Abstract
Background
In Denmark and many other European countries, harvest records suggest a marked decline in European brown hare numbers, a decline often attributed to the agricultural practice. In the present study, we analyse the association between agricultural land-use, predator abundance and winter severity on the number of European brown hares harvested in Denmark in the years 1955 through 2000.
Results
Winter cereals had a significant negative association with European brown hare numbers. In contrast to this, root crop area was positively related to their numbers. Remaining crop categories were not significantly associated with the European brown hare numbers, though grass out of rotation tended to be positively related. The areas of root crop production and of grass out of rotation have been reduced by approximately 80% and 50%, respectively, while the area of winter cereals has increased markedly (>70%). However, European brown hare numbers were primarily negatively associated with the number of red fox. Finally, we also found a positive association between mild winters and European brown hare numbers.
Conclusion
The decline of Danish European brown hare populations can mainly be attributed to predation by red fox, but the development in agricultural land-use during the last 45 years have also affected the European brown hare numbers negatively. Additionally, though mild winters were beneficial to European brown hares, the increasing frequency of mild winters during the study period was insufficient to reverse the negative population trend.
Similar content being viewed by others
Background
In most countries in Western Europe, the landscape has undergone dramatic changes during the last century due to changes in the agricultural practices. In Denmark, both the uncultivated land and the semi-cultivated land, such as permanent grass areas, have been reduced dramatically, reflecting the general intensification of agriculture [1]. Additionally, fields have become larger, which has resulted in widespread fragmentation of remaining habitats, and today the landscape appears as a mosaic of natural habitats surrounded by cultivated land [1, 2]. These changes in agriculture have affected a number of wildlife species living in this man-made landscape. For instance, the shift in agricultural practice has severely influenced the diversity and abundance of insects with concomitant consequences for the dynamics of a wide range of farmland birds [3, 4], including the grey partridge (Perdix perdix) [5]. Among mammals, the European brown hare (Lepus europaeus) in particular has experienced a dramatic decline in many European countries [reviewed by [6]], including Denmark [7, 8].
Despite its currently declining numbers, the European brown hare is still common and one of the most important game species throughout the country [8]. The dynamics of European brown hares seem resilient to even heavy hunting pressure [9], though local population dynamic data may be needed to ensure sustainable harvest [10]. In Denmark hunting of European brown hares is generally assumed to be without regulating effect [8]. The European brown hare is a typical grass steppe herbivore, and inhabits primarily open landscapes, including cultivated farmland [11], which is the predominant landform in Denmark [1]. The species is rather sedentary, and has generally small home ranges [e.g. [12]]. This site fidelity makes European brown hares highly susceptible to changes in their surrounding habitats, and the general decline in the European brown hare populations in Europe is mainly being attributed to changes in agriculture practice and land-use [reviewed by [6, 12]].
European brown hares are important prey primarily for mammalian predators. In Northern Europe, the red fox (Vulpes vulpes) is the main predator on European brown hares, and foxes have been reported to influence the dynamics of several European brown hare populations substantially [13–17]. Also, infectious diseases such as the European brown hare syndrome virus, pseudotuberculosis, pasteurellosis and coccidiosis are present in many European brown hare populations [18–20]. Haerer et al. [19], however, concluded that diseases were not responsible for the decline of brown hare populations in Switzerland. Similarly, Frölich et al. [20] found that compared to red foxes, infectious diseases seemed to play a minor role in the dynamics of European brown hare populations in Germany.
An increasing number of papers have documented the importance of climate for a number of life history traits and abundance of many terrestrial vertebrates [21], and European brown hare populations are affected negatively by cold winters and cold springs [12, 22–24]. Factors regulating vertebrate populations may, however, exhibit large spatial variation, and even among proximate populations spatial variation and gradients in vertebrate population dynamics may exist [e.g. [25]].
In this study, we analyse and contrast the simultaneous associations between agricultural land-use, the number of red fox harvested, winter severity and the population dynamics of European brown hares across 11 Danish districts during 45 years.
Results
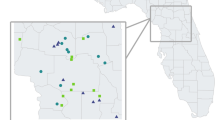
In the period 1955–2000 the European brown hare harvest record declined steadily in all the Danish districts but one: On the island Bornholm the European brown hare population declined until the late 1980s, but has increased markedly since then, and has now reached a level higher than that of 1955 (Fig. 1).
We found statistical significant direct density-dependence (X t-1 ) in the European brown hare time series (G = 281.4, df = 2, P < 0.0001). Additionally, the effect of district was also significant (G = 769.0, df = 1, P < 0.0001).
From 1955 to 2000, agricultural land-use changed markedly in Denmark, resulting in large temporal shifts in the areas covered by the different crop categories (Fig. 2). The areas covered with grass and green fodder in rotation and in particular the areas out of rotation have been reduced, the latter by approximately 50% since the mid 1950s. An even more dramatic decline has been observed for the root crops in the same period, a decline by more than 80% (Fig. 2). In Storstrøm, however, the area covered with root crops has been relatively stable over the years. In general, cereals were the dominant crop category in 1955 through 2000, with a shift from a predominance of spring cereals to winter cereals in the 1980s (Fig. 2).
The areas covered with winter cereals had a marked negative association with the number of European brown hares (Table 2), whereas root crops had a marked positive relation. Neither spring cereals, nor winter and spring rape seemed to be associated with European brown hare numbers (Table 2). Similarly, neither grass areas in or out of rotation were significantly related to European brown hare numbers, though the latter tended to have a positive association (Table 2).
The number of red foxes harvested in year t-1 had a marked negative effect on the number of European brown hares harvested the following year (Table 2), whereas red fox number in year t seemed unimportant for the European brown hare numbers.
Mild winters, i.e. high winter state of the large-scale climatic phenomenon the North Atlantic Oscillation [NAO; [26]], had a significant positive effect on the European brown hare numbers (Table 2).
Discussion
Despite its high reproductive potential [e.g. [27]], the Danish European brown hare has, according to annual harvest records, declined dramatically since 1955. Studies of Danish European brown hare populations indicate that its reproductive success is low compared to that of con-specifics in other countries [27], and has, in turn, declined from the 1950s to the 1990s [28]. Hansen [27] suggested that the low reproductive success observed in Danish European brown hares might be attributed to the agricultural practice. Using data covering almost half a century, our analyses suggest that the dramatic decline in the Danish European brown hares can be attributed mainly to the negative effect of red foxes, but also to the agricultural land-use. The area of winter cereal production has increased dramatically during the last century, and became the dominating crop in the early 1990s. We also found a significant positive association between root crops, a crop type that has declined dramatically in the second half of the last century, and European brown hare numbers.
European brown hares mainly forage on grasses and herbs [12], and cereals such as wheat are preferred during winter [29, 30], which seems to contradict the results of our analyses. However, as European brown hares choose to feed on specific crops according to plant phenology [12, 31], cereals, although important in winter, still occupy large areas when no longer of nutritional value to European brown hares, which may result in low availability of food especially during summer. Similarly, rape is avoided in the diet [29], but European brown hares may spend a substantial fraction of their time in rape fields during winter [30] prior to the development of glucosinulates [see [29]]. Apart from winter cereals and root crops, the crop categories did not affect the European brown hare numbers significantly. The lacking effect of grass and green fodder areas, especially those out of rotation, was unexpected as other studies have shown that hares prefer grass areas year-round [12, 30]. This, however, may be attributed to the fact that we were unable to separate grass areas into those e.g. with and without cattle, factors that might affect European brown hare use of grass areas [12].
In a recent study, Fox [32] showed that farmland birds seemed to benefit from the reduced application of pesticides and inorganic fertilisers seen in Denmark since the early 1980s. The continuing decline in European brown hare numbers in that period therefore suggests that hares are not directly affected by the use of such substances, but rather respond to the loss of suitable habitat and space. European brown hares mainly move along field margins [12, 33], and the increasing field size [34] combined with the general loss of suitable habitats possibly force hares to aggregate in the remaining patches of non-agricultural and non-urbanized land. Here, density-dependence may perpetuate the negative population development as European brown hares aggregate in the few, remaining pockets of suitable habitat. In line with this, Frylestam [23] reported an inverse relationship between fertility and density in European brown hares, which he attributed to shortage of food at least in some parts of the year, which also has been suggested in a number of other studies [12, 24, 27]. Hence, agricultural land-use, especially the increasing use of winter cereals and the marked reduction in root crops, but probably also grass areas out of rotation, seem to have contributed to the European brown hare decline in Denmark. However, the single-most important parameter for the number of European brown hares was the number of red foxes. Hence, our results are consistent with other studies reporting that red foxes may affect European brown hare populations negatively through predation [13–17]. This relationship is also particular evident from the positive development in the European brown hare population on the island Bornholm following the outbreak of sarcoptic mange there [35]. To elaborate the fox-hare interaction further, we reran the analyses including all variables but the red fox variables, and added the delayed AR term (i.e. X t-2 ). In seven of the 11 districts the delayed density dependence was significant (P < 0.05), suggesting important inter-specific interactions [36, 37]. The significant association with red fox numbers in year t-1 (and not year t ) most likely reflects that compared to adults, juvenile European brown hares suffer more from predation [e.g. [19]]. Hence, high red fox numbers in year t result in low harvest of European brown hares in year t , which affects the reproductive potential of the populations, and, hence, the number of European brown hares the following year (year t+1 ). Also, the significant effect of district may point to differences in habitat quality, but also differences in the history of the sarcoptic mange, i.e. time since the eruption of the mange, among districts [see [35]].
Both European brown hare over-winter survival [24] and reproductive rate [23] increases with temperature, which may be attributed to improved forage availability following mild winters [see [38, 39]]. Our analyses revealed similar results as mild winters affected the European brown hares positively. There may, however, also be a negative effect of mild winters, namely through transmission of diseases and parasites, which may be enhanced under mild climatic conditions [18]. Nevertheless, the overall effect of mild winters seemed positive. The upward trend in the NAO since the 1960s [40], however, was not sufficient to reverse the European brown hare decline, but may have decelerated it.
Conclusion
Our analyses have provided important insight into the structure of the European brown hare dynamics in Denmark, and documented important patterns within the mechanisms regulating European brown hares. Hence, we have documented that the decline of European brown hares in Denmark mainly can be attributed to predation by red fox, but also to changes in agricultural land-use. Additionally, though mild winters were beneficial to European brown hares, the increasing frequency of mild winters during the study period was insufficient to reverse the negative population trend.
Methods
European brown hare and red fox density indices
As indices of European brown hare and red fox density, we used the harvest records from 14 Danish counties from 1955–2000. In 1970, the geographical borders of some of the counties were changed, which in two cases lead to substantial changes in area [7]. This, together with inconsistency in crop registration, forced us to lump together some counties, and we therefore present analyses of European brown hare harvest records from 11 districts (Fig. 1). European brown hare and red fox harvest records were expressed as the number of animals shot per hectare per year. Harvest records may seem rather crude indicators of density compared to e.g. spotlight surveys and line transects [see [41]]. Unfortunately, no alternative indices of European brown hare density in Denmark are available. Harvest records, however, may still be useful indicators of the long-term trends in European brown hare numbers [41].
Sarcoptic mange was first encountered in Denmark in the early 1980s, and is now present in seven of the 11 districts [35]. In one district (Bornholm; Fig. 1), the disease has practically eliminated the entire red fox population, and red fox hunting has been prohibited here since 1991. Consequently, we restrict our analyses of the Bornholm district to 1955 through 1990. In all other districts, red fox hunting is open from 1 September to 31 January, and for European brown hares from 1 October to 31 December. Neither red fox nor European brown hare hunting is quota-based.
Agricultural land-use
Time series quantifying agricultural land-use data, i.e. annual crop areas in hectares, covering the period 1955–81 were obtained from Statistics Denmark. Data for the period 1982–2000 were obtained from the official website of Statistics Denmark http://www.statistikbanken.dk. The annual crop data were grouped into seven categories (Table 1).
No data on the fraction of winter and spring cereals, respectively, were available for 1955–61, and these data points were omitted in the analyses. Similarly, prior to 1982 no data on the fraction of winter barley and spring barley were available. However up until 1968, winter barley was only sown on a few thousand hectares in Denmark, when it was forbidden due to its function as reservoir for mildew attacking spring barley [42]. The use of mildew resistant winter barley was permitted in 1982. Hence, from 1955–81 barley was regarded as spring barley. Finally, no data on the fraction of winter and spring rye are available from 1955–61 and 1979–98, but in Denmark spring rye is generally sown on very few hectares only [42], and we regarded rye as winter rye unless otherwise specified [see also [32]]. Oat is sown as spring crop only. No data on the areas covered with grass and green fodder in or out of rotation were available for 1994, and the data point was interpolated, i.e. 1994 equalled the average of 1993 and 1995.
Climatic variability
As an index of winter severity, we used the winter state of the North Atlantic Oscillation [NAO; [26]]. The climatic phenomenon NAO is defined as the difference in sea-level pressure between Portugal and Iceland, affecting the temperature, precipitation and wind across the Northern Hemisphere [26, 43, 44]. The NAO, thus, integrates the effects of a number of abiotic factors, and therefore seems particularly useful when analysing the dynamics of larger, endothermic animals with relatively large buffer-capacity against climatic variability.
The relationship between the winter NAO (December t-1 through March t ) and temperature, precipitation and wind is particularly strong in Northern Europe [45]. When NAO is in its high state, the winter climate in Northern Europe is warm, wet, and windy, while when in its low state winters are cold and dry [44]. The NAO winter index has shown an upward trend since the 1960s, which accounts for a substantial fraction of the general warming in the Northern Hemisphere [40]. The NAO index data are available from the Climate Analysis Section, National Center for Atmospheric Research, USA http://www.cgd.ucar.edu/~jhurrell/nao.html.
Data analyses
To remove heteroscedasticity in the system, data were loge-transformed, i.e. X (t) = ln(N (t) + 1), where N (t) is the number of European brown hares harvested in year (t) . To allow the direct comparison of regression coefficients, variables were standardized prior to the analyses (i.e., [X (t) -µ(1955–2000)]/s(1955–2000)). Regression coefficients therefore express the rate of change in standard deviation units of the independent variable per one standard deviation unit of the dependent variable [46]. To obtain stationarity in the time series [see [47] for details], data were detrended following [48] by including year as covariate in all models.
For each district, we tested for the presence of multi-collinearity among parameters prior to the analyses by means of condition indices and variance proportions [49], but multi-collinearity was not observed in any districts (condition index < 12.47; [see [49] for details]).
The European brown hare time series were tested for non-linearity in X (t) vs. X (t-1) by applying the likelihood ratio test [50]. In all districts, linearity in X (t) vs. X (t-1) was not rejected λ< 7.76, P > 0.05). We analysed the data using a first-order autoregressive (AR(1)) mixed model approach with district as fixed factor:
X (t) ~(µ,V),
where

and

where LAND_USE represents the seven crop categories (see Table 1). Because of the inclusion of red fox numbers, we did not include a delayed AR term (i.e. X t-2 ), as inclusion of both delayed density dependence and predator abundance can be seen as redundant [37]. All analyses were performed in SAS 8.2, using the PROC MIXED procedure with restricted maximum-likelihood estimation of regression coefficients [49]. Model reduction was conducted using log-likelihood ratio tests [51].
References
Bach H, Christensen N, Kristensen P: 2002, Copenhagen, Denmark, National Environmental Research Institute, 409: 1-368.The state of the environment in Denmark,Technical report,
Caspersen OH: Landbrug og landskab fra 1800 til 2000. Grænser i landskabet. Edited by: HelsT, FrandsenJN, FritzbøgerB and OlesenCR. 2001, Denmark, Odense University Press, 111-134.
Fuller RJ, Gregory RD, Gibbons DW, Marchant JH, Wilson JD, Carter N: Population declines and range concentrations among lowland farmland birds in Britian. Conserv Biol. 1995, 9: 1425-1441. 10.1046/j.1523-1739.1995.09061425.x.
Chamberlain DE, Fuller RJ, Bunce RGH, Duckworth JC, Shrubb M: Changes in abundance of farmland birds in relation to the timing of agricultural intensification in England and Wales. J Appl Ecol. 2000, 37: 771-788. 10.1046/j.1365-2664.2000.00548.x.
Benton TG, Bryant DM, Cole L, Crick HQP: Linking agricultural practice to insect and bird populations: a historical study over three decades. J Appl Ecol. 2002, 39: 673-687. 10.1046/j.1365-2664.2002.00745.x.
Edwards PJ, Fletcher MR, Berny P: Review of the factors affecting the decline of the European brown hare, Lepus europaeus (Pallas, 1778) and the use of wildlife incident data to evaluate the significance of paraguat. Agr Ecosyst Environ. 2000, 79: 95-103. 10.1016/S0167-8809(99)00153-X.
Strandgaard H, Asferg T: The Danish bag record II. Danish Review of Game Biology. 1980, 11: 1-112.
Madsen J, Asferg T, Clausager I, Noer H: 1996, Copenhagen, Denmark, National Environmental Research Institute, 6: 1-112.Status og jagttider for danske vildtarter,Technical report,
Pepin D: Variation in survival of brown hare (Lepus europaeus) leverets from different farmland areas in the Paris Basin (France). J Appl Ecol. 1989, 26: 13-24.
Marboutin E, bray Y, Péroux R, Mauvy B, Lartiges A: Population dynamics in European hare: breeding parameters and sustainable harvest rates. J Appl Ecol. 2004, 40: 580-591. 10.1046/j.1365-2664.2003.00813.x.
Frylestam B: Utilisation of farmland habitats by European hares (Lepus europaeus, Pallas) in southern Sweden. Swedish Wildlife Research. 1980, 11: 271-284.
Tapper SC, Barnes RFW: Influence of farming practice on the ecology of the Brown hare (Lepus europaeus). J Appl Ecol. 1986, 23: 39-52.
Pielowski Z: The role of foxes in the reduction of the European hare population. Ecology and management of European hare populations. Edited by: PielowskiZ and PucekZ. 1976, Warszawa, Polish Hunting Association, 135-148.
Lindström E, Andrèn H, Angelstam P, Widèn P: Influence of predators on Hare populations in Sweden: a critical review. Mammal Rev. 1986, 16: 151-156.
Goszczynski J, Wasilewski M: Predation of foxes on a hare population in central Poland. Acta Theriol. 1992, 4: 329-338.
Reynolds JC, Tapper SC: Predation by foxes Vulpes vulpes on brown hares Lepus europaeus in central southern England, and its potential impact on annual population growth. Wildlife Biol. 1995, 1: 145-158.
Reynolds JC, Tapper SC: The ecology of the red fox Vulpes vulpes in relation to small game in rural southern England. Wildlife Biol. 1995, 1: 105-117.
Rattenborg EE: Diseases in the hare (Lepus europaeus) population in Denmark - epidemiological description and analyses of post-mortem data. 1994, The Royal Veterinary & Agricultural University, Copenhagen, Denmark, 1-114.
Haerer G, Nicolet J, Bacciarini L, Gottstein B, Giacometti M: Causes of mortality, zoonoses and reproductive performance in European brown hare in Switzerland. Schweizer Archiv Für Tierheilkunde. 2001, 143: 193-201.
Frolich K, Wisser J, Schmüser H, Fehlberg U, Neubauer H, Grunow R, Nikolaou K, Priemer J, Thiede S, Streich WJ, Speck S: Epizootiologic and ecologic investigations of European brown hares (Lepus europaeus) in selected populations from Schleswig-Holstein, Germany. J Wild Dis. 2003, 39: 751-761.
Mysterud A, Stenseth NC, Yoccoz NG, Ottersen G, Langvatn R: The response of terrestrial ecosystems to climate variability associated with the North Atlantic Oscillation. The North Atlantic Oscillation - climatic significance and environmental impact. Edited by: Hurrell James W, Kushnir Yochanan, Ottersen Geir and Visbeck Martin. 2003, Washington, DC, American Geophysical Union, 235-262.
Andersen J: Studies in Danish Hare populations. I. Population fluctuations. Danish Review of Game Biology. 1957, 3: 85-131.
Frylestam B: Structure, size, and dynamics of three European Hare populations in Southern Sweden. Acta Theriol. 1979, 24: 449-464.
Marboutin E, Hansen K: Survival rates in a nonharvested Brown hare population. J Wildl Manage. 1998, 62: 772-779.
Stenseth NC, Bjørnstad ON, Saitoh T: Seasonal forcing on the dynamics of Clethrionomys rufocanos: Modelling geographic gradients in population dynamics. Res Popul Ecol. 1998, 40: 85-95.
Hurrell JW: Decadal trends in the North Atlantic Oscillation: Regional temperatures and precipitation. Science. 1995, 269: 676-679.
Hansen K: Reproduction in European hare in a Danish farmland. Acta Theriol. 1992, 37: 27-40.
Hansen K, Hartmann P: 1994, Copenhagen, Denmark, Ministry of Environment and Energy, 125: 1-20.Unge harer i jagtudbyttet - en indikator for bestandens tilstand,Technical report,
Frylestam B: Agricultural land use effects on the winter diet of Brown Hares (Lepus europaeus Pallas) in southern Sweden. Mammal Rev. 1986, 16: 157-161.
Hansen K, Sørensen PL, Daugaard O: Bestandsstørrelse og afgrødevalg hos hare (Lepus europaeus). 1989, Vildtbiologisk Station, Kalø, Landbrugsministeriets Vildtforvaltning, 1-12.
Chapuis JL: Comparison of the diets of two sympatric lagomorphs, Lepus europaeus Pallas and Oryctolagus cuniculus (L.) in an agrosystem of the Ile-de-France. Zeitschrift für Säugetierkunde. 1990, 55: 176-185.
Fox AD: Has Danish agriculture maintained farmland bird populations?. J Appl Ecol. 2004, 41: 427-439. 10.1111/j.0021-8901.2004.00917.x.
Lewandowski K, Nowakowski JJ: Spatial distribution of Brown hare Lepus europaeus populations in habitats of various types of agriculture. Acta Theriol. 1993, 38: 435-442.
Brandt J, Holmes E, Larsen D, Madsen MM: Småbiotoper i det danske agerland 1991. Landscape Ecological Papers. 1996, 3: 1-153.
Forchhammer MC, Asferg T: Invading parasites cause a structural shift in red fox dynamics. Proc R Soc Lond B. 2000, 267: 779-786. 10.1098/rspb.2000.1071.
Stenseth NC, Falck W, Chan KS, Bjørnstad ON, O' Donoghue M, Tong H, Boonstra R, Boutin S, Krebs CJ, Yoccoz NG: From patterns to processes: Phase and density dependencies in the Canadian lynx cycle. Proc Natl Acad Sci. 1998, 95: 15430-15435. 10.1073/pnas.95.26.15430.
Post E, Forchhammer MC: Pervasive influence of large-scale climate in the dynamics of a terrestrial vertebrate community. BMC Ecol. 2001, 1:5:
Forchhammer MC, Clutton-Brock TH, Lindstrom J, Albon SD: Climate and population density induce long-term cohort variation in a northern ungulate. J Anim Ecol. 2001, 70: 721-729. 10.1046/j.0021-8790.2001.00532.x.
Mysterud A, Stenseth NC, Yoccoz NG, Langvatn R, Steinheim G: Nonlinear effects of large-scale climatic variability on wild and domestic herbivores. Nature. 2001, 410: 1096-1099. 10.1038/35074099.
Hurrell JW, Kushnir Y, Visbeck M: The North Atlantic Oscillation. Science. 2001, 291: 603-605. 10.1126/science.1058761.
Langbein J, Hutchings MR, Harris S, Stoate C, Tapper SC, Wray S: Techniques for assessing the abundance of Brown Hares Lepus europaeus. Mammal Rev. 1999, 29: 93-116. 10.1046/j.1365-2907.1999.00040.x.
Jensen KM, Reenberg A: Landbrugsatlas Danmark. 1986, København, Det Kongelige Geografiske Selskab og C.A. Reitzels Forlag, 1-118.
Lamb PJ, Peppler RA: North Atlantic Oscillation: Concept and an application. Bull Amer Meteorol Soc. 1987, 68: 1218-1225. 10.1175/1520-0477(1987)068<1218:NAOCAA>2.0.CO;2.
Hurrell JW, Van Loon H: Decadal variations in climate associated with the North Atlantic Oscillation. Clim Change. 1997, 36: 301-326. 10.1023/A:1005314315270.
Ottersen G, Planque B, Belgrano A, Post E, Reid PC, Stenseth NC: Ecological effects of the North Atlantic Oscillation. Oecologia. 2001, 128: 1-14. 10.1007/s004420100655.
Sokal RR, Rohlf FJ: Biometrics. 1995, New York, W.H. Freeman and Company, 1-887. Third
Priestley MB: Spectral analysis and time series. 1984, London, Academic Press, 1-890. Third
Forchhammer MC, Stenseth NC, Post E, Langvatn R: Population dynamics of Norwegian red deer: density-dependence and climatic variation. Proc R Soc Lond B. 1998, 265: 341-350. 10.1098/rspb.1998.0301.
Inc. SAS Institute: The SAS system for Windows, version 8e. 2000, Cary, NC
Tong H: Non-linear time series. 1993, Oxford, Clarendon Press, 1-564.
Littel RC, Milliken GA, Stroup WW, Wolfinger RD: SAS Systems for Mixed Models. 1996, Cary, NC, USA, SAS Institute Inc.
Acknowledgements
We extend our sincere thanks to Eric Post and two anonymous referees for valuable comments and improvements to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
NMS designed the study, participated in data preparation, carried out the statistical analyses, and drafted the manuscript. TA participated in data preparation. MCF supported the data analyses and contributed to the writing. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Schmidt, N.M., Asferg, T. & Forchhammer, M.C. Long-term patterns in European brown hare population dynamics in Denmark: effects of agriculture, predation and climate. BMC Ecol 4, 15 (2004). https://doi.org/10.1186/1472-6785-4-15
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-6785-4-15