Abstract
Background
Little information is available on the circadian sequela of an immune challenge in the brain of aged rats. To assess them, we studied 24-hour rhythms in hypothalamic and striatal norepinephrine (NE) content, hypothalamic and striatal dopamine (DA) turnover and hypophysial NE and DA content, in young (2 months) and aged (18–20 months) rats killed at 6 different time intervals, on day 18th after Freund's adjuvant or adjuvant's vehicle administration.
Results
Aging decreased anterior and medial hypothalamic NE content, medial and posterior hypothalamic DA turnover, and striatal NE concentration and DA turnover. Aging also decreased NE and DA content in pituitary neurointermediate lobe and augmented DA content in the anterior pituitary lobe. Immunization by Freund's adjuvant injection caused: (i) reduction of DA turnover in anterior hypothalamus and corpus striatum; (ii) acrophase delay of medial hypothalamic DA turnover in old rats, and of striatal NE content in young rats; (iii) abolition of 24-h rhythm in NE and DA content of neurointermediate pituitary lobe, and in DA content of anterior lobe, of old rats.
Conclusions
The decline in catecholamine neurotransmission with aging could contribute to the decrease of gonadotropin and increase of prolactin release reported in similar groups of rats. Some circadian responses to immunization, e.g. suppression of 24-h rhythms of neurointermediate lobe NE and DA and of anterior lobe DA were seen only in aged rats.
Similar content being viewed by others
Background
In 1974, Pittendrigh and Daan first described the changes caused by aging in the circadian timing system of rodents [1]. Since then, a bulk of information has accumulated indicating that reduced amplitude, shorter free-running periods and desynchronization of circadian rhythms are associated with advanced age in rodents as well as in humans (for references see [2]). Both the efficacy of input and output pathways from the central nervous system circadian pacemaker, located in the hypothalamic suprachiasmatic nuclei, and the functioning of the central pacemaker itself, change with advancing age. In addition, some of the decline in overt circadian rhythmicity may be due to deteriorating function of the body's aging effector systems [2].
An aspect of circadian organization in aged subjects less known is the modification in amplitude or phase of circadian rhythms during the different phases of the response to an immune challenge. Since aging is associated with declines in multiple areas of immune function [3], it seems feasible that differences in the circadian response to an immune challenge with age occur.
Adjuvant arthritis is an experimental model for rheumatoid arthritis, induced by the intradermal injection of heat-killed Mycobacterium tuberculosis in incomplete Freund's adjuvant to rats [4]. In the classical adjuvant-induced arthritis model, polyarthritis is accompanied by a widespread systemic disease. In this experimental model we previously reported the effect of aging on circadian organization of plasma prolactin, growth hormone (GH), thyrotropin (TSH), insulin, follicle-stimulating hormone (FSH), luteinizing hormone (LH) and testosterone, studied during the acute phase of inflammatory disease of the joints (18 days after Freund's adjuvant injection) in rats [5]. As a continuation of those studies we now report, in comparable groups of animals, the changes in 24-hour organization of hypothalamic and striatal norepinephrine (NE) content and dopamine (DA) turnover and hypophysial NE and DA content. The results support the view that 24-h rhythms and levels of hypothalamic, striatal and hypophysial catecholamines are age-dependent, as are some of the responses to Freund's adjuvant administration.
Results
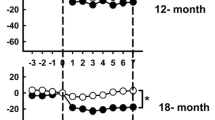
Figure 1 shows the 24-h changes in hypothalamic NE content in young and old rats injected with Freund's adjuvant or its vehicle 18 days earlier. Rhythm parameters as analyzed by Cosinor are summarized in Table 1. In the three hypothalamic regions examined significant 24-h changes of NE content occurred, as shown by individual oneway analysis of variance (ANOVA) (Fig. 1). In the experimental groups in which data fitted a cosine function, acrophases coincided with the second half of activity span or the first half of rest span (04:06 – 13:08 h. Table 1). A factorial ANOVA taking age as a main factor revealed a significantly lower NE content in anterior and medial hypothalamus of aged rats (F1,126= 8.76, p < 0.004, and F1,122 = 4.83, p < 0.03, respectively). In Cosinor, mesor values of NE content in anterior hypothalamus and amplitude values in posterior hypothalamus of old rats were significantly lower than their respective younger counterparts (Table 1).
Twenty-four h changes of hypothalamic NE content in young and old rats injected with Freund's adjuvant or its vehicle 18 days earlier. Groups of 5–7 rats were killed by decapitation at six different time intervals throughout a 24-h cycle. Shown are the means + SEM. The groups in which significant differences among time intervals were detected by a one-way ANOVA are indicated by their F values in the Figure. For further statistical analysis, see text.
Figure 2 depicts the 24-h changes in hypothalamic DA turnover. Individual one-way ANOVA's indicated significant time-of-day changes in the anterior hypothalamus of all experimental groups, in the medial hypothalamus of young rats treated with Freund's adjuvant and of old rats treated with adjuvant's vehicle, and in the posterior hypothalamus of young and old rats treated with adjuvant's vehicle. Acrophases occurred during the second half of activity span or first half of rest span (04:00 – 13:47 h), except for the medial hypothalamus of Freund's adjuvant-treated young rats whose acrophases were at the beginning of the activity span (20:17 h. Table 1). A factorial ANOVA taking age as a main factor indicated that medial and posterior hypothalamic DA turnover was significantly lower in old rats (F1,124 = 5.29, p < 0.01 and F1,121 = 6.41, p < 0.001, respectively). Mean values (pg/mg) of 3,4-dihydroxyphenylacetic acid (DOPAC) and DA in young rats were: 38 ± 2.7 and 147 ± 11 (anterior hypothalamus); 83 ± 5.5 and 72 ± 4 (medial hypothalamus); 10 ± 1 and 27 ± 2.3 (posterior hypothalamus). Mean values (pg/mg) of DOPAC and DA in old rats were: 31 ± 2 and 122 ± 9 (anterior hypothalamus); 65 ± 6.6 and 68 ± 5 (medial hypothalamus); 7 ± 1 and 26 ± 1.7 (posterior hypothalamus). In every hypothalamic region of old rats DOPAC concentration was lower than that of young rats (p < 0.05, Student's t test). When immunization was taken as a main factor in factorial ANOVA, a significant reduction in DA turnover was found in the anterior hypothalamus of Freund's adjuvant-treated rats (F1,123 = 3.99, p < 0.04). Mean values (pg/mg) of DOPAC and DA in the anterior hypothalamus of adjuvant's vehicle- and Freund's adjuvant-treated rats were: 37 ± 2.3 and 125 ± 13, and 33 ± 1.9 and 130 ± 12, respectively.
Twenty-four h changes of hypothalamic DA turnover in young and old rats injected with Freund's adjuvant or its vehicle 18 days earlier. Groups of 5–7 rats were killed by decapitation at six different time intervals throughout a 24-h cycle. Shown are the means + SEM. The groups in which significant differences among time intervals were detected by a one-way ANOVA are indicated by their F values in the Figure (n.s.: not significant F). For further statistical analysis, see text.
Figure 3 depicts striatal NE content and DA turnover. Striatal NE content varied significantly on a 24-h basis. Acrophases coincided with the second half of activity span or the first half of rest span (06:29 – 12:16 h), except for young rats treated with Freund's adjuvant in which acrophase occurred late in the rest span (17:15 h) (Table 2). Differences in acrophases between immunized and non-immunized young rats were significant (Table 2). Analyzed as a main factor in a factorial ANOVA, old rats had lower striatal NE concentrations (F1,121 = 8.76, p < 0.003). In every case, old rats showed smaller mesor values of striatal NE content than their younger counterparts (Table 2). Amplitude decreased significantly in old rats injected with Freund's adjuvant (Table 2).
Twenty-four h changes of NE content and DA turnover in corpus striatum of young and old rats injected with Freund's adjuvant or its vehicle 18 days earlier. Groups of 5–7 rats were killed by decapitation at six different time intervals throughout a 24-h cycle. Shown are the means + SEM. The groups in which significant differences among time intervals were detected by a one-way ANOVA are indicated by their F values in the Figure (n.s.: not significant F). For further statistical analysis, see text.
Time-of-day changes in striatal DA turnover were not significant as indicated by individual one-way ANOVA's (Fig. 3). In a factorial ANOVA, the effects of both main factors "age" and "immunization" were significant, aging and Freund's adjuvant decreasing striatal DA turnover (F1,122 = 5.97, p < 0.01 and F1,123 = 13.4, p < 0.00001, respectively). Mean values (pg/mg) of striatal DOPAC and DA in young and old rats were: 437 ± 52 and 3123 ± 225, and 189 ± 21 and 2631 ± 202, respectively. Differences in striatal DOPAC concentration between young and old rats were significant (p < 0.01, Student's t test). Mean values (pg/mg) of striatal DOPAC and DA in adjuvant's vehicle-and Freund's adjuvant-treated rats were: 411 ± 54 and 2417 ± 256, and 215 ± 25 and 2152 ± 234, respectively. The decrease in striatal DOPAC concentration after Freund's adjuvant administration was significant (p < 0.01, Student's t test).
Hypophysial NE and DA levels are depicted in Fig. 4. NE levels of the neurointermediate lobe showed significant 24-h variations in all groups except for old rats receiving Freund's adjuvant. Acrophases were at 02:31 – 03:34 h (Table 3). In a factorial ANOVA in which age was analyzed as a main factor, a significant depression of NE content was detected in aged rats (F1,123 = 10.1, p < 0.0001). Mesor values were significantly lower in old rats. Amplitude in old rats receiving adjuvant's vehicle was significantly lower than that of their respective young counterparts (Table 3).
Twenty-four h changes of NE and DA content in hypophysial neurointermediate lobe and of DA content of hypophysial anterior lobe in young and old rats injected with Freund's adjuvant or its vehicle 18 days earlier. Groups of 5–7 rats were killed by decapitation at six different time intervals throughout a 24-h cycle. Shown are the means + SEM. The groups in which significant differences among time intervals were detected by a one-way ANOVA are indicated by their F values in the Figure (n.s.: not significant F). For further statistical analysis, see text.
Significant 24-h changes in DA content of the neurointermediate lobe occurred in the 4 experimental groups, except for old rats receiving Freund's adjuvant (Fig. 4); acrophases were at 02:47 – 04:23 h (Table 3). A factorial ANOVA indicated a depressive effect of aging on neurointermediate lobe DA content (F1,126 = 28.4, p < 0.00001). In Cosinor, mesor and amplitude values in old rats receiving adjuvant's vehicle were significantly lower than those of their respective young counterparts (Table 3).
Figure 4, lower panel, depicts DA levels in the anterior hypophysis. Significant 24-h changes were observed in all groups except for old rats administered with Freund's adjuvant. Only changes in young animals injected with adjuvant's vehicle fit a cosine function, with acrophase at 10:31 h. In a factorial ANOVA, aging augmented anterior hypophysial DA levels (F1,122 = 37.8, p < 0.00001).
Discussion
The present study, performed in rats sacrificed at 6 different time intervals during a 24-h cycle, documented the following effects of aging on hypothalamic, striatal and hypophysial catecholamines: (i) lower NE content in anterior and medial hypothalamus; (ii) lower DA turnover in medial and posterior hypothalamus; (iii) lower striatal NE concentration and DA turnover; (iv) lower NE and DA content of the neurointermediate pituitary lobe; (v) augmented anterior hypophysial DA content. In addition, the identified effects of Freund's adjuvant administration were: (i) reduction of DA turnover in the anterior hypothalamus; (ii) acrophase delay of DA turnover in medial hypothalamus of old rats; (iii) acrophase delay of striatal NE content of young rats; (iv) decreased striatal DA turnover; (v) abolished 24-h variations of neurointermediate lobe NE and DA content, and of anterior lobe DA content, in old rats.
Most previous studies on the effect of aging on brain and peripheral catecholamines in rats have been obtained as single time points in a 24-h cycle. Decreases in NE content in limbic areas, spinal cord, medulla oblongata and pons, and less consistently, in the hypothalamus and corpus striatum of old rats were identified [6–12]. In aging male rats, levels of hypothalamic NE either decreased [6, 13–16] or remained unchanged [9, 17, 18].
Our present results indicate that aged rats had lower NE content in the anterior and medial hypothalamus. In addition, hypothalamic NE levels showed significant 24-h changes with acrophases at the second half of activity span or first half of rest span. In the posterior hypothalamus of old rats, amplitude of rhythm was significantly lower than in their respective younger counterparts. Since noradrenergic neurons stimulate LH releasing hormone release [19], the decline in noradrenergic stimulation with aging could contribute to the age-related decrease of FSH and LH described previously in similar groups of rats [5]. It should be noted that a strong positive correlation did exist between the rate constant of NE loss measured in rats and the magnitude of the age-related depletion in NE concentrations within specific brain regions [20].
The present results also support the existence of a lower DA turnover in medial and posterior hypothalamus of aged rats. This was the consequence of a decrease of both DA and DOPAC concentration, being most marked in the case of the latter. DA released by tuberoinfundibular dopaminergic neurons in the hypothalamic periventricular and arcuate nuclei tonically inhibits pituitary prolactin secretion. In turn, prolactin stimulates DA secretion from dopaminergic neurons, forming a feedback loop [21, 22]. This autoregulatory feedback control of prolactin is altered in the aged rat, as evidenced by increased circulating concentrations of prolactin and decreased activity of these neurons [22, 23]. In addition, tuberoinfundibular DA neurons of old rats failed to respond to exogenous prolactin administration [24].
Prolactin increases occur with aging in male rats of most strains, including Wistar [25] and Long-Evans [26] rats, but not in all, e.g. Sprague-Dawley [27] rats. Most studies have reported a decrease in DA content in the hypothalamus and median eminence of aging male rats, including Wistar [14, 15], Long-Evans [28], Sprague-Dawley [9] and F344 [16] rats. In the present study, medial hypothalamic, as well as median eminence DA levels (data not shown), decreased with age. Since the decreases in DOPAC levels exceeded those of DA, DA turnover rate also decreased. Our results agree with measurements of hypothalamic DA turnover in aged Wistar [14] and Long-Evans [28] rats at single time-points in a 24-h cycle.
In vivo, secretion of DA into hypophysial portal blood has been reported to decrease [29] or to increase with aging [30]. Long-term (12 months) testosterone replacement in 24-month-old male Wistar rats increased DA release in the medial preoptic area nearly to levels observed in 3-month-old rats [31]. These findings suggest that DA is involved in the age-related decline in male reproductive function, as indicated by the low FSH, LH and testosterone levels reported in our previous study [5].
In rats, the concentrations of DA in the adenohypophysis increases progressively with age. The increase in the DA content is not a consequence of reduced metabolism of DA, nor is it a static pool because as in young rats, adenohypophysial DA is rapidly decreased by pharmacological treatments that reduce the activity of tuberoinfundibular DA neurons. In aged rats, the amount of DA associated with light particles was 5 times that found in young rats, whereas the amount of DA associated with heavy particles was the same as that in young rats [32]. Therefore, an increased concentration of DA in anterior hypophysis, like that reported in the present study in old rats, does not necessarily result in inhibition of prolactin secretion. It is interesting that, in addition to DA, impairments are observed in the processing (binding, accumulation and intracellular distribution) of hypothalamic hormones in the adenohypophysis of old rats. Taken together, these observations are supportive of the view that the neuroendocrine/endocrine changes appearing with age result from a complex balance of functional alterations occurring at each level, central and peripheral, of the axis.
In the present study, striatal DA and DOPAC levels and turnover rate decreased significantly in aged rats. The results agree with the bulk of information obtained in aged rodents indicating decreased levels and turnover rate of DA [8, 9, 16, 28, 33–36]. Generally, the decreased levels of midbrain DA and DOPAC detectable in aged rats have little correlation with age-related changes in the density of dopaminergic receptor binding or the density of DA uptake sites [37].
Significant amounts of NE and DA are present in the neurointermediate lobe of the hypophysis. DA nerve terminals belong to centrally located neurons whereas the origin of NE fibers is in part peripheral, as shown by the 40–50% decrease of posterior pituitary NE content after the bilateral removal of the sympathetic superior cervical ganglia [38, 39]. Activation of central noradrenergic input to the magnocellular nuclei augments arginine vasopressin release [40] whereas that of peripheral noradrenergic input decreased arginine vasopressin release [39]. Data presented herein indicate that reduced concentrations of DA occurred in the neurointermediate lobe of the pituitary gland of old rats as compared to those of young rats. Our present results also indicate that NE levels in neurointermediate lobe showed significant 24-h variations in all groups except for old rats receiving Freund's adjuvant. Acrophases were at about the middle of the activity span (at 02:47 – 04:23 h), in concordance with the previously reported maxima in tyrosine hydroxylase activity in the superior cervical, stellate and celiac-superior mesenteric ganglia of young and old rats [41]. Therefore, the central and peripheral sources of noradrenergic innervation of hypophysial neurointermediate lobe seem to have similar 24-h patterns of activation. NE content in neurointermediate lobe and tyrosine hydroxylase in sympathetic ganglia [41] was lowest in old rats.
A number of studies have indicated an age-related reduction in immune function associated with cell mediated immunity in both experimental animals and humans. In advancing age alterations were mainly observed in T cell mediated immunity including decreased proliferative responsiveness of T cells to mitogens, decreased T cell-dependent humoral immune responses, lowered resistance to tumor cell challenge, decreased graft-vs.-host reactivity, delayed skin allograft rejection time, impaired delayed hypersensitivity, reduced cytolytic immune response, altered cytokine production after stimulation, and decreased natural killer cell activity (for references see [42]). Most of the studies were performed at single time points in a 24-h cycle, thus overseeing any effect the circadian system can have on the responses.
In the present study, the injection of Freund's adjuvant to young and old rats was employed as an antigenic challenge. The adjuvant-induced arthritis that follows is considered to be a model for T-lymphocyte-dependent, autoimmune diseases [43] and the central symptoms found partly constitute the "sickness behavior", i.e., the behavioral changes that accompany the immune reaction [44]. One feature of sickness behavior addressed experimentally in the present study was the modification of the 24-hour pattern of hypothalamic, striatal and hypophysial catecholamines occurring 18 days after the injection of complete Freund's adjuvant to young and old rats.
Changes in circadian rhythms are apparent at an early phase of experimental arthritis in rats and persist thereafter [45–49]. In the present study Freund's adjuvant administration brought about, 18 days later, a decrease of DA turnover in the anterior hypothalamus and striatum. It also caused a number of circadian alterations, including acrophase delay of DA turnover in medial hypothalamus of old rats, acrophase delay of striatal NE content of young rats and abolished 24-h variations of neurointermediate lobe NE and DA content, and anterior lobe DA content, in old rats. Experimental evidence suggests that symptomatology after Freund's adjuvant administration are a part of a defense response to antigenic challenge and are mediated by the neural effects of cytokines like interleukin (IL)-l, IL-6, IL-2, granulocyte-macrophage colony-stimulating factor and interferon-α [44, 50]. Since we previously reported that immunosuppression restored rhythmicity of several of the neuroendocrine parameters examined in Freund's adjuvant-injected rats [51], the immune-related nature of the studied phenomena seems to be warranted. Suprachiasmatic nuclei themselves may be sensitive to immune-derived signals. Presumably, the chronic stress condition given by mycobacterial adjuvant injection is instrumental in inhibiting a number of circadian rhythms at early and late phases of disease.
Conclusions
Aged rats had lower NE content in the anterior and medial hypothalamus, smaller amplitude of 24-h rhythm in posterior hypothalamic NE content, lower DA turnover in medial and posterior hypothalamus, augmented adenohypophysial DA and decreased NE and DA content in pituitary neurointermediate lobe. These results are probably instrumental for the neuroendocrine/endocrine changes appearing with age. Aged rats had also significantly decreased striatal DA and DOPAC levels and turnover rate when measured at 6 different time points along a 24-h cycle, thus agreeing with the bulk of information obtained at single time points on the occurrence of age-related decreased levels and turnover rate of DA in corpus striatum.
With advancing age alterations were mainly reported on T cell mediated immunity. We hereby reported that during Freund's adjuvant-induced arthritis (a T-lymphocyte-dependent autoimmune disease) a decrease of DA turnover occurred in the anterior hypothalamus and striatum. We also observed that responses to immunization like suppression of 24-h rhythms of neurointermediate lobe NE and DA and of anterior lobe DA were only seen in aged rats. Collectively, our results indicate that 24-h rhythms and levels of hypothalamic, striatal and hypophysial catecholamines are age-dependent, as are some of the responses to Freund's adjuvant administration.
Materials and Methods
Animals
Experiments were carried out in adult male Wistar rats, kept under light between 0800 and 2000 h daily. Light intensity at the level of the animal cages was about 200 lux. Rats had access to food and water ad libitum. Adequate measures were taken to minimize pain or discomfort, in accordance with the principles and procedures outlined in European Communities Council Directives (86/609/EEC).
Groups of young (2 months) and aged (18–20 months) rats were injected s.c. with Freund's complete adjuvant (0.5 mg heat-killed Mycobacterium butyricum/rat) or its vehicle (0.5 ml paraffin oil containing 15% mannide monooleate) 3 h after light on (HALO) (i.e., at 11:00 h). The effect of varying the time of Freund's adjuvant injection on day-night differences of submaxillary lymph node ornithine decarboxylase activity (an index of lymph node proliferative response) was examined in a previous work [52]. Immunization performed during daylight (5 HALO) or at night (18 HALO) resulted in similar day-night differences in ornithine decarboxylase activity, indicating that changes in lymph node proliferative responses were relatively independent of the biological time of mycobacterial antigen exposure [52]. We maintained a similar injection schedule as in several previous studies conducted on Freund's adjuvant effects on immune and endocrine responses [53, 54]; thus, rats included in the present study were injected with Freund's adjuvant at 3 HALO (11:00 h).
Although arthritis is induced most easily in inbred Lewis rats, it is also produced, to a milder extent, in Wistar rats [55–58]. Rats injected with Freund's adjuvant vehicle were included as a control of any inflammatory reaction the adjuvant's oil alone might cause [59–61]. The course of adjuvant-induced arthritis was followed by behavioral observations including those of spontaneous behavior-mobility, exploring, rearing and scratching [4, 43]. Eighteen days after Freund's adjuvant injection a lack of mobility and exploring behavior, an increase in scratching behavior and signs of hyperalgesia were clearly established in young and old rats as compared with their respective adjuvant's vehicle-injected groups. As reported previously by using plethysmography [62], old rats exhibited less behavioral signs of inflammation (spontaneous behavior-mobility, exploring, scratching) than young rats.
On day 18 after injection, groups of 5–7 rats were killed by decapitation at 6 different time intervals throughout a 24-hour cycle. The brains were quickly removed and the hypothalamus, corpus striatum and pituitaries were taken out. The hypothalamus was further sectioned in the frontal plane, the anterior and posterior regions comprising one-third of the block each [63].
Catecholamine assays
Tissue was weighed and homogenized in chilled (0–1°C) 2 M acetic acid. After centrifugation (at 15 000 × g for 30 min, at 5°C), the samples were analyzed by high performance liquid chromatography using electrochemical detection (Coulochem, 5100A, ESA; USA). AC-18 reverse phase column eluted with a mobile phase (pH 4, 0.1 M sodium acetate, 0.1 M citric acid, 0.7 mM sodium octylsulphate and 0.57 mM EDTA containing 10% methanol, v/v), was employed. Flow rate was 1 ml/min, at a pressure of 2200 psi. Fixed potentials against H2/H+ reference electrode were: conditioning electrode: -0.4 V; preoxidation electrode: +0.10 V; working electrode: +0.35 V. Catecholamine concentrations were calculated from the chromatographic peak heights by using external standards. The linearity of the detector response for NE, DA and DOPAC (a major catabolite of DA) was tested within the concentration ranges found in hypothalamic supernatants. The turnover of DA was assessed by the DOPAC/DA ratio [64]. Although DOPAC concentration depends on the balance between catabolite synthesis and clearance, its acidic nature slows substantially its clearance from tissue, so that DOPAC concentration reflects an integral of past DA release.
Statistical analysis
Statistical analysis of results was performed by a one-way ANOVA, a two-way factorial ANOVA or one-tailed Student's t test, as stated. Cosinor analysis was used to analyze general rhythmic parameters, i.e., acrophase (the maximum of the cosine function fit to the experimental data), mesor (the statistical estimate of the 24-h time series mean) and amplitude (half the difference between maximal and minimal values of the derived cosine curve). Percent of rhythm defined the part of variation that could be explained by a cosine function. Statistical analysis of Cosinor parameters was carried out by standard procedures [65]. Statistical significance of the derived cosine curves was tested against the null hypothesis (i.e., amplitude = 0) [66]; p values lower than 0.05 were considered evidence for statistical significance.
References
Pittendrigh CS, Daan S: Circadian oscillations in rodents: A systematic increase of their frequency with age. Science. 1974, 186: 548-551.
Kolker D, Turek F: Circadian rhythms and sleep in aging rodents. In Functional Neurobiology of Aging. Edited by Hof PR, Mobbs CV. San Diego: Academic Press;. 2001, 869-882.
Solomon GF, Morley JE: Psychoneuroimmunology and aging. In Psychoneuroimmunology. Edited by Ader R, Felten DL, Cohen N. San Diego:Academic Press;. 2001, 701-717.
Pearson C, Wood F: Studies of polyarthritis and other lesions induced in rats by injection of mycobacterial adjuvant. I. General clinical and pathologic characteristics and some modifying factors. Arthr Rheum. 1959, 2: 440-459.
Garcia Bonacho M, Esquifino AI, Castrillon P, Reyes Toso C, Cardinali DP: Age-dependent effect of Freund's adjuvant on 24-hour rhythms in plasma prolactin, growth hormone, thyrotropin, insulin, follicle-stimulating hormone, luteinizing hormone and testosterone in rats. Life Sci. 1999, 66: 1969-1977. 10.1016/S0024-3205(00)00522-1.
Ponzio F, Brunello N, Algeri S: Catecholamine synthesis in brain of ageing rats. J Neurochem. 1978, 30: 1617-1620.
Ida Y, Tanaka M, Kohno Y, Nakagawa R, Iimori K, Tsuda A, et al: Effects of age and stress on regional noradrenaline metabolism in the rat brain. Neurobiol Aging. 1982, 3: 233-236. 10.1016/0197-4580(82)90044-6.
Ponzio F, Calderini G, Lomuscio G, Vantini G, Toffano G, Algeri S: Changes in monoamines and their metabolite levels in some brain regions of aged rats. Neurobiol Aging. 1982, 3: 23-29. 10.1016/0197-4580(82)90057-4.
Carfagna N, Trunzo F, Moretti A: Brain catecholamine content and turnover in aging rats. Exp Gerontol. 1985, 20: 265-269. 10.1016/0531-5565(85)90051-8.
Roubein IF, Embree LJ, Jackson DW: Changes in catecholamine levels in discrete regions of rat brain during aging. Exp Aging Res. 1986, 12: 193-196.
Huguet F, Comoy E, Piriou A, Bohuon C: Age-related changes of noradrenergic-NPY interaction in rat brain: norepinephrine, NPY levels and alpha-adrenoceptors. Brain Res. 1993, 625: 256-260. 10.1016/0006-8993(93)91066-2.
Miguez JM, Aldegunde M, Paz-Valinas L, Redo J, Sanchez-Barcelo E: Selective changes in the contents of noradrenaline, dopamine and serotonin in rat brain areas during aging. J Neural Transm. 1999, 106: 1089-1098. 10.1007/s007020050225.
Miller AE, Shaar CJ, Riegle GD: Aging effects on hypothalamic dopamine and norepinephrine content in the male rat. Exp Aging Res. 1976, 2: 475-480.
Simpkins JW, Mueller GP, Huang HH, Meites J: Evidence for depressed catecholamine and enhanced serotonin metabolism in aging male rats: Possible relation to gonadotropin secretion. Endocrinology. 1977, 100: 1672-1678.
Bhaskaran D, Radha E: Monoamine levels and monoamine oxidase activity in different regions of rat brain as a function of age. Mech Ageing Dev. 1983, 23: 151-160. 10.1016/0047-6374(83)90064-7.
Steger RW, De Paolo LV, Shepherd AM: Effects of advancing age on hypothalamic neurotransmitter content and on basal and norepinephrine-stimulated LHRH release. Neurobiol Aging. 1985, 6: 113-116. 10.1016/0197-4580(85)90027-2.
Lorens SA, Hata N, Handa RJ, Van de Kar LD, Guschwan M, Goral J, et al: Neurochemical, endocrine and immunological responses to stress in young and old Fischer344 male rats. Neurobiol Aging. 1990, 11: 139-150. 10.1016/0197-4580(90)90047-4.
Rodriguez-Gomez JA, de la RC, Machado A, Cano J: The effect of age on the monoamines of the hypothalamus. Mech Ageing Dev. 1995, 77: 185-195. 10.1016/0047-6374(94)01525-Q.
Kalra SP, Kalra PS: Neural regulation of luteinizing hormone secretion in the. rat. Endocr Rev. 2001, 4: 311-351.
Estes KS, Simpkins JW: Age-related alterations in dopamine and norepinephrine activity within microdissected brain regions of ovariectomized Long Evans rats. Brain Res. 1984, 298: 209-218. 10.1016/0006-8993(84)91420-3.
Moore KE: Interactions between prolactin and dopaminergic neurons. Biol Reprod. 1987, 36: 47-58.
Demarest KT, Moore KE, Riegle GD: Responsiveness of tuberoinfundibular dopamine neurons in the aged female rat to the stimulatory actions of prolactin. Neuroendocrinology. 1987, 45: 227-232.
Demarest KT, Moore KE, Riegle GD: Adenohypophysial dopamine content and prolactin secretion in the aged male and female rat. Endocrinology. 1985, 116: 1316-1323.
MohanKumar PS, MohanKumar SM, Quadri SK, Voogt JL: Effects of chronic bromocriptine treatment on tyrosine hydroxylase (TH) mRNA expression, TH activity and median eminence dopamine concentrations in ageing rats. J Neuroendocrinol. 2001, 13: 261-269. 10.1046/j.1365-2826.2001.00621.x.
Simpkins JW, Millard WJ: Influence of age on neurotransmitter function. Endocrinol Metab Clin North Am. 1987, 16: 893-917.
Riegle GD, Meites J: Effect of aging on LH and prolactin after LHRH, L-DOPA, methyl-dopa, and stress in male rats. Proc Soc Exp Biol Med. 1976, 151: 507-511.
Amoroso S, Di Renzo GF, Maurano F, Maida P, Taglialatela M, Annunziato L: Lack of evidence for an impairment of tuberoinfundibular dopaminergic neurons in aged male rats of the Sprague-Dawley strain. Exp Aging Res. 1987, 13: 85-87.
Demarest KT, Riegle GD, Moore KE: Characteristics of dopaminergic neurons in the aged male rat. Neuroendocrinology. 1980, 31: 222-227.
Hotta H, Ito H, Matsuda K, Sato A, Tohgi H: Age-related changes in rates of basal secretion of immunoreactive vasoactive intestinal polypeptide and dopamine into pituitary stalk blood from the hypothalamus in anesthetized male rats. Jpn J Physiol. 1991, 41: 317-325.
Gudelsky GA, Nansel DD, Porter JC: Dopaminergic control of prolactin secretion in the aging male rat. Brain Res. 1980, 204: 446-450. 10.1016/0006-8993(81)90606-5.
Sato Y, Shibuya A, Adachi H, Kato R, Horita H, Tsukamoto T: Restoration of sexual behavior and dopaminergic neurotransmission by long term exogenous testosterone replacement in aged male rats. J Urol. 1998, 160: 1572-1575. 10.1097/00005392-199810000-00117.
Arita J, Reymond MJ, Porter JC: Evidence for alteration in the processing of dopamine in the anterior pituitary gland of aged rats: Receptors and intracellular compartmentalization of dopamine. Endocrinology. 1984, 114: 974-979.
Finch CE: Agerelated changes in brain catecholamines: A synopsis of findings in C57BL/6J mice and other rodent models. In Parkinson's disease. 11. Aging and Neuroendocrine Relationship. Edited by Finch CE, Potter DE, Kenny AD. New York: Plenum Press;. 1978, 15-39.
Strong R, Samorajski T, Gottesfeld Z: High-affinity uptake of neurotransmitters in rat neostriatum: Effects of aging. J Neurochem. 1984, 43: 1766-
Moretti A, Carfagna N, Trunzo F: Effect of aging on monoamines and their metabolites in the rat brain. Neurochem Res. 1987, 12: 1035-1039.
Venero JL, Machado A, Cano J: Turnover of dopamine and serotonin and their metabolites in the striatum of aged rats. J Neurochem. 1991, 56: 1940-1948.
Burwell RD, Lawler CP, Gallagher M: Mesostriatal dopamine markers in aged Long-Evans rats with sensorimotor impairment. Neurobiol Aging. 1995, 16: 175-186. 10.1016/0197-4580(94)00157-X.
Saavedra J: Central and peripheral catecholamine innervation of the rat intermediate and posterior pituitary lobes. Neuroendocrinology. 1985, 40: 281-284.
Romeo HE, Spinedi E, Esquifino AI, Estivariz F, Cardinali DP: Anterograde nerve degeneration after superior cervical ganglionectomy co-exists with a decrease of arginine vasopressin release in rats. Neuroendocrinology. 1991, 54: 346-352.
Sladek JR, Sladek CD: Neurological control of vasopressin release. Fed Proc. 1985, 44: 66-71.
Brusco LI, GarciaBonacho M, Esquifino AI, Cardinali DP: Diurnal rhythms in norepinephrine and acetylcholine synthesis of sympathetic ganglia, heart and adrenals of aging rats: Effect of melatonin. J Auton Nerv Syst. 1998, 74: 49-61. 10.1016/S0165-1838(98)00134-9.
Bellinger DI, Madden KS, Lorton D, ThyagaRajan S, Felten DL: Age-related alterations in neural-immune interactions and neural strategies in immunosenescence. In Psychoneuroimmunology. Edited by Ader R, Felten DL, Cohen N. San Diego: Academic Press;. 2001, 241-286.
Calvino B, Crepon-Bernard MO, Le Bars D: Parallel clinical and behavioural studies of adjuvant-induced arthritis in the rat: possible relationship with 'chronic pain'. Behav Brain Res. 1987, 24: 11-29. 10.1016/0166-4328(87)90032-5.
Kent S, Bluthe RM, Kelley KW, Dantzer R: Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992, 13: 24-28. 10.1016/0165-6147(92)90012-U.
Neidhart M, Fluckiger EW: Hyperprolactinaemia in hypophysectomized or intact male rats and the development of adjuvant arthritis. Immunology. 1992, 77: 449-455.
Sarlis NJ, Chowdrey HS, Stephanou A, Lightman SL: Chronic activation of the hypothalamo-pituitary-adrenal axis and loss of circadian rhythm during adjuvant-induced arthritis in the rat. Endocrinology. 1992, 130: 1775-1779.
Holmes MC, French KL, Seckl JR: Modulation of serotonin and corticosteroid receptor gene expression in the rat hippocampus with circadian rhythm and stress. Brain Res Mol Brain Res. 1995, 28: 186-192. 10.1016/0169-328X(94)00207-U.
Cardinali DP, Brusco LI, Selgas L, Esquifino AI: Diurnal rhythms in ornithine decarboxylase activity and norepinephrine and acetylcholine synthesis in submaxillary lymph nodes and spleen of young and aged rats during Freund's adjuvant-induced arthritis. Brain Res. 1998, 789: 283-292. 10.1016/S0006-8993(98)00015-8.
Duvilanski BH, Selgas L, Garcia-Bonacho M, Esquifino AI: Daily variations of amino acid concentration in mediobasal hypothalamus, in rats injected with Freund's adjuvant. Effect of cyclosporine. J Neuroimmunol. 1998, 87: 189-196. 10.1016/S0165-5728(98)00108-8.
Besedovsky HO, del Rey A: Cytokines as mediators of central and peripheral immune-neuroendocrine interactions. In Psychoneuroimmunology. Edited by Ader R, Felten DL, Cohen N. San Diego CA: Academic Press;. 2001, 1-17.
Esquifino AI, Selgas L, Vara E, Arce A, Cardinali DP: Twenty-four hour rhythms of hypothalamic corticotropin-releasing hormone, thyrotropin-releasing hormone, growth hormone-releasing hormone and somatostatin in rats injected with Freund's adjuvant. Biol Signals Recept. 1999, 8: 178-190. 10.1159/000014588.
Cardinali D, Della Maggiore V, Selgas L, Esquifino A: Diurnal rhythm in ornithine decarboxylase activity and noradrenergic and cholinergic markers in rat submaxillary lymph nodes. Brain Res. 1996, 711: 153-162. 10.1016/0006-8993(95)01346-6.
Neidhart M: Bromocriptine microcapsules inhibit ornithine decarboxylase activity induced by Freund's complete adjuvant in lymphoid tissues of male rats. Endocrinology. 1989, 125: 2846-2852.
Selgas L, Pazo D, Arce A, Esquifino AI, Cardinali DP: Circadian rhythms in adenohypophysial hormone levels and hypothalamic monoamine turnover in mycobacterial-adjuvant-injected rats. Biol Signals. 1998, 7: 15-24. 10.1159/000014524.
Pearson CM: Development of arthritis, periarthritis and periostitis in rats given adjuvant. Proc Soc Exp Biol Med. 1956, 91: 95-103.
Holoshitz J, Matiau J, Cohen I: Arthritis induced in rats by cloned T lymphocytes responsive to mycobacteria but not to collagen type II. J Clin Invest. 1984, 73: 211-215.
Knight B, Katz DR, Isenberg DA, Ibrahim MA, Le Page S, Hutchings P, et al: Induction of adjuvant arthritis in mice. Clin Exp Immunol. 1992, 90: 459-465.
Tanaka H, Ueta Y, Yamashita U, Kannan H, Yamashita H: Biphasic changes in behavioral, endocrine, and sympathetic systems in adjuvant arthritis in Lewis rats. Brain Res Bull. 1996, 39: 33-37. 10.1016/0361-9230(95)02037-3.
Lussier A, De Medicis R, Tetreault L: Adjuvant arthritis: influence of the adjuvant volume and composition on the non-specific inflammation. Int J Tissue React. 1981, 3: 11-15.
Kleinau S, Erlandsson H, Holmdahl R, Klareskog L: Adjuvant oils induce arthritis in the DA rat. I. Characterization of the disease and evidence for an immunological involvement. J Autoimmun. 1991, 4: 871-880.
Lorentzen JC, Glaser A, Jacobsson L, Galli J, Fakhrai-rad H, Klareskog L, et al: Identification of rat susceptibility loci for adjuvant-oil-induced arthritis. Proc Natl Acad Sci USA. 1998, 95: 6383-6387. 10.1073/pnas.95.11.6383.
Cardinali DP, Brusco LI, Garcia BM, Esquifino AI: Effect of melatonin on 24-hour rhythms of ornithine decarboxylase activity and norepinephrine and acetylcholine synthesis in submaxillary lymph nodes and spleen of young and aged rats. Neuroendocrinology. 1998, 67: 349-362. 10.1159/000054333.
Moreno ML, Villanua MA, Esquifino AI: Serum prolactin and luteinizing hormone levels and the activities of hypothalamic monoamine oxidase A and B and phenylethanolamine-N-methyl transferase are changed during sexual maturation in male rats treated neonatally with melatonin. J Pineal Res. 1992, 13: 167-173.
Thiblin I, Finn A, Ross SB, Stenfors C: Increased dopaminergic and 5-hydroxytryptaminergic activities in male rat brain following long-term treatment with anabolic androgenic steroids. Br J Pharmacol. 1999, 126: 1301-1306.
Nelson W, Tong YL, Lee JK, Halberg F: Methods for cosinor-rhythmometry. Chronobiohgia. 1979, 6: 305-323.
Cornélissen G, Halberg F, Stebbings J, Halberg E, Caradente F, Bartholomew H: Chronobiometry with pocket calculators and computer systems. La Ricerca din Lab. 1980, 10: 333-385.
Acknowledgements
This work was supported by grants from DGES, PB97-0257, Spain, University of Buenos Aires, Beca Ramón Carrillo – Arturo Oñativia, Ministerio de Salud, Argentina and Agencia Nacional de Promoción Científica y Tecnológica, Argentina (PICT 6153).
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Cano, P., Cardinali, D.P., Chacon, F. et al. Age-dependent changes in 24-hour rhythms of catecholamine content and turnover in hypothalamus, corpus striatum and pituitary gland of rats injected with Freund's adjuvant. BMC Physiol 1, 14 (2001). https://doi.org/10.1186/1472-6793-1-14
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-6793-1-14