Abstract
Background
This study tested a low-volume (20–30 μl/20–30 μg DNA) jet injection method for intradermal delivery of a DNA vaccine. Jet injection offers the advantages of a needle-less system, low-cost, rapid preparation of the injected DNA solution, and a simple delivery system. More than one construct can be injected simultaneously and the method may be combined with adjuvants.
Results
Low-volume jet injection targeted delivery of a DNA solution exclusively to the dermis and epidermis of rabbits. A three injection series of plasmid DNA, encoding the Hepatitis B Surface Antigen stimulated a humoral immune response in 2/5 rabbits. One rabbit developed a significant rise in antibody titer after 1 injection and one following 2 injections. There were no significant differences between jet injection and particle bombardment in the maximal antibody titers or number of injections before response. A three injection series of the same plasmid DNA by particle bombardment elicited a significant rise in antibody titer in 3/5 rabbits. One rabbit developed antibody after 1 injection and two after 3 injections. In contrast, 0/5 rabbits receiving DNA by needle and syringe injection responded. In the jet injection and particle bombardment groups, gene expression levels in the skin did not predict response. While immune responses were similar, luciferase gene expression levels in the skin following particle bombardment were 10–100 times higher than jet injection.
Conclusion
Low-volume jet injection is a simple, effective methodology for intradermal DNA immunization.
Similar content being viewed by others
Background
Jet injection is a relatively simple and low-cost method for gene transfer into somatic tissues [1]. Aside from the injector, no special equipment or solutions are required. Plasmid DNA is suspended at the desired concentration in one of several types of solution and injected into tissue. More than one plasmid construct can be injected simultaneously. Injection of antigen encoding plasmids can be combined with either adjuvants or plasmids encoding immune enhancing cytokines. Low-volume jet injection deliberately limits the volume of the injected solution and is utilized to target delivery to small tissue areas [2].
In mice, multiple different routes of DNA immunization have been used successfully [3]. Particle bombardment of skin is reported to be superior to both intra-dermal and intra-muscular needle and syringe injection for induction of immunity in mice when compared directly [4–8]. In Aotus monkeys and pigs, intra-dermal administration of DNA vaccine by needle and syringe is reported to be more effective at inducing an immune response than intra-muscular administration by needle and syringe [9, 10]. High-volume jet injection (500 μl) of either 100 or 1000 μg DNA into ovine muscle or mammary gland [11, 12] or 500 μg DNA into four separate muscle sites in rabbits [13] induces humoral immunity.
Multiple studies have demonstrated that particle bombardment of skin elicits immune responses in larger animals [14–18]. Similarly, high-volume jet injection (100 μl) of either 50 or 250 μg DNA into the skin of primates induces immunity [19]. Delivery to the oral mucosa by jet injection elicited a higher IgA response than intranasal delivery in mice [20]. However, none of these studies compared different intracutaneous DNA delivery methods within the same study.
This investigation tested the ability of low-volume jet injection to target delivery of a DNA vaccine to the skin for the purpose of inducing immunity. A direct comparison of the new methodology to the established techniques of particle bombardment and needle and syringe injection was performed. The experiments demonstrated that low-volume jet injection successfully induced immunity in rabbits. Moreover, there were no significant differences between jet injection and particle bombardment in the maximal antibody titers or number of injections before response. In contrast no rabbits receiving DNA vaccine by needle and syringe injection developed an antibody response. In the low-volume jet injection and particle bombardment groups, gene expression levels in the skin did not predict the magnitude of the immune response. While immune responses were comparable, luciferase gene expression levels in the skin following particle bombardment were 10–100 times higher than low-volume jet injection.
Results and discussion
Low-volume jet injection targets delivery of a plasmid DNA solution to the epidermis and dermis of rabbits
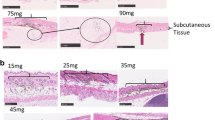
Injection path lengths were measured following low-volume jet injection of a 1% India ink solution. Injection paths averaged between 0.5 mm and 0.75 mm (Figure 1). The injection paths did not extend through the dermis. These experiments show that low-volume jet injection specifically targeted delivery of a DNA solution to the skin and that the injection paths did not reach into the underlying tissue.
Penetration depth of low-volume jet injection into rabbit skin. The injection paths of three separate low-volume jet injections of a 1% India ink solution at 3 Bar into rabbit skin are illustrated in panels A (1 path) and B (2 paths). Penetration depths averaged between 0.5 mm and 0.75 mm. Arrows indicate injection points into the epidermis.
Optimal injection parameters for low-volume jet injection, particle bombardment and needle and syringe injection
Optimal injection parameters for each technique were established by comparing relative mean expression levels of the luciferase reporter gene in the skin 48 hours after injection. Injection parameters that yielded the highest levels of luciferase expression were selected as optimal. Particle bombardment produced the highest mean levels of luciferase activity (Table 1). Mean luciferase activity following low-volume jet injection was 10–100 fold lower than following particle bombardment. Mean luciferase activity following needle and syringe injection was 50 fold lower than jet injection.
Maximal antibody titers and number of injections before response following low-volume jet injection, particle bombardment, and needle and syringe delivery of a DNA vaccine to the skin
Injections of plasmid DNA encoding the Hepatitis B Surface Antigen (HBSAg) were performed at Days 0, 14 and 28. Antibody titers were determined prior to any injection (Day 0), two weeks after each of the three injections (Days 14, 28, and 42), and four weeks after the third and final injection (Day 56). By days 14 and 28 two of five rabbits immunized by low-volume jet injection demonstrated a statistically significant and titratable response at serial time points that persisted through day 56 (Figure 2A) (ANOVA, P = 0.009 at the 1:250 dilution; P = 0.003 at the 1:2,500 dilution at day 56). The number of injections before response and the maximal antibody responses were comparable to rabbits that received DNA immunization by particle bombardment. By days 14 (1) and 42 (2) three of five rabbits immunized by particle bombardment demonstrated a statistically significant response at serial time points (Figure 2B). One rabbit expired during anesthesia at the day 28-injection time point. At day 56 the immune response was statistically significant only at the 1:250 dilution in the two remaining rabbits (ANOVA, P < 0.001 at the 1:250 dilution; P = 0.609 at the 1:2,500 dilution). There was no statistically significant difference in maximal antibody titers between low-volume jet injection and particle bombardment (ANOVA, P = 0.875) (Figure 3). In contrast to immunization with either low-volume jet or particle bombardment, there was no significant titratable antibody response in rabbits that received DNA immunization by needle and syringe injection (ANOVA, P = 0.062 at the 1:250 dilution; P = 0.140 at the 1:2,500 dilution at day 56). Longer-term experiments and larger groups of rabbits will be required to determine if persistence of antibody is different between the low-volume jet injected and particle bombardment groups.
Development of antibody response following low-volume jet injection and particle bombardment. Injections performed at days 0, 14 and 28. Serum drawn prior to each injection and at days 42 and 56. Data presented for individual rabbits. Five rabbits (Rabbits 1–5) received DNA immunization by low-volume jet injection and five rabbits (Rabbits 6–10) received DNA immunization by particle bombardment. Antibody detected by ELISA and presented as optical density (O.D.). Serum dilution 1:250. A. Low volume jet injection. Statistically significant rises in antibody titer were detected at four serial time points in Rabbit 1 (light gray squares; ANOVA, P = 0.005) and three serial time points in Rabbit 2 (black squares; ANOVA, P < 0.001) as compared to negative control sera. B. Particle bombardment injection. Statistically significant rises in antibody titer were detected at two serial time points in Rabbit 6 (black squares; ANOVA, P < 0.001), Rabbit 7 (light gray squares; ANOVA, P < 0.001), and Rabbit 8 (medium gray squares; ANOVA, P 0 = 0.003) as compared to negative control sera. Rabbit 6 expired during anesthesia after the day 28 injection.
Comparison of maximal antibody titers following low-volume jet injection (0.35 ± 0.13) and particle bombardment (0.33 ± .05) at a 1:250 serum dilution. Maximal antibody titers are defined as the highest antibody titer that was measured in each individual rabbit. Mean ± S.E is shown. There was no significant difference in maximal antibody titers between the two methods (ANOVA, P = 0.875).
The experiments demonstrate that intradermal low-volume jet injection of a DNA vaccine can be utilized to elicit immune responses. The low-volume jet injection method was comparable to the more established technique of particle bombardment and was significantly better than the established technique of needle and syringe injection.
Conclusion
Low-volume jet injection is an effective new methodology for intradermal DNA immunization that has advantages over the established techniques of needle and syringe injection and particle bombardment. It is more effective than needle and syringe injection and is simpler to perform than particle bombardment because the plasmid DNA solution can be injected directly rather than being precipitated onto specific particles. High levels of gene expression were not required for successful intradermal genetic immunization by low-volume jet injection. Gene expression levels following particle bombardment were 10–100 fold higher than those found following low-volume jet injection but the immune response was equivalent. The disassociation between local gene expression levels in the injected skin and immune response is consistent with a previous report [16].
Materials and methods
Plasmids
A plasmid encoding the HBSAg under the control of the Human Cytomegalovirus Immediate Early gene I (HCMVIE1) enhancer/promoter (pRc/CMV-HBs(S) was obtained from Aldevron (Fargo, ND) [20]. A plasmid encoding the firefly luciferase gene under the control of the HCMVIE1 enhancer/promoter (pHCMVIE1-luciferase) was used to measure relative gene expression levels following gene transfer by low-volume jet injection, particle bombardment and needle and syringe injection [2]. Plasmids were propagated in DH5α cells and purified by Bio 101 Merlin Core Services (Carlsbad, CA).
Animals, skin preparation, and injection schedules
Twenty-five male New Zealand White rabbits were obtained at 2–3 kg in weight. Animals were purchased from a commercial vendor (Covance, Denver, PA). Health status was evaluated quarterly by the vendor and the animals were free of tested pathogens (other than Bordetella bronchiseptica and rotavirus but these were not expected to interfere with the current study). The rabbits were in excellent health and not symptomatic. The rabbits were anesthetized with 40 mg/kg Ketamine (100 mg/ml,) and 3 mg/kg Xylazine (20 mg/ml) prior to each injection. In preparation for injection, the dorsal skin was shaved, treated with a depilatory, and washed with a chlorhexidine scrub followed by 70% alcohol. Following injections 0.05 mg/kg Buprenorphine (0.3 mg/ml) was given for analgesia.
To establish skin penetration depth following low-volume jet injection, one rabbit was injected at 6 different sites with a 1% India ink solution. The skin was removed at necropsy and examined under a dissecting microscope to measure the length of the injection paths. To establish optimal injection conditions for low-volume jet injection, two rabbits were injected at a total of 15 sites per rabbit with pHCMVIE1-luciferase and euthanized 48 h later. The injected skin was removed at necropsy and tested for luciferase activity. When using particle bombardment the goal is to deliver the majority of gold particles into the epithelial cell layer rather than the deeper, less cellular dermis [21]. To establish skin penetration depth following particle bombardment, four rabbits were injected at eight sites on each rabbit. The skin was removed at necropsy and fixed in 10% neutral formalin, embedded by standard methods, and 5-micron sections were prepared. Hematoxylin and eosin stained sections were examined under the microscope to determine the penetration depth. Gold particles were found distributed primarily in the epidermis and minimally in the superficial dermis of the rabbit skin (data not shown). To establish optimal injection conditions for particle bombardment, six rabbits were injected at a total of 12 sites per rabbit with pHCMVIE1-luciferase and euthanized 48 h later. The injected skin was removed at necropsy and tested for luciferase activity. To establish optimal conditions for intra-dermal needle and syringe injection, two rabbits were injected at a total of 7 sites per rabbit with pHCMVIE1-luciferase and euthanized 48 h later. The injected skin was removed at necropsy and tested for luciferase activity.
For the immunization experiments, rabbits were injected at three time points at two weeks intervals: 0 days, 14 days and 28 days. Three ml blood was obtained from an ear vein prior to each injection and two weeks and four weeks following the third and final injection. One of the five rabbits that received particle bombardment injection died under anesthesia prior to the second injection. A necropsy was performed, no abnormalities were found, and the death was attributed to anesthesia.
Optimization of low-volume jet injection, particle bombardment and needle and syringe injection conditions
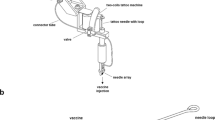
Low-volume jet injection was performed with a prototype low-volume jet injector with adjustable injection volume and pressure. The plasmid DNA solution at 1 μg/μl was prepared in phosphate buffered saline (PBS). The injector is manufactured with a refillable liquid reservoir that holds 180 μl and can rapidly deliver multiple injections to the same site (EMS, Konstanz, Germany) [2, 22]. For optimization of the jet injection experiments, three different injection volume settings (5-μl, 6-μl and 7-μl) at three different pressures (1,2 and 3-Bar) were tested. When using low-volume jet injection for intradermal delivery the goal is contain the delivery solution within the epidermis and dermis but optimize gene expression levels. Conditions for optimal gene expression were established by testing luciferase activity in the skin 48 h following injection of pHCMVIE1-luciferase. Four rapid injections at each volume were used for total DNA delivery of 20 μl /20 μg plasmid DNA, 24 μl/24 μg plasmid DNA, and 28 μl/28 μg plasmid DNA. The injected fluid remained within the dermis under all conditions. The delivery volume with the highest mean luciferase expression was chosen as the optimal delivery condition for the immunization studies. This optimal setting of 28 μl/28 μg plasmid DNA delivered at 3-Bar was utilized for each immunization time point.
A Helios Gene Gun System, (Bio-Rad Laboratories, Hercules, CA) was utilized for particle bombardment. Plasmid DNA was precipitated onto gold particles 1.6 microns in diameter following standard protocols [21]. Optimization conditions tested included the helium pressure, concentration of polyvinylpyrrolidone (PVP), amount of carrier gold and DNA concentration as described previously [23]. Conditions for optimal gene expression were established by testing luciferase activity in the skin 48 h following injection of pHCMVIE1-luciferase. The set of conditions (pressure/PVP concentration/gold and DNA amounts) with the highest mean luciferase expression and optimal skin penetration was chosen as the optimal combination for immunization studies. This optimal setting of one μg plasmid DNA prepared with 0.05 mg/ml PVP/0.50 mg gold delivered at 360 psi was utilized for each immunization time point.
A plasmid DNA solution at 1 μg/μl was prepared in PBS. A 29-gauge needle and 1 ml syringe were utilized for intra-dermal injection. Conditions for optimal gene expression in the injected skin were established prior to the genetic immunization experiments. When using needle and syringe injection for intradermal delivery the goal is contain the delivery solution within the epidermis and dermis but optimize gene expression levels. Conditions for optimal gene expression were established by testing luciferase activity in the skin 48 h following injection of pHCMVIE1-luciferase. A maximum of 20 μl/20 μg plasmid DNA could be injected and kept contained within the epidermis and dermis. This volume yielded the highest luciferase values. The optimal condition of twenty μl (20 μg plasmid DNA) was delivered at each immunization time point.
Luciferase Assay
Two days after injection rabbits were euthanized and the area of injected skin excised. Skin tissue was homogenized in lysis reagent buffer (Luciferase Assay System, Promega, Madison, WI). For each injection sample, luciferase activity was measured in duplicate using a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA) [2].
Enzyme-linked immunosorbent assay (ELISA)
Affinity-purified recombinant Hepatitis B antigen produced in Saccharomyces cerevisae following transformation with the plasmid pCGA7 was used for detection of antibody responses using an ELISA (Aldevron, Fargo, ND) [20]. The recombinant antigens were diluted in 0.1 M carbonate buffer (pH 9.6) and coated at 1 ug/100 ul per well in 96-well microtitre plates (Dynex Technologies, INC. Chantilly, VA). Adjoining negative control wells were coated with 0.1 M carbonate buffer (pH 9.6) alone. The plates were incubated at 4°C overnight. Plates were washed three times in phosphate buffered saline (PBS) (pH 7.4) and blocked with 5% bovine serum albumin (BSA) in PBS before serum was added. One hundred ul of PBS-Tween-20 with 1% BSA was added to each well. To quantify the antibody response at the different time points after injection(s) for each rabbit, 100 ul of diluted serum samples at 1:25, 1:250 and 1:2,500 were added to separate wells in duplicate. Assays for all time points were performed simultaneously. The entire assay was repeated at least twice for all samples. Rabbit sera obtained prior to any injection were utilized as negative controls. The plate was incubated at 37°C for 1 h. After additional washing, phosphatase-conjugated goat anti-rabbit IgG antibody was added into each sample well and incubated for 1 h at 37°C. Diethanolamine (DEA) buffer phosphatase substrate (Kirkegaard Perry Lab, Gaithersburg, Maryland) was added into all wells and the plates were incubated for 30 min at room temperature in the dark. The reaction was terminated after the appearance of color using 3 N NaOH. The absorbance was measured at 410 nm using a microplate reader (Emax, Sunnyvale, CA). The mean value for each set of duplicate wells was determined. Specific binding to the Hepatitis B antigen was determined by subtracting the mean value from the negative control wells that were coated with buffer alone from the mean value from the Hepatitis B-coated experimental wells. Rabbits defined as demonstrating an immune response exhibited a statistically significantly higher mean absorbance reading following injection measured at serum dilutions of 1:250 and 1:2,500 at a minimum of two serial time points following injection as compared to mean values for negative control serum.
Statistical analysis
Means and standard errors (S.E.) for the luciferase expression assays and antibody responses as measured by ELISA were calculated (SPSS 9.0, SPSS, Inc., Chicago, IL). Mean antibody responses were compared by ANOVA (SPSS 9.0). P ≤ 0.05 was considered a statistically significant difference.
References
Furth PA, Kerr D, Wall R: Gene transfer by jet injection into differentiated tissues of living animals and in organ culture. Mol Biotechnol. 1995, 4: 121-127.
Cartier R, Ren SV, Walther W, Stein U, Lewis A, Schlag PM, Li M, Furth PA: In vivo gene transfer by low-volume jet injection. Anal Biochem. 2000, 282: 262-265. 10.1006/abio.2000.4619.
Babiuk LA, van Drunen Littel-van den Hurk, Babiuk SL: Immunization of animals: from DNA to the dinner plate. Vet Immunol Immunopathol. 1999, 72: 189-202. 10.1016/S0165-2427(99)00132-4.
Weiss R, Leitner WW, Scheiblhofer S, Chen D, Bernhaupt A, Mostbock S, Thalhammer S, Lyon JA: Genetic vaccination against malaria infection by intradermal and epidermal injections of a plasmid containing the gene encoding the Plasmodium berghei circumsporozoite protein. Infect Immun. 2000, 68: 5914-5919. 10.1128/IAI.68.10.5914-5919.2000.
Lodmell DL, Ray NB, Ulrich JT, Ewalt LC: DNA vaccination of mice against rabies virus: effects of the route of vaccination and the adjuvant monophosphoryl lipid A (MPL). Vaccine. 2000, 18: 1059-1066. 10.1016/S0264-410X(99)00352-7.
Bennett AM, Phillpotts RJ, Perkins SD, Jacobs SC, Williamson ED: Gene gun mediated vaccination is superior to manual delivery for immunisation with DNA vaccines expressing protective antigens from Yersinia pestis or Venezuelan Equine Encephalitis virus. Vaccine. 1999, 18: 588-596. 10.1016/S0264-410X(99)00317-5.
Choi AH, Knowlton DR, McNeal MM, Ward RL: Particle bombardment-mediated DNA vaccination with rotavirus VP6 induces high levels of serum rotavirus IgG but fails to protect mice against challenge. Virology. 1997, 232: 129-138. 10.1006/viro.1997.8552.
Yoshida A, Nagata T, Uchijima M, Higashi T, Koide Y: Advantage of gene gun-mediated over intramuscular inoculation of plasmid DNA vaccine in reproducible induction of specific immune responses. Vaccine. 2000, 18: 1725-1729. 10.1016/S0264-410X(99)00432-6.
Gramzinski RA, Millan CL, Obaldia N, Hoffman SL, Davis HL: Immune response to a hepatitis B DNA vaccine in Aotus monkeys: a comparison of vaccine formulation, route, and method of administration. Mol Med. 1998, 4: 109-118.
van Rooij EM, Haagmans BL, de Visser YE, de Bruin MG, Boersma W, Bianchi AT: Effect of vaccination route and composition of DNA vaccine on the induction of protective immunity against pseudorabies infection in pigs. Vet Immunol Immunopathol. 1998, 66: 113-126. 10.1016/S0165-2427(98)00186-X.
Jenkins M, Kerr D, Fayer R, Wall R: Serum and colostrum antibody responses induced by jet-injection of sheep with DNA encoding a Cryptosporidium parvum antigen. Vaccine. 1995, 13: 1658-1664. 10.1016/0264-410X(95)00121-G.
Kerr DE, Furth PA, Powell AM, Wall RJ: Expression of gene-gun injected plasmid DNA in the ovine mammary gland and in lymph nodes draining the injected site. Animal Biotechnology. 1996, 7: 33-45.
Davis HL, Michel ML, Mancini M, Schleef M, Whalen RG: Direct gene transfer in skeletal muscle: plasmid DNA-based immunization against the hepatitis B virus surface antigen. Vaccine. 1994, 12: 1503-1509. 10.1016/0264-410X(94)90073-6.
Han R, Cladel NM, Reed CA, Peng X, Budgeon LR, Pickel M, Christensen ND: DNA vaccination prevents and/or delays carcinoma development of papillomavirus-induced skin papillomas on rabbits. J Virol. 2000, 74: 9712-9716. 10.1128/JVI.74.20.9712-9716.2000.
Allen TM, Vogel TU, Fuller DH, Mothe BR, Steffen S, Boyson JE, Fuller J, Hanke T, Sette A, Altman JD, et al: Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J Immunol. 2000, 164: 4968-4978.
Oliveira SC, Harms JS, Rosinha GM, Rodarte RS, Rech EL, Splitter GA: Biolistic-mediated gene transfer using the bovine herpesvirus-1 glycoprotein D is an effective delivery system to induce neutralizing antibodies in its natural host. J Immunol Methods. 2000, 245: 109-118. 10.1016/S0022-1759(00)00267-2.
Vanrompay D, Cox E, Vandenbussche F, Volckaert G, Goddeeris B: Protection of turkeys against Chlamydia psittaci challenge by gene gun-based DNA immunizations. Vaccine. 1999, 17: 2628-2635. 10.1016/S0264-410X(99)00053-5.
Braun RP, Babiuk LA, Loehr BI, van Drunen Littel-van den Hurk: Particle-mediated DNA immunization of cattle confers long-lasting immunity against bovine herpesvirus-1. Virology. 1999, 265: 46-56. 10.1006/viro.1999.0032.
Haensler J, Verdelet C, Sanchez V, Girerd-Chambaz Y, Bonnin A, Trannoy E, Krishnan S, Meulien P: Intradermal DNA immunization by using jet-injectors in mice and monkeys. Vaccine. 1999, 17: 628-638. 10.1016/S0264-410X(98)00242-4.
Lundholm P, Asakura Y, Hinkula J, Lucht E, Wahren B: Induction of mucosal IgA by a novel jet delivery technique for HIV-1 DNA. Vaccine. 1999, 17: 2036-2042. 10.1016/S0264-410X(98)00404-6.
Helios Gene Gun System Instruction Manual. Bio-Rad Laboratories. Rev. B & C. 2001
Walther W, Stein U, Fichtner I, Malcherek L, Lemm M, Schlag PM: Nonviral in vivo gene delivery into tumors using a novel low volume jet-injection technology. Gene Ther. 2001, 8: 173-180. 10.1038/sj/gt/3301350.
Xiao W, Brandsma JL: High efficiency, long-term clinical expression of cottontail rabbit papillomavirus (CRPV) DNA in rabbit skin following particle-mediated DNA transfer. Nucleic Acids Res. 1996, 24: 2620-2622. 10.1093/nar/24.13.2620.
Acknowledgements
This work was supported in part by EMS Medical GmbH (Konstanz, Germany) (to P.A.F.) and NIH CA68033 (to P.A.F.). The authors thank Thomas Nellessen and Wolfgang Merkle at EMS GmbH for their support and Ms. Jessica H. Walker, Mr. John Glidewell, and Mr. Justin Mulholland for excellent technical assistance in the particle bombardment experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
S.R. performed the low-volume jet and needle and syringe injections, collected tissues, analyzed luciferase expression levels, prepared the serum for analysis, developed and performed the ELISA. M.L. assisted with the low-volume jet and needle and syringe injections and development of the ELISA. J.M.S. prepared the gold particles for injection, performed the particle bombardment injections, anesthetized the rabbits and prepared the skin for injection, collected the blood from the rabbits, wrote the materials and methods section on particle bombardment, and edited the manuscript. L.J.D. assisted with the development of the animal protocols and particle bombardment procedure and edited the manuscript. P.A.F. designed the experiments, supervised the performance of the low-volume jet and needle and syringe injections, luciferase assays and the ELISA, performed the statistical analyses, prepared the figures, and wrote the manuscript for publication. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ren, S., Li, M., Smith, J.M. et al. Low-volume jet injection for intradermal immunization in rabbits. BMC Biotechnol 2, 10 (2002). https://doi.org/10.1186/1472-6750-2-10
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-6750-2-10