosis, consistent with obesity observed in humans and rodent models of DIO [1, 6, 7]. Of note, there seemed to be no decrease in physical activity in the zebrafish overfed Artemia (data not shown), indicating that the development of obesity is most likely to be a result of the increased intake of a high-fat diet.
There are several advantages to the DIO zebrafish model. First, zebrafish (AB line) respond well to the Artemia diet because almost all of the zebrafish overfed Artemia developed obesity. The C57BL/6J (B6) line has been widely used for DIO in mice because these mice are very susceptible to obesity when fed a high-fat diet [6–9]. However, there are variations in adiposity among individual B6 mice [46, 47]. Similarly, when outbred Sprague-Dawley rats are fed a high-fat diet, about half become obese, while the other half are resistant to DIO [6–10]. The relatively homogenous responses of zebrafish to overfeeding with Artemia suggest that these fish represent an excellent alternative model species for experimental research on DIO. Furthermore, the dietary protocol to induce obesity in zebrafish is simple and can be applied to other zebrafish lines. For example, we are currently applying the protocol to the Casper line, which have transparent abdomens, even in the adult stage [48]. Using DIO Casper zebrafish, visceral AT can be visualized in a living animal under a fluorescent microscope by staining the AT with a fluorescent dye, such as Nile Red [49]. This feature makes it possible to monitor the short- and long-term effects of a therapeutic intervention on the amount of visceral AT in live DIO zebrafish. Finally, zebrafish are small and easy to maintain in large stocks because of their high fecundity, thus making zebrafish amenable for medium-to-high throughput screening for early drug discovery [50].
One limitation of the zebrafish DIO model is the apparent absence of brown AT [51]. The development of obesity in mammals not only depends on the balance between food intake and calorie utilization, but also on the balance between white AT and brown AT [51]. Therefore, zebrafish DIO may not be suitable to identify signaling pathways related to brown AT.
Comparative transcriptomics of visceral AT revealed common pathophysiological pathways in zebrafish and mammalian obesity
Genome-wide expression assays using DNA microarrays allow rapid screening and quantification of differences in large groups of functionally related genes and are thus well-suited to studies of pathways dysregulated in obesity [52]. We compared the visceral AT expression profiles of zebrafish, rat, mouse and human obesity. The comparative transcriptome analysis revealed that several genes involved in blood coagulation, platelet activation, fatty acid metabolism, cholesterol efflux, and triglyceride metabolism were dysregulated in both zebrafish and mammalian obesity. IL-6, IL-1β and APOH were identified as regulatory factors involved in blood coagulation and platelet activation in zebrafish and mammalian obesity. Similarly, SREBP1, PPARα/γ, NR3H1 and LEP were identified as common regulatory factors for fatty acid metabolism, cholesterol efflux and triglyceride metabolism in zebrafish and mammalian obesity.
Visceral AT dysfunction can play a causal role in the prothrombotic state observed in obesity by affecting hemostasis, coagulation and fibrinolysis [53–55]. It has also been shown that IL-6 and IL-1β, secreted from visceral AT, induce the biosynthesis of fibrinogen from visceral AT and liver. Fibrinogen is a substrate of F2 (thrombin) in the final step of the coagulation cascade, and its presence is essential for platelet aggregation [53]. Of interest, plasma fibrinogen levels were found to be significantly higher in obese subjects than in age- and sex-matched non-obese individuals [53]. Significant correlations have been reported between fibrinogen and BMI and the waist-to-hip ratio [53]. It has also been reported that substantial weight loss reduces fibrinogen levels more effectively than modest weight reduction [53]. Consistent with these reports, OF and CR significantly induced and reduced, respectively, the expression of fibrinogen in zebrafish (Figures 3A and 5A, Additional files 1 and 2: Supplemental Table S1 and S2). APOH binds to activated protein C (APC), an anticoagulant enzyme that is activated by activation of the protein C zymogen by the thrombin-thrombomodulin complex on the surface of endothelial cells, platelets and monocytes [56]. However, the effect of APOH binding to APC is inconclusive and the functional role of APOH in obesity remains to be elucidated.
Dyslipidemia is commonly seen in obesity [57, 58] and is characterized by an increased flux of free fatty acids, elevated TG levels, low high-density lipoprotein cholesterol levels and increased low-density lipoprotein levels [57]. Fatty acid metabolism, cholesterol efflux and triglyceride metabolism are closely related to these pathways. This study revealed that SREBP1, PPARα/γ, NR3H1 and LEP are key regulatory factors in these pathways and are expressed in zebrafish and mammalian obesity. PPARs mediate adaptive metabolic responses to increased systemic lipid availability and are activated by endogenous or dietary lipids [59]. PPARα promotes lipid clearance by increasing tissue fat oxidization [59, 60] while PPARγ promotes lipid storage in white AT, as well as preadipocyte differentiation to mature adipocytes [59]. SREBP1 is an important transcription factor that regulates the transcription of many lipid genes and participates in adipocyte differentiation by stimulating PPARγ [61, 62]. NR3H1, also known as liver X receptor A (LXRA), has been shown to regulate lipid and carbohydrate homeostasis [63]. LEP, an adiposity hormone produced by white AT, reflects total fat mass [64]. Although we did not detect a statistically significant difference between OF8W and OF1W in terms of the mRNA expression level of lep measured by qPCR (data not shown), the expression of apoa1, a transcriptional target gene of leptin [65], was significantly induced and reduced by OF and CR, respectively (Figures 4A and 5B) [Additional files 1, 2 and 7: Supplemental Tables S1 and S2, and Figure S1], suggesting that leptin protein levels were likely to be increased in zebrafish DIO. It is noteworthy that the functional importance of SREBP1, PPARα/γ, NR3H1 and LEP in obesity has been shown in many genetic studies [2, 11, 63, 66].
Conclusions
Here, we have shown that the DIO zebrafish model shares common pathophysiological pathways with mammalian obesity and can be used to identify putative pharmacological targets of human obesity. For example, we are currently performing genome-wide expression profiling of the DIO zebrafish liver. The zebrafish model can also be used to study the hypothalamic-pituitary axis, the main link between the central nervous and the endocrine system [67]. Further studies are needed to examine whether complications related to obesity such as insulin resistance, cardiovascular diseases and cancer can be studied in DIO zebrafish.
Methods
Feeding zebrafish
Adult zebrafish (AB line, ZIRC, Eugene, OR, USA) were kept at 28°C under a 14-h light:10-h dark cycle, and water conditions of environmental quality were maintained according to an established protocol [68]. Zebrafish at 3.5 mpf were assigned into two dietary groups (overfeeding and maintenance groups) with approximately five fish per 2-L tank. Zebrafish in the overfeeding group were fed three times per day with freshly hatched Artemia (corresponding to 60 mg cysts/fish/day). Zebrafish in the maintenance group were fed freshly hatched Artemia (corresponding to 5 mg cysts/fish/day) once per day. For calorie restriction, the zebrafish were fed freshly hatched Artemia (corresponding to 2.5 mg cysts/fish/day) for 2 weeks after being overfed for 8 weeks. Commercial flake food (Hikari Tropical Fancy Guppy, Kyorin, Hyogo, Japan) consists of 9% fat, 17% carbohydrate and 59% protein, whereas Artemia nauplii consist of 22% fat, 16% carbohydrate and 44% protein (dry weight basis).
The percentage of consumed Artemia was estimated by counting Artemia before and after feeding. Briefly, 1 mL of water was collected from 1700 mL of water containing freshly hatched Artemia corresponding to 100 or 8.5 mg cysts. The numbers of hatched Artemia were counted three times to determine a mean count. After counting, the samples were returned to the 2-L tank. Then, five fish were transferred to the tank for feeding. After 2 hours, the number of Artemia was counted as before feeding. Zebrafish fed 5 or 60 mg of Artemia per day consumed about 80 or 50% of the provided Artemia, respectively. Zebrafish fed 2.5 mg of Artemia per day consumed almost all of the provided Artemia.
Measurement of BMI and plasma TG
The body weight and length of zebrafish were measured weekly throughout the study. Zebrafish length was measured from the head to the end of the body. Blood was withdrawn from the dorsal artery of the zebrafish at the indicated times after an overnight fast to measure plasma TG (TG L-type assay, Wako, Tokyo, Japan).
Oil Red O staining
Zebrafish were fixed using 4% formaldehyde solution in PBS. The fixed samples were rapidly frozen in liquid nitrogen-cooled isopentane, embedded in Tissue-Tek (Sakura Finetek Europe, Zoeterwoude, Netherlands) and cut using a cryostat. After drying, the sections were fixed in 4% formalin and rinsed with distilled water. The sections were then immersed in a working solution of Oil Red O (Wako) for 15 min and rinsed with distilled water. Sections were also counterstained using Mayer's hematoxylin to visualize the nuclei.
Nile Red staining
A stock solution of Nile Red (Wako) was prepared by dissolving 10 mg of Nile Red in 10 mL acetone. Zebrafish were transferred into 1000 mL of swimming water containing 100 μg of Nile Red and incubated overnight in the dark at 28°C. After incubation, the fish were rinsed in fresh water and observed with a fluorescence microscope (MZ 16F, Leica, Tokyo, Japan) using a GFP2 filter (Leica).
DNA microarray analysis of visceral AT of DIO zebrafish
The visceral (omental) AT of male DIO zebrafish stained with Nile Red was collected by surgical extraction under a fluorescence microscope. The AT was stored in RNA-later (Applied Biosystems, Foster City, CA, USA). Total RNA was then extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA), qualified by an Agilent Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA) and quantified using a spectrophotometer (NanoDrop ND-100, Wilmington, DE, USA). Three hundred nanograms of total RNA from each visceral AT depot were converted into labeled cRNA using the Low RNA Input Fluorescent Linear Amplification Kit (Agilent). Cy3-labeled cRNA (1.5 μg) was hybridized to Agilent Zebrafish Whole Genome Oligo Microarrays (G2518A) according to the manufacturer's protocol. The microarray has 22,000 different probes. Each probe is spotted twice distantly in a microarray. The hybridized microarrays were scanned (Agilent G2565BA) and analyzed using Feature Extraction software (Agilent). The data were normalized according to the manufacturer's protocol (Agilent). RankProd analysis [28] was performed using Bioconductor [69] to identify differentially expressed genes between two groups by calculating the FDR. Differentially expressed genes (FDR <15% for both duplicated probes) were then converted to human orthologs using the Life Science Knowledge Bank (World Fusion, Tokyo, Japan). The gene symbols of human orthologs were used for functional analysis.
DNA microarray analysis of visceral AT in mammalian obesity
The microarray data ([GEO:GDS8700] [30], [GEO:GSE11790] [31] and [GEO;GSE15524] [32]) were downloaded from Gene Expression Omnibus [29]. We used microarray data that were filtered and normalized and applied the data to RankProd analysis [28] to identify differentially expressed genes between obese and control mammals. The conversion to human orthologs and functional analysis were performed as described above for visceral AT from DIO zebrafish. The microarray data of zebrafish DIO have been deposited in NCBI GEO and can be downloaded from GEO main page (http://www.ncbi.nlm.nih.gov/geo/) with accession number GSE18566. GSEA and SNEA were performed using Pathway Studio 7 (Ariadne Genomics, Rockville, MD, USA). Pathway Studio is a program used to visualize and analyze biological pathways and gene regulation networks. This program includes the ResNet database of more than 500,000 functional relationships and the MedScan tool for automatic extraction of information from the scientific literature. Pathway Studio 7 draws networks based on the extracted information and was used to draw Figures 3, 4 and 5.
GSEA was used to determine the list of biological pathways dysregulated in obesity using the Kolmogorov-Smirnov enrichment algorithm with 400 random permutations to determine statistical significance. SNEA was used to identify molecules regulating expression of genes in the coagulation cascade (blood coagulation and platelet activation) and in lipid metabolism (fatty acid metabolism, cholesterol efflux and triglyceride metabolism), both of which were identified by GSEA to be dysregulated in zebrafish and mammalian obesity. SNEA built sub-networks of genes related to the coagulation cascade or the lipid metabolism and identified the central "seed" of each network prioritized by the p-value based on the criterion "Expression Targets" in the ResNet database.
qPCR analysis
Total RNAs from zebrafish visceral AT were used to generate cDNAs using an iScript Select cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). qPCR was done using an ABI Prism 7300 (Life Technologies, Carlsbad, CA, USA) with SYBR Green Realtime PCR Master Mix Plus (Toyobo, Osaka, Japan). The thermal cycling condition comprised an initial step at 95°C for 1 min followed by 40 cycles of 95°C for 15 sec, 60°C for 15 sec and 72°C for 45 sec. The primers used in this study are shown in [Additional file 8: Supplementary Table S7]. Data were normalized by the quantity of glyceraldehyde-3-phosphate dehydrogenase (gapdh). This allowed us to account for any variability in the initial template concentration as well as the conversion efficiency of the reverse transcription reaction.
Statistical Analysis
Results are expressed as means ± SEM. Differences between groups were tested for statistical significance using Student's t-test.
References
Bessesen DH: Update on obesity. J Clin Endocrinol Metab. 2008, 93 (6): 2027-2034. 10.1210/jc.2008-0520.
Bell CG, Walley AJ, Froguel P: The genetics of human obesity. Nat Rev Genet. 2005, 6 (3): 221-234. 10.1038/nrg1556.
Adan RA, Vanderschuren LJ, ElF S: Anti-obesity drugs and neural circuits of feeding. Trends Pharmacol Sci. 2008, 29 (4): 208-217. 10.1016/j.tips.2008.01.008.
Speakman JR: Obesity: the integrated roles of environment and genetics. J Nutr. 2004, 134 (8 Suppl): 2090S-2105S.
Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Perusse L, Bouchard C: The human obesity gene map: the 2005 update. Obesity (Silver Spring). 2006, 14 (4): 529-644. 10.1038/oby.2006.71.
Casper RC, Sullivan EL, Tecott L: Relevance of animal models to human eating disorders and obesity. Psychopharmacology (Berl). 2008, 199 (3): 313-329. 10.1007/s00213-008-1102-2.
Speakman J, Hambly C, Mitchell S, Krol E: Animal models of obesity. Obes Rev. 2007, 8 (Suppl 1): 55-61. 10.1111/j.1467-789X.2007.00319.x.
West DB, York B: Dietary fat, genetic predisposition, and obesity: lessons from animal models. Am J Clin Nutr. 1998, 67 (3 Suppl): 505S-512S.
Buettner R, Scholmerich J, Bollheimer LC: High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring). 2007, 15 (4): 798-808. 10.1038/oby.2007.608.
Butler AA, Cone RD: The melanocortin receptors: lessons from knockout models. Neuropeptides. 2002, 36 (2-3): 77-84. 10.1054/npep.2002.0890.
Powell DR: Obesity drugs and their targets: correlation of mouse knockout phenotypes with drug effects in vivo. Obes Rev. 2006, 7 (1): 89-108. 10.1111/j.1467-789X.2006.00220.x.
Chiang SH, MacDougald OA: Will fatty worms help cure human obesity?. Trends Genet. 2003, 19 (10): 523-525. 10.1016/j.tig.2003.08.002.
Schlegel A, Stainier DY: Lessons from "lower" organisms: what worms, flies, and zebrafish can teach us about human energy metabolism. PLoS Genet. 2007, 3 (11): e199-10.1371/journal.pgen.0030199.
Lieschke GJ, Currie PD: Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007, 8 (5): 353-367. 10.1038/nrg2091.
Song Y, Cone RD: Creation of a genetic model of obesity in a teleost. FASEB J. 2007, 21 (9): 2042-2049. 10.1096/fj.06-7503com.
Tanaka T, Oka T, Shimada Y, Umemoto N, Kuroyanagi J, Sakamoto C, Zang L, Wang Z, Nishimura Y: Pharmacogenomics of cardiovascular pharmacology: pharmacogenomic network of cardiovascular disease models. J Pharmacol Sci. 2008, 107 (1): 8-14. 10.1254/jphs.08R03FM.
Kinkel MD, Prince VE: On the diabetic menu: Zebrafish as a model for pancreas development and function. Bioessays. 2009, 31 (2): 139-152. 10.1002/bies.200800123.
Wang Z, Nishimura Y, Shimada Y, Umemoto N, Hirano M, Zang L, Oka T, Sakamoto C, Kuroyanagi J, Tanaka T: Zebrafish beta-adrenergic receptor mRNA expression and control of pigmentation. Gene. 2009, 446 (1): 18-27. 10.1016/j.gene.2009.06.005.
Watanabe K, Nishimura Y, Oka T, Nomoto T, Kon T, Shintou T, Hirano M, Shimada Y, Umemoto N, Kuroyanagi J, et al: In vivo imaging of zebrafish retinal cells using fluorescent coumarin derivatives. BMC Neurosci. 2010, 11 (1): 116-10.1186/1471-2202-11-116.
Song Y, Golling G, Thacker TL, Cone RD: Agouti-related protein (AGRP) is conserved and regulated by metabolic state in the zebrafish, Danio rerio. Endocrine. 2003, 22 (3): 257-265. 10.1385/ENDO:22:3:257.
Gorissen M, Bernier NJ, Nabuurs SB, Flik G, Huising MO: Two divergent leptin paralogues in zebrafish (Danio rerio) that originate early in teleostean evolution. J Endocrinol. 2009, 201 (3): 329-339. 10.1677/JOE-09-0034.
Nishio S, Gibert Y, Bernard L, Brunet F, Triqueneaux G, Laudet V: Adiponectin and adiponectin receptor genes are coexpressed during zebrafish embryogenesis and regulated by food deprivation. Dev Dyn. 2008, 237 (6): 1682-1690. 10.1002/dvdy.21559.
Jones KS, Alimov AP, Rilo HL, Jandacek RJ, Woollett LA, Penberthy WT: A high throughput live transparent animal bioassay to identify non-toxic small molecules or genes that regulate vertebrate fat metabolism for obesity drug development. Nutr Metab (Lond). 2008, 5: 23-10.1186/1743-7075-5-23.
Bengtson DA, Leger P, Sorgeloos P: Use of artemia as a food source for aquaculture. 1991, CRC Press, 29:
Moussavi N, Gavino V, Receveur O: Could the quality of dietary fat, and not just its quantity, be related to risk of obesity?. Obesity (Silver Spring). 2008, 16 (1): 7-15. 10.1038/oby.2007.14.
Pannevis MC, Earle KE: Maintenance energy requirement of five popular species of ornamental fish. J Nutr. 1994, 124 (12 Suppl): 2616S-2618S.
Ahima RS: Adipose tissue as an endocrine organ. Obesity (Silver Spring). 2006, 14 (Suppl 5): 242S-249S. 10.1038/oby.2006.317.
Breitling R, Armengaud P, Amtmann A, Herzyk P: Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 2004, 573 (1-3): 83-92. 10.1016/j.febslet.2004.07.055.
Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M, Marshall KA: NCBI GEO: archive for high-throughput functional genomic data. Nucleic Acids Res. 2009, D885-890. 10.1093/nar/gkn764. 37 Database
Li S, Zhang HY, Hu CC, Lawrence F, Gallagher KE, Surapaneni A, Estrem ST, Calley JN, Varga G, Dow ER, et al: Assessment of diet-induced obese rats as an obesity model by comparative functional genomics. Obesity (Silver Spring). 2008, 16 (4): 811-818. 10.1038/oby.2007.116.
Poussin C, Hall D, Minehira K, Galzin AM, Tarussio D, Thorens B: Different transcriptional control of metabolism and extracellular matrix in visceral and subcutaneous fat of obese and rimonabant treated mice. PLoS One. 2008, 3 (10): e3385-10.1371/journal.pone.0003385.
MacLaren RE, Cui W, Lu H, Simard S, Cianflone K: Association of adipocyte genes with ASP expression: a microarray analysis of subcutaneous and omental adipose tissue in morbidly obese subjects. BMC Med Genomics. 2010, 3: 3-10.1186/1755-8794-3-3.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al: Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005, 102 (43): 15545-15550. 10.1073/pnas.0506580102.
Kotelnikova E, Yuryev A, Mazo I, Daraselia N: Computational approaches for drug repositioning and combination therapy design. J Bioinform Comput Biol. 2010, 8 (3): 593-606. 10.1142/S0219720010004732.
Badman MK, Flier JS: The gut and energy balance: visceral allies in the obesity wars. Science. 2005, 307 (5717): 1909-1914. 10.1126/science.1109951.
Spiegelman BM, Flier JS: Obesity and the regulation of energy balance. Cell. 2001, 104 (4): 531-543. 10.1016/S0092-8674(01)00240-9.
Cummings DE, Overduin J: Gastrointestinal regulation of food intake. J Clin Invest. 2007, 117 (1): 13-23. 10.1172/JCI30227.
Volkoff H: The role of neuropeptide Y, orexins, cocaine and amphetamine-related transcript, cholecystokinin, amylin and leptin in the regulation of feeding in fish. Comp Biochem Physiol A Mol Integr Physiol. 2006, 144 (3): 325-331. 10.1016/j.cbpa.2005.10.026.
Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, et al: Zebrafish hox clusters and vertebrate genome evolution. Science. 1998, 282 (5394): 1711-1714. 10.1126/science.282.5394.1711.
Mommsen TP, Mojsov S: Glucagon-like peptide-1 activates the adenylyl cyclase system in rockfish enterocytes and brain membranes. Comp Biochem Physiol B Biochem Mol Biol. 1998, 121 (1): 49-56. 10.1016/S0305-0491(98)10110-4.
Amole N, Unniappan S: Fasting induces preproghrelin mRNA expression in the brain and gut of zebrafish, Danio rerio. Gen Comp Endocrinol. 2009, 161 (1): 133-7. 10.1016/j.ygcen.2008.11.002.
Huang H, Vogel SS, Liu N, Melton DA, Lin S: Analysis of pancreatic development in living transgenic zebrafish embryos. Mol Cell Endocrinol. 2001, 177 (1-2): 117-124. 10.1016/S0303-7207(01)00408-7.
Mathieu M, Trombino S, Argenton F, Larhammar D, Vallarino M: Developmental expression of NPY/PYY receptors zYb and zYc in zebrafish. Ann N Y Acad Sci. 2005, 1040: 399-401. 10.1196/annals.1327.073.
Novak CM, Jiang X, Wang C, Teske JA, Kotz CM, Levine JA: Caloric restriction and physical activity in zebrafish (Danio rerio). Neurosci Lett. 2005, 383 (1-2): 99-104. 10.1016/j.neulet.2005.03.048.
Volkoff H, Peter RE: Feeding behavior of fish and its control. Zebrafish. 2006, 3 (2): 131-140. 10.1089/zeb.2006.3.131.
Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B: Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab. 2002, 282 (4): E834-842.
Koza RA, Nikonova L, Hogan J, Rim JS, Mendoza T, Faulk C, Skaf J, Kozak LP: Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006, 2 (5): e81-10.1371/journal.pgen.0020081.
White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, Bourque C, Dovey M, Goessling W, Burns CE, et al: Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008, 2 (2): 183-189. 10.1016/j.stem.2007.11.002.
Greenspan P, Mayer EP, Fowler SD: Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985, 100 (3): 965-973. 10.1083/jcb.100.3.965.
Barros TP, Alderton WK, Reynolds HM, Roach AG, Berghmans S: Zebrafish: an emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br J Pharmacol. 2008, 154 (7): 1400-1413. 10.1038/bjp.2008.249.
Gesta S, Tseng YH, Kahn CR: Developmental origin of fat: tracking obesity to its source. Cell. 2007, 131 (2): 242-256. 10.1016/j.cell.2007.10.004.
Nadler ST, Attie AD: Please pass the chips: genomic insights into obesity and diabetes. J Nutr. 2001, 131 (8): 2078-2081.
Mertens I, Van Gaal LF: Obesity, haemostasis and the fibrinolytic system. Obes Rev. 2002, 3 (2): 85-101. 10.1046/j.1467-789X.2002.00056.x.
Nieuwdorp M, Stroes ES, Meijers JC, Buller H: Hypercoagulability in the metabolic syndrome. Curr Opin Pharmacol. 2005, 5 (2): 155-159. 10.1016/j.coph.2004.10.003.
Faber DR, de Groot PG, Visseren FL: Role of adipose tissue in haemostasis, coagulation and fibrinolysis. Obes Rev. 2009, 10 (5): 554-563. 10.1111/j.1467-789X.2009.00593.x.
Sola E, Navarro S, Medina P, Vaya A, Estelles A, Hernandez-Mijares A, Espana F: Activated protein C levels in obesity and weight loss influence. Thromb Res. 2009, 123 (5): 697-700. 10.1016/j.thromres.2008.07.017.
Kolovou GD, Anagnostopoulou KK, Cokkinos DV: Pathophysiology of dyslipidaemia in the metabolic syndrome. Postgrad Med J. 2005, 81 (956): 358-366. 10.1136/pgmj.2004.025601.
Unger RH, Clark GO, Scherer PE, Orci L: Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010, 1801 (3): 209-214.
Sugden MC, Zariwala MG, Holness MJ: PPARs and the orchestration of metabolic fuel selection. Pharmacol Res. 2009, 60 (3): 141-150. 10.1016/j.phrs.2009.03.014.
Yoon M: The role of PPARalpha in lipid metabolism and obesity: focusing on the effects of estrogen on PPARalpha actions. Pharmacol Res. 2009, 60 (3): 151-159. 10.1016/j.phrs.2009.02.004.
Vazquez-Vela ME, Torres N, Tovar AR: White adipose tissue as endocrine organ and its role in obesity. Arch Med Res. 2008, 39 (8): 715-728. 10.1016/j.arcmed.2008.09.005.
Lefterova MI, Lazar MA: New developments in adipogenesis. Trends Endocrinol Metab. 2009, 20 (3): 107-114. 10.1016/j.tem.2008.11.005.
Dahlman I, Nilsson M, Jiao H, Hoffstedt J, Lindgren CM, Humphreys K, Kere J, Gustafsson JA, Arner P, Dahlman-Wright K: Liver X receptor gene polymorphisms and adipose tissue expression levels in obesity. Pharmacogenet Genomics. 2006, 16 (12): 881-889. 10.1097/01.fpc.0000236334.49422.48.
Das UN: Obesity: genes, brain, gut, and environment. Nutrition. 2010, 26 (5): 459-473. 10.1016/j.nut.2009.09.020.
Swartz-Basile DA, Lu D, Basile DP, Graewin SJ, Al-Azzawi H, Kiely JM, Mathur A, Yancey K, Pitt HA: Leptin regulates gallbladder genes related to absorption and secretion. Am J Physiol Gastrointest Liver Physiol. 2007, 293 (1): G84-90. 10.1152/ajpgi.00389.2006.
Schmidt C, Gonzaludo NP, Strunk S, Dahm S, Schuchhardt J, Kleinjung F, Wuschke S, Joost HG, Al-Hasani H: A meta-analysis of QTL for diabetes-related traits in rodents. Physiol Genomics. 2008, 34 (1): 42-53. 10.1152/physiolgenomics.00267.2007.
Toro S, Wegner J, Muller M, Westerfield M, Varga ZM: Identification of differentially expressed genes in the zebrafish hypothalamic-pituitary axis. Gene Expr Patterns. 2009, 9 (4): 200-208. 10.1016/j.gep.2008.12.007.
Westerfield M: A guide for the laboratory use of zebrafish (Danio rerio). 2007, University of Oregon Press
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al: Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004, 5 (10): R80-10.1186/gb-2004-5-10-r80.
Acknowledgements
This work was supported, in part, by the New Energy and Industrial Technology Development Organization, the Ministry of Education, Science, Sports and Culture, the Organization for Pharmaceutical Safety and Research, the Mie Medical Research Foundation and Mie Prefecture. We express our gratitude to Monte Westerfield and Zoltan M. Varga for teaching us the fundamental techniques for zebrafish research. We also thank K. Nishiguchi, C. Suzuki, Y. Yoshikawa T. Murata and A. Kamakura for their experimental assistance and R. Ikeyama, Y. Yoshida and K. Ito for secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
TO carried out the experiments and helped to draft the manuscript. YN carried out qPCR experiments and drafted the manuscript. LZ carried out the assessment of feeding. MH participated in the design of the study. YS helped to draft the manuscript. ZW, NU and JK helped to carry out the experiments. NN conceived the study. TT conceived the study and drafted the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
12899_2010_168_MOESM6_ESM.XLS
Additional file 6: Supplemental Table S6. Genes differentially expressed in visceral AT in the same direction in at least two obesity models (XLS 28 KB)
12899_2010_168_MOESM7_ESM.PDF
Additional file 7: Supplemental Figure S1. Validation of the differential gene expression by qPCR analysis. Total RNA was extracted from zebrafish visceral AT and qPCR analysis was performed to validate the differential expression identified by microarray analysis. Results represent means ± S.E.M. of each group. P-value was calculated by Student's t-test using OF1W (N = 6) vs. OF8W (N = 4). *p < 0.05. (PDF 117 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Oka, T., Nishimura, Y., Zang, L. et al. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol 10, 21 (2010). https://doi.org/10.1186/1472-6793-10-21
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-6793-10-21