Abstract
Background
Combination of CHD (chromo-helicase-DNA binding protein)-specific polymerase chain reaction (PCR) with electrophoresis (PCR/electrophoresis) is the most common avian molecular sexing technique but it is lab-intensive and gel-required. Gender determination often fails when the difference in length between the PCR products of CHD-Z and CHD-W genes is too short to be resolved.
Results
Here, we are the first to introduce a PCR-melting curve analysis (PCR/MCA) to identify the gender of birds by genomic DNA, which is gel-free, quick, and inexpensive. Spilornis cheela hoya (S. c. hoya) and Pycnonotus sinensis (P. sinensis) were used to illustrate this novel molecular sexing technique. The difference in the length of CHD genes in S. c. hoya and P. sinensis is 13-, and 52-bp, respectively. Using Griffiths' P2/P8 primers, molecular sexing failed both in PCR/electrophoresis of S. c. hoya and in PCR/MCA of S. c. hoya and P. sinensis. In contrast, we redesigned sex-specific primers to yield 185- and 112-bp PCR products for the CHD-Z and CHD-W genes of S. c. hoya, respectively, using PCR/MCA. Using this specific primer set, at least 13 samples of S. c. hoya were examined simultaneously and the Tm peaks of CHD-Z and CHD-W PCR products were distinguished.
Conclusion
In this study, we introduced a high-throughput avian molecular sexing technique and successfully applied it to two species. This new method holds a great potential for use in high throughput sexing of other avian species, as well.
Similar content being viewed by others
Background
Real-time PCR (polymerase chain reaction) is a well-established method for RNA quantification [1, 2] and genomic DNA analysis [3–6]. Several analytical methods have been employed to detect PCR products. The most popular, the TaqMan assay [7], is a hybridization-based method with high specificity, but it is relatively expensive. Another method is based on the double-stranded DNA binding ability [8, 9] of fluorescent dye SYBR Green I. The amount of PCR products (amplicons) can be detected in real-time in both methods by measuring fluorescence levels from the cycle threshold (Ct). Both types of analyses are potentially fast and sensitive but the SYBR Green I dye is more cost-effective than the TaqMan probe.
Recently, SYBR green real-time quantitative PCR has been applied to investigate the mRNA expression in birds. For example, the dosage compensation of Z-linked gene expressions was reported [10, 11]. However, to our knowledge, real-time PCR has not been applied in genomic DNA studies such as the molecular sexing of birds. In general, the molecular sexing of birds depends on the difference in the length of CHD genes when comparing a universal CHD primer pair P2/P8 [12]. Traditionally, a single CHD-Z band is found in males and two bands in females [12] after electrophoresis (female, ZW; male, ZZ) when analyzing CHD-Z and CHD-W genes. Since real-time PCR can monitor the PCR products quantitatively and robustly without the need for gel electrophoresis, the advantages of real-time PCR can be utilized to evaluate avian molecular sexing using the P2/P8 primers. However, SYBR green real-time PCR alone cannot distinguish the individual fluorescence values from different PCR products mixed in the same well. Therefore, this disadvantage of SYBR green real-time PCR alone hinders its utility in molecular sexing using CHD-based primers.
Fortunately, the fluorescence detection problem of SYBR green real-time PCR may be hurdled by subsequent coupling with SYBR green-based melting curve analysis (MCA). MCA was originally used to confirm PCR product identity and to differentiate between specific and non-specific PCR products [13]. Although MCA can be used to differentiate between specific PCR products of CHD-Z and CHD-W genes, genes vary among species and the difference in length of the PCR products of CHD-Z and CHD-W in some cases will be too close to allow for precise sexing. For example, the length of CHD-Z and CHD-W products are too close in the case of the Black-faced Spoonbill (Platalea minor) [14], Tawny owl [12] and kiwi ratite (Apteryx spp) [15]. We found similarly close length differences in at least nine species of birds with CHD-Z and CHD-W gene records in Genbank: in the CHD-Z and CHD-W genes (accession nos. = length difference) for Accipiter gentilis AB096144, AB096143 (5-bp), Milvus migrans AB096142, AB096141 (2-bp), Circaetus gallicus AY313610, AY313609 (9-bp), Spizaetus nipalensis AB096150, AB096149 (8-bp), Gyps indicus DQ156155, DQ156156 (6-bp), Gyps bengalensis DQ156153, DQ156154 (5-bp), Accipiter nisus AB096152, AB096151 (4-bp), Circus spilonotus AB096146, AB096145 (4-bp), and Aquila chrysaetos AB096148, AB096147 (3-bp), respectively. Therefore, a difference between the bands would be too difficult to resolve using agarose gel electrophoresis. Alternatively, polyacrylamide gels may provide sufficient resolution to discriminate between CHD-Z and CHD-W products for some species [12, 15]. Yet, it is still time-consuming and it makes high-throughput screening of avian molecular sexing more difficult.
In this study, two different species of birds, Spilornis cheela hoya and Pycnonotus sinensis, were chosen as examples to evaluate the P2/P8 primer and a redesign of the sex-specific primers for CHD-Z and CHD-W genes of S. c. hoya using real-time PCR/MCA. Overall, the aim of this study was to develop a rapid and high-throughput SYBR green-based real-time PCR assay that would distinguish male and female birds via real-time PCR and unique melting curve profiles.
Methods
Sample collection and blood DNA extraction
A scientific and banding permit number 96YULI032 was issued by the Council of Agriculture of Taiwan pursuant to the Wildlife Conservation Law of Taiwan. Thirteen banded Formosan Crested Serpent Eagles (Spilornis cheela hoya, S. c. hoya), numbered Bd6 and Bds12~24, were collected with official permission from the Kenting National Park (21°55' N and 120°49' E), Taiwan. Bd6 and Bd15 of S. c. hoya were used for the female positive control of molecular sexing based upon their behavior of playing parts in incubation, brooding and feeding. Two Chinese Bulbuls (Pycnonotus sinensis, P. sinensis) numbered 3424 (female) and 927 (male) were provided by the Taiwan Endemic Species Research Institute. Blood DNA was extracted immediately after sample collection using a Qiagen blood kit and stored at -20°C until needed.
PCR and primary molecular sexing by P2/P8 primers
The universal P2/P8 primer pair [12] for avian molecular sexing was used for S. c. hoya and P. sinensis species. The primer sequence information is: forward primer P2 (5'-TCTGCATCGCTAAATCCTTT-3') and the reverse primer P8 (5'-CTCCCAAGGATGAGRAAYTG-3'). DNA samples were added to a modified PCR reaction mixture (10 μl) containing 1× PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.7U Taq enzyme (Invitrogen corp.), 0.16 μM primers mix (1:1), and 10–20 ng DNA. The PCR program was followed: 94°C (4 min); 5 cycles of 94°C (30 s), 47°C (30 s), 72°C (30 s); 49 cycles of 94°C for (30 s), 46°C (20 s), 72°C (20 s); and 72°C (5 min).
TA cloning and nucleotide sequence analysis for CHD-Z and CHD-W genes
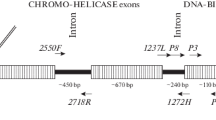
For S. c. hoya, P2/P8 PCR products were gel isolated (MiniElute gel extraction kit, QIAGEN), cloned (pGEM -T Easy TA Cloning, Promega) and sequenced. The female and male birds were selected for CHD-W and CHD-Z abundant sources because the CHD-Z and CHD-W PCR products were hard to resolve in regular agarose gel. The sequencing results for the P2/P8 PCR products of S. c. hoya are shown in Fig. 1. For P. sinensis, the CHD-Z and CHD-W PCR products were easy to resolve in regular agarose gel without TA cloning technique. The length difference between CHD-Z and CHD-W PCR products using P2/P8 primers is 13- and 52-bp for S. c. hoya and P. sinensis, respectively.
Secondary molecular sexing
An alignment performed with Biology Workbench 3.2 at the San Diego Supercomputer Center (SDSC) [16] is shown in Fig. 1. The primers for CHD-Z- and CHD-W-specific PCR of S. c. hoya were redesigned as follows: CHD-W-F: 5-GAGATGGAGTCACTATCAGATCC-3, CHD-W-R: 5-GGTTTTCACACATGGCACA-3; CHD-Z-F: 5-CATTAAAGCTGATCTGGAATTTC, CHD-Z-R: 5-TTTTTTCCTTTTCTGAACACATATTT-3. The lengths for PCR products amplified by CHD-W-F/CHD-W-R and CHD-W-F/CHD-W-R are 185- and 112-bp, respectively. The same PCR conditions as described above were used and the PCR program was modified: denaturation for 3 min at 95°C followed by 45 cycles of denaturation at 95°C for 30 s; annealing at 58°C for 30 s; and extension at 72°C for 20 s.
Real-time PCR and melting curve analysis (MCA)
Real-time PCR was performed using MyiQ (Bio-Rad Laboratories, Hercules, CA). The programs for each PCR reaction, including P2/P8 primers for S. c. hoya and P. sinensis, and CHD-Z- and CHD-W-specific primers for S. c. hoya, are described above. After completion of the PCR reaction, a melting curve was recorded by holding at 95°C for 1 min, cooling to 55°C 1 min, and then heating slowly at 0.5°C/s until 95°C under maximal ramp rate by default in MyiQ instrument (80 repeats of counts). The melting peaks were plotted by the -dF/dT versus T formula (F is fluorescence; T is temperature).
Control analysis of amplification product
To confirm the presence and purity of amplicons, PCR products were resolved on a 4% agarose gel, stained with ethidium bromide, and photographed.
Results
Molecular sexing of S. c. hoya and P. sinensis using P2/P8 primers and its gel electrophoresis
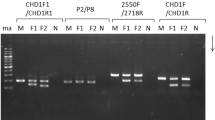
To test the feasibility of the P2/P8 primers in sexing of S. c. hoya and P. sinensis, we first performed a regular PCR reaction using this primer pair followed by gel electrophoresis, as shown in Fig. 2. For S. c. hoya, the CHD-W and CHD-Z gene products were unsolved in 3% agarose gel (Fig. 2A). After cloning as described in Materials and Methods, the CHD-W and CHD-Z genes of S. c. hoya were sequenced and submitted to Genbank with accession nos. DQ885238 and DQ885237, respectively. The difference in length (13-bp) between these CHD-W and CHD-Z genes of S. c. hoya was too short to resolve in 4% agarose gel. In contrast, in the case of P. sinensis, two distinct PCR products corresponding to CHD-Z and CHD-W genes were amplified and could be resolved in regular agarose gels (Fig. 2B). These two bands—i.e. CHD-W and CHD-Z gene products—were purified, sequenced and submitted to Genbank with accession nos. EF582413 and EF582412, respectively. There is a 52-bp length difference between the CHD-W and CHD-Z genes of P. sinensis. Accordingly, the P2/P8 primer set is informative for molecular sexing of P. sinensis but it is not suitable for the sex-specific primers for S. c. hoya. In short, the gender identification of S. c. hoya needs to be improved.
Real-time PCR/MCA of S. c. hoya and P. sinensis using P2/P8 primers
Real-time PCR analyses for S. c. hoya (Fig. 3A) and P. sinensis (Fig. 3C) demonstrated that the PCR reactions were successful. Reaction products were also confirmed by gel electrophoresis (data not shown). However, SYBR green-fluorescence of PCR products for CHD-Z and CHD-W genes were counted totally rather than individually. Consequently, the real-time PCR results cannot clearly provide evidence that CHD-Z or/and CHD-W genes are present individually or are present in both of them.
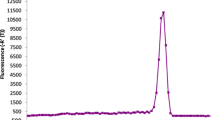
Real-time PCR/MCA using primer sets P2/P8. DNAs were chosen from S. c. hoya sample Bd6 (A, B) and P. sinensis sample 3424 (C, D), which are the ecology female control. (A, C) Real-time PCR data. (B, D) Data of MCA. The Tm of P2/P8 PCR products of S. c. hoya and P. sinensis is about 80.0 and 80.5°C, respectively. These data are collected from duplicated experiments. RFU, Relative fluorescence unit.
After performing MCA on the PCR products of CHD-Z and CHD-W genes of S. c. hoya amplified by P2/P8 primers in the same well, we found that there was a single peak (Tm) at 80°C for the P2/P8 PCR products in S. c. hoya, which could be distinguished from a nonspecific amplification with Tm at 72-74°C (Fig. 3B). The nonspecific PCR products were the primer-dimers confirmed by gel electrophoresis (data not shown). To our surprise, only a single peak at 80.5°C was found in P. sinensis for P2/P8 amplification (Fig. 3D) although the length difference of CHD-Z and CHD-W was 52-bp and two distinct bands were visualized (as shown in Fig. 2B). Therefore, it is unsuitable to perform SYBR green-based real-time PCR with MCA by P2/P8 primers in the example of S. c. hoya and P. sinensis.
Redesign of sex-specific primers in S. c. hoya
In order to improve molecular sexing using real-time PCR and melting curve assay, we redesigned the sex-specific primers. In the example of S. c. hoya, we aligned CHD-Z and CHD-W gene sequences from Genbank accession nos. DQ885238 and DQ885237 and designed sex-specific primers for both of them (Fig. 1). The PCR products of CHD-Z and CHD-W genes are 185-bp and 112-bp, respectively. This elongated length difference between each of them was designed to make it more suitable for melting curve assay. Since the primer sets for CHD-Z and CHD-W genes overlapped, it was not suitable to perform PCR reactions in the same PCR-well (data not shown).
Validation of sex-specific primers in S. c. hoya
To examine the effect of sex-specific primers on the molecular sexing by our protocol, we performed PCR reactions using CHD-Z and CHD-W primers in different PCR-wells. With our protocol, the female bird (ZW genotype) is positive for both PCR reactions using CHD-Z-F/R and CHD-W-F/R primers. However, the male bird (ZZ genotype) is positive only for PCR reactions using CHD-Z-F/R primers and not for PCR reactions using CHD-W-F/R primers. For instance, the predicted female birds named Bd15 and Bd17 showed a single band for both CHD-W (Fig. 4A) and CHD-Z (Fig. 4B) PCR products with correct size 185-bp and 112-bp, respectively. For predicted male birds, the Bd16, Bd21 and Bd22 showed a single band of CHD-Z PCR products with the correct size of 112-bp (Fig. 4B). In this case, the male birds showed some weak bands for non-specific PCR products and primer-dimers using CHD-W-F/R primers (Fig. 4A). The size of these non-specific PCR products was not unlike that of CHD-W-F/R primers. Some of these bands (marked with a star symbol) were sequenced and found to be unrelated to CHD genes by BLAST analysis (data not shown).
Representative gel view and MCA using sex-specific primers of S. c. hoya. PCR products with 185- and 112-bp amplified by (A) CHD-W-F/R and (B) CHD-Z-F/R primers in different PCR-wells were run in 4% agarose gel, respectively. The arrow of Fig. 4A indicates the remaining primers or primer-dimers of PCR. Non-specific products of W-185 indicated with star symbols were only occurred in male samples. (C, D) demonstration for high-throughput molecular sexing of multiple S. c. hoya samples using MCA. All data are performed in duplicate. (C) 7 females (Bds 12, 14, 15, 17, 19, 20, and 23) and (D) 6 males (Bds 13, 16, 18, 21, 22, and 24) were included. Z-112 and W-185 represent the primer mixtures for CHD-Z-F/CHD-Z-R (112-bp) and CHD-W-F/CHD-W-R (185-bp). The Tm value for Z-112 and W-185 is 75.0°C and 79°C, respectively. The Tm of non-specific products of W-185 is greatly larger than 79°C.
High-throughput molecular sexing of S. c. hoya- Real-time PCR/MCA
As real-time PCR is inherent with the high-throughput feature, we further evaluated the potential of our protocol for high-throughput avian molecular sexing by performing real-time PCR reactions on 13 different samples simultaneously. As shown in Fig. 4C, seven different individuals of S. c. hoya were identified to be females, as they exhibited both CHD-Z- and CHD-W-specific peaks at 75°C (Z-112) and 79°C (W-185), respectively. On the other hand, six different individuals of S. c. hoya were identified as males, as only the CHD-Z-specific peak at 75°C was shown in Fig. 4D. Furthermore, it was noted that the non-specific products (W-185) amplified by CHD-W-specific primers were discriminate to the CHD-Z- and CHD-W-specific products because of the wrong Tm value (Fig. 4D). Taken together, although only thirteen samples of S. c. hoya were tested in this study, our results clearly demonstrate the feasibility of our protocol for high-throughput molecular sexing of birds. 4). Further details on all the tested samples shown in Fig. 4 are shown individually in the supplementary information (see additional file 1).
Discussion
Application of real-time PCR/MCA for traditional PCR-electrophoresis methods using CHD gene-related primers
P2/P8 [12], 1237L/1272H [17] and 2550F/2718R [18] primer sets have been reported in the molecular sexing of birds. However, they no study has used melting curve asssay for high-throughput molecular sexing. Theoretically, MCA is able to provide the Tm value for each PCR product even if different PCR products are mixed in the same well. The use of MCA eliminates the necessity for agarose gel electrophoresis because the melting temperature (Tm) of the specific amplicon is analogous to the detection of an electrophoretic band. In this study, we focused on the issue in the case of P2/P8 primers. The fact that MCA cannot detect the individual PCR products of CHD-Z and CHD-W genes by P2/P8 primers could be due to the short length difference (13-bp for S. c. hoya) between these two DNA fragments (Fig. 3B).
Recently, the lengths of CHD-W and CHD-Z genes of forty-four avian species have been reported [19]. After calculation, we found that the average length difference of CHD-W and CHD-Z genes using P2/P8 primers among these species is about 40-bp (n = 44, ranging from 10- to 80-bp). Here, we chose P. sinensis as an example to mimic the most common PCR length difference of CHD-Z and CHD-W genes using P2/P8 primers. Its PCR length difference is 52-bp but two peaks with different Tm values were still not shown by MCA (Fig. 3D). Perhaps 52-bp is still too short for resolution in melting curve assays for CHD-W and CHD-Z genes of P. sinensis. In addition, the base composition and the concentration of SYBR green may play a role in Tm value and the resolution of MCA [8]. More importantly, MCA is also dependant on base pair positioning and juxtapositioning. It is possible that MCA using P2/P8 primers cannot be applied to the sexing of most bird species. In fact, our data indicated the limitations of P2/P8 primers, underlying the necessity of sex-specific primers for avian molecular sexing. Accordingly, we chose S. c. hoya as the example to evaluate the effect of sex-specific primers (Fig. 1) in avian molecular sexing by SYBR green-based real-time PCR followed by MCA. However, these melting curve assays were performed by a MyiQ real-time PCR machine (Bio-rad). We cannot exclude the possibility that some brands of real-time PCR machines may have different resolution. Also, high resolution melting (HRM) analysis [20, 21] may resolve subtle differences even with short length differences.
Improvement for real-time PCR combined with MCA
Using our redesigned sex-specific primers CHD-Z-F/CHD-Z-R and CHD-W-F/CHD-W-R of S. c. hoya, we calculated a 73-bp length difference between two PCR products (Fig. 1). This length difference proved to be long enough for resolution by MCA as shown in the results of Fig. 4. Furthermore, this gender identification method had validated by anatomical inspection (see additional file 2). However, it is possible that cross-interference of four primers mixed together in the same well hampered optimal PCR reaction (data not shown). Therefore, PCR reaction amplified by CHD-Z-F/CHD-Z-R and CHD-W-F/CHD-W-R was analyzed in different PCR-wells. This strategy proved to be successful (Figs. 4A and 4B) to determine the gender of S. c. hoya in a high-throughput manner (Figs. 4C and 4D).
Nonspecific peaks in MCA
Nonspecific amplification is mainly caused by nonspecific primers but sometimes may also be due to a low annealing temperature and high concentrations of template DNA, Mg2+, polymerase, or dNTPs [22]. Fortunately, in our study the Tm shift derived from the PCR product polymerization can be distinguished from nonspecific amplification. Accordingly, the nonspecific amplifications have some distinct peaks in the melting curves and show some bands with different MWs from PCR products of CHD-Z and CHD-W genes (Figs. 4B and 4D).
Other methods for molecular avian sexing
Increasing evidence [15, 18, 23–25] shows that the gender of some avian species cannot be identified by the P2/P8 PCR-based protocol [12] due to the limited difference in the length of intron for CHD-Z and CHD-W. This limitation has been overcome by some methods such as redesigned PCR primers [18] and our proposed approach, as well as PCR-RFLP [23, 25], RAPD [26] and AFLP [27] fingerprintings. Some of these methods may also have the potential for high-throughput application of avian molecular sexing, but their gel electrophoresis step has to be improved. In contrast, our proposed PCR/MCA method is gel-free and only an extra SYBR green I is required compared to a regular PCR cocktail.
Conclusion
Real-time PCR using SYBR green fluorescence followed by melting temperature determination is a simple and effective way to identify the gender of birds. The essential condition for this powerful molecular sexing method is the presence of CHD-Z and CHD-W sequences allowing the design of sex-specific primers. This work serves as a novel model for the development and use of a one-step, SYBR green-based real-time PCR and melting curve procedure for the rapid and specific detection and verification of the gender of birds using sex-specific or P2/P8 primer sets. Due to its real-time nature, 96- or 384-well plates are available for the real-time PCR machine to perform molecular sexing of birds in a high-throughput manner.
Abbreviations
- NCBI:
-
National Center for Biotechnology Information
- BLAST:
-
Basic Local Alignment Search Tool
- MCA:
-
melting curve assay
- CHD:
-
chromo-helicase-DNA binding protein
References
Buckhaults P, Zhang Z, Chen YC, Wang TL, St Croix B, Saha S, Bardelli A, Morin PJ, Polyak K, Hruban RH, Velculescu VE, Shih IM: Identifying tumor origin using a gene expression-based classification map. Cancer research. 2003, 63 (14): 4144-4149.
Salani R, Beuberger I, Kurman RJ, Bristow R, Chang HW, Wang TL, Shih IM: Expression of extracellular matrix proteins in ovarian serous tumors. International Journal of Gynecological Pathology. 2007, 26 (2): 141-146. 10.1097/01.pgp.0000229994.02815.f9.
Randegger CC, Hachler H: Real-time PCR and melting curve analysis for reliable and rapid detection of SHV extended-spectrum beta-lactamases. Antimicrobial agents and chemotherapy. 2001, 45 (6): 1730-1736. 10.1128/AAC.45.6.1730-1736.2001.
Wang BG, Huang HY, Chen YC, Bristow RE, Kassauei K, Cheng CC, Roden R, Sokoll LJ, Chan DW, Shih IM: Increased plasma DNA integrity in cancer patients. Cancer research. 2003, 63 (14): 3966-3968.
Ponchel F, Toomes C, Bransfield K, Leong FT, Douglas SH, Field SL, Bell SM, Combaret V, Puisieux A, Mighell AJ, Robinson PA, Inglehearn CF, Isaacs JD, Markham AF: Real-time PCR based on SYBR-Green I fluorescence: an alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions. BMC biotechnology. 2003, 3: 18-10.1186/1472-6750-3-18.
Skow A, Mangold KA, Tajuddin M, Huntington A, Fritz B, Thomson RB, Kaul KL: Species-level identification of staphylococcal isolates by real-time PCR and melt curve analysis. Journal of clinical microbiology. 2005, 43 (6): 2876-2880. 10.1128/JCM.43.6.2876-2880.2005.
Holland PM, Abramson RD, Watson R, Gelfand DH: Detection of specific polymerase chain reaction product by utilizing the 5'----3' exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci U S A. 1991, 88 (16): 7276-7280. 10.1073/pnas.88.16.7276.
Giglio S, Monis PT, Saint CP: Demonstration of preferential binding of SYBR Green I to specific DNA fragments in real-time multiplex PCR. Nucleic Acids Res. 2003, 31 (22): e136-10.1093/nar/gng135.
Ririe KM, Rasmussen RP, Wittwer CT: Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Analytical biochemistry. 1997, 245 (2): 154-160. 10.1006/abio.1996.9916.
McQueen HA, McBride D, Miele G, Bird AP, Clinton M: Dosage compensation in birds. Curr Biol. 2001, 11 (4): 253-257. 10.1016/S0960-9822(01)00070-7.
Kuroiwa A, Yokomine T, Sasaki H, Tsudzuki M, Tanaka K, Namikawa T, Matsuda Y: Biallelic expression of Z-linked genes in male chickens. Cytogenet Genome Res. 2002, 99 (1-4): 310-314. 10.1159/000071609.
Griffiths R, Double MC, Orr K, Dawson RJ: A DNA test to sex most birds. Mol Ecol. 1998, 7 (8): 1071-1075. 10.1046/j.1365-294x.1998.00389.x.
Lyon E: Mutation detection using fluorescent hybridization probes and melting curve analysis. Expert review of molecular diagnostics. 2001, 1 (1): 92-101. 10.1586/14737159.1.1.92.
Cheng YH, Kuo TF, Lee DN, Weng CF: Sex Identification of the Black-faced Spoonbill (Platalea minor). Zoological Studies. 2006, 45 (1): 104-113.
Huynen L, Miles J, Lambert D: Unusual electrophoretic mobility of a DNA fragment of the universal 'non-ratite' sexing marker CHD allows sexing of New Zealand's endangered kiwi ratite Apteryx spp. IBIS. 2006, 148 (1): 167-168. 10.1111/j.1474-919X.2006.00474.x.
Biology Workbench 3.2 at the San Diego Supercomputer Center (SDSC) . [http://workbench.sdsc.edu/]
Kahn NW, John JST, Quinn TW: Chromosome-specific intron size differences in the avian CHD gene provide an efficient method for sex identification in birds. The Auk. 1998, 115 (4): 1074-1078.
Fridolfsson A, Ellegren H: A simple and universal method for molecular sexing of non-ratite birds. Journal of Avian Biology. 1999, 30 (1): 116-121. 10.2307/3677252.
Jensen T, Pernasetti FM, Durrant B: Conditions for rapid sex determination in 47 avian species by PCR of genomic DNA from blood, shell-membrane blood vessels, and feathers. Zoo Biology. 2003, 22: 561-571. 10.1002/zoo.10101.
Wittwer CT, Reed GH, Gundry CN, Vandersteen JG, Pryor RJ: High-resolution genotyping by amplicon melting analysis using LCGreen. Clinical chemistry. 2003, 49 (6 Pt 1): 853-860. 10.1373/49.6.853.
Montgomery J, Wittwer CT, Palais R, Zhou L: Simultaneous mutation scanning and genotyping by high-resolution DNA melting analysis. Nature protocols. 2007, 2 (1): 59-66. 10.1038/nprot.2007.10.
Carbonari M, Sbarigia D, Cibati M, Fiorilli M: Optimization of PCR performance. Trends Genet. 1993, 9 (2): 42-43. 10.1016/0168-9525(93)90177-J.
Sacchi P, Soglia D, Maione S, Meneguz G, Campora M, Rasero R: A non-invasive test for sex identification in short-toed Eagle (Circaetus gallicus). Molecular and cellular probes. 2004, 18 (3): 193-196. 10.1016/j.mcp.2004.01.002.
De volo SB, Reynolds RT, Topinka JR, May B, Antolin MF: Population genetics and genotyping for mark-recapture studies of Northern Goshawks (Accipiter gentilis) on the Kaibab plateau, Arizona. J Raptor Res. 2005, 39 (3): 286-295.
Reddy A, Prakash V, Shivaji S: A rapid, non-invasive, PCR-based method for identification of sex of the endangered Old World vultures (white-backed and long-billed vultures) - Implications for captive breeding programmes. Current Science. 2007, 92 (5): 659-662.
Wu CP, Horng YM, Wang RT, Yang KT, Huang MC: A novel sex-specific DNA marker in Columbidae birds. Theriogenology. 2007, 67 (2): 328-333. 10.1016/j.theriogenology.2006.08.001.
Huang CW, Cheng YS, Rouvier R, Yang KT, Wu CP, Huang MC: AFLP fingerprinting for paternity testing in ducks. Br Poult Sci. 2007, 48 (3): 323-330. 10.1080/00071660701370459.
Acknowledgements
This work was supported in part by the National Science Council of Taiwan under grants NSC96-2622-B-037-003-CC3 and NSC95-2622-B-037-002-CC3 to H-W Chang, and by funds from Kaohsiung Medical University for student training in summer 2007. The authors are also grateful for the help received from representatives at Kenting National Park, Taiwan and the support of real-time PCR machine from Prof. Steven Shoei-Lung Li. We are grateful for the help of Miss Samantha Benton and Mr. Joel Stocker in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Authors' contributions
H-WC and C-C Cheng designed the experiment and wrote the manuscript. C-AC and D-LG worked on melting curve assays. C-C Chang improved the real-time PCR performance. S-HS, C-HW, Y-CC worked on DNA extraction and bioinformatics. T-CC, C-TY and, C-LT provided the samples and ecological information. All authors had read and approved the final manuscript.
Electronic supplementary material
12896_2007_281_MOESM2_ESM.pdf
Additional file 2: Validation the results of our proposed PCR/MCA method by anatomical inspection. The anatomical structure and molecular gender identification of female samples of S. c. hoya were presented. (PDF 311 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chang, HW., Cheng, CA., Gu, DL. et al. High-throughput avian molecular sexing by SYBR green-based real-time PCR combined with melting curve analysis. BMC Biotechnol 8, 12 (2008). https://doi.org/10.1186/1472-6750-8-12
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-6750-8-12