Abstract
Background
It is speculated that blood transfusion may induce adverse consequences after cancer surgery due to immunosuppression. This study was intended to assess the impact of perioperative blood transfusion on the prognosis of patients who underwent lung cancer resection.
Methods
Eligible studies were identified through a computerized literature search. The pooled relative risk ratio (RR) with 95% confidence interval (CI) was calculated using Review Manager 5.1 Software.
Results
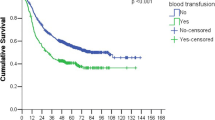
Eighteen studies with a total of 5915 participants were included for this meta-analysis. Pooled analysis showed that perioperative blood transfusion was associated with worse overall survival (RR: 1.25, 95% CI: 1.13-1.38; P <0.001) and recurrence-free survival (RR: 1.42, 95% CI: 1.20-1.67; P <0.001) in patients with resected lung cancer.
Conclusions
Perioperative blood transfusion appears be associated with a worse prognosis in patients undergoing lung cancer resection. These data highlight the importance of minimizing blood transfusion during surgery.
Similar content being viewed by others
Background
Lung cancer is one of the most common cancers worldwide. Surgical resection is the most effective and potentially curative therapeutic option for this disease. Despite improvements in surgical and anesthetic techniques, a great number of patients need perioperative blood transfusions. The immunosuppression from blood products has led to concerns about its effects on the postoperative outcome of surgical oncology patients [1]. Some reports suggested that perioperative blood transfusion was associated with worse long-term oncological outcomes after surgery for lung cancer [2–5], but other studies failed to find such an association [6–9].
In the light of these conflicting findings, we performed a meta-analysis to elucidate the correlation between perioperative blood transfusion and prognosis in patients undergoing lung cancer resection.
Methods
The study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) [10].
Literature search
A computerized search of the literature was performed by searching Medline, EMBASE, OVID, Cochrane database, and China National Knowledge Infrastructure from the time of inception to December 2013. The following medical subject heading terms were used: “lung cancer,” “blood transfusion,” “prognosis,” and “survival”. Only studies on humans and in the Chinese and English languages were eligible for inclusion. Reference lists of all identified articles were manually searched for additional studies.
Inclusion and exclusion criteria
Inclusion criteria for primary studies were as follows: (i) the correlation between perioperative allogenenic blood transfusion and prognosis in patients undergoing lung cancer resection; and (ii) data available on overall survival (OS) or recurrence-free survival (RFS) with a median follow-up of at least 24 months. For duplicate publications reported by the same authors, either the one of higher quality or the most recent publication was selected. Abstracts, letters, editorials, expert opinions and reviews without original data were excluded from analysis.
Data extraction
Two reviewers (LW and HL) independently extracted the following parameters from each study: first author, year of publication, country of origin, study population characteristics, study design, inclusion and exclusion criteria, numbers of participants, relative risk ratio (RR) or hazard ratio (HR) with 95% confidence interval (CI) for OS and RFS. All relevant texts, tables and figures were reviewed for data extraction. If additional data were needed, the authors were contacted to provide full details.
The quality of each included study was assessed using the Newcastle-Ottawa Scale consisting of three factors: patient selection, comparability of the study groups, and outcome assessment [11]. Studies achieving 6 or more stars were considered to be of higher quality.
Outcome measurement
The primary outcomes of this study were OS and RFS.
Statistical analysis and synthesis
The RR with 95% CI was used to evaluate the association between perioperative blood transfusions and RFS or OS. To do this, the HR was directly considered as RR. DerSimonian-Laird random-effect model was used to calculate the overall effect estimates. The RR was transformed to a natural log scale and then calculated for standard errors (SEs). Where HR was not reported, published data and figures from original papers were used to calculate the HR according to the methods described by Parmar et al. [12]. Heterogeneity across studies was evaluated with I2 statistics, with values up to 25%, 25%–50%, and above 50% indicating low, moderate, and high levels of heterogeneity. The RR was calculated by a random-effects model when the P value was less than 0.1. Otherwise, a fixed-effects model was used. Examination of publication bias was performed using a funnel plot based on the primary outcome. Sensitivity analyses were carried out by using the following subgroups: (i) studies of high quality; (ii) studies of patients with stage I disease; and (iii) studies containing more than 200 patients. All analyses were performed using the statistical software Review Manager version 5.1 (The Cochrane Collaboration, Software Update, Oxford).
Results
Eligible studies
We identified 647 potentially relevant records. After excluding studies that did not fulfill our inclusion criteria, 18 studies with a total of 5915 participants were included in the final meta-analysis [2, 3, 5–9, 13–23]. The main features of the included studies are summarized in Table 1. Of these studies, eight studies were conducted in the USA [2, 3, 6–8, 13, 22, 23], two in Italy [6, 17], one in Finland [9], one in Poland [15], one in the United Kingdom [16], one in Spain [18], one in China [19], one in France [20], and one in Greece [21]. The number of patients ranged from 105 to 636 in each study. The transfusion rate in these reports ranged from 9.4 to 55.4%.
There was 100% agreement between the two reviewers.
Primary outcomes
Data on OS were available from 14 studies. Univariate analysis alone was done in 2 [6, 18] of the 14 studies. Multivariate analysis was done in the remaining 12 series [3, 8, 9, 13–17, 20–23]. In one study [15], the authors stated that there was no significant impact of transfusion in the multivariate analysis, but the statistic necessary for meta-analysis (RR, CI) was not reported; we therefore extracted the survival data from the Kaplan-Meier curve. The pooled data indicated that perioperative blood transfusion was associated with a worse OS (RR: 1.25, 95% CI: 1.13-1.38; P < 0.001) (Figure 1) in patients with resected lung cancer. As the test for heterogeneity was significant (I2 = 60%, P =0.002), a random-effects model was used to calculate the RR. Additional analyses in which the RR of the multivariate Cox model was pooled did not change the results significantly (RR: 1.27, 95% CI: 1.12-1.43; P < 0.001; I2 = 61%, P =0.004).
Data on DFS were available from10 studies. Univariate analysis alone was done in 1 [6] of the 10 studies. Multivariate analysis was done in the remaining 9 series [2, 5–8, 13, 14, 19, 22, 23]. In two studies [5, 19], the authors stated that transfusion was an independent predictor of poor RFS, but the statistic necessary for meta-analysis (RR, CI) was not reported; we therefore extracted the survival data from the Kaplan-Meier curve. The pooled data indicated that perioperative blood transfusions was associated with a worse DFS (RR: 1.42, 95% CI: 1.20-1.67; P <0.001), with significantly heterogeneity between studies (I2 = 51%, P = 0.03) (Figure 2). Additional analyses in which the RR of the multivariate Cox model was pooled did not change the results significantly (RR: 1.64, 95% CI: 1.37-1.1.96; P <0.001; I2 = 0%, P =0.58).
Sensitivity analysis
As Table 2 shows, the results derived from three subgroups were all consistent with those derived from overall meta-analysis.
Publication bias
Visual assessment of a funnel plot of the studies used in the meta-analysis reporting on OS is shown in Figure 3. Two of the studies lay outside the limits of the 95% CI, indicating evidence of publication bias.
Discussion
Blood transfusion is life saving in many circumstances but it also poses significant adverse effects, including incompatibility, transmission of viral diseases, coagulopathy, and allergic reactions [1]. In addition, it confers a significant cost and is an increasingly pressured resource. In 1982, Burrows and Tartter reported a higher recurrence rate in transfused patients after colon cancer resection as compared with matched untransfused patients [24]. Since then, numerous studies have addressed the effect of perioperative blood transfusion on patient survival after cancer surgery. Chung et al. [25] reviewed 20 studies that examined the effect of blood transfusion on prognosis after resection for colorectal carcinoma and found that transfusion was associated with an increased risk of tumor recurrence and cancer-related death. Also, in the field of hepatocellular carcinoma surgery, a recent meta-analysis conducted by Liu et al. [26] compared 22 studies that included 5635 patients and demonstrated that perioperative blood transfusion was associated with adverse clinical outcomes, including increased deaths, recurrences and complications. For lung cancer surgery, this subject is particularly relevant because of high transfusion rates ranging from 9.4% to 55.4%, as demonstrated in the present study. To the best of our knowledge, our study provides the first meta-analysis on the effect of perioperative blood transfusion on long-term outcomes after lung cancer surgery, for it included 18 studies with a sufficiently large sample size (n = 5915). The results show that perioperative blood transfusion has an unfavorable impact on prognosis in terms of OS and RFS.
Consistent with the clinical observations, experimental animal data indicate that blood transfusion facilitates tumor growth [27]. The most popular hypothesis is that blood transfusion-associated immunosuppressive alterations, such as the decreased helper/suppressor T-lymphocyte ratio, decreased natural killer cell function, defective antigen presentation and decreased cell-mediated immunity, might decrease tumor surveillance and worsen the prognosis [1]. In addition, there is evidence that transfusion has a significant impact on postoperative morbidity. In a retrospective analysis of 432 patients undergoing pneumonectomy for thoracic malignancies, the incidence of infectious complications was 13.7% in transfused patients and 5.6% in non-transfused patients (P =0.004) [20]. Infection induces the release of cytokines and chemokines including tumor necrosis factor-alpha, interleukin 6, and interleukin 8, which have been proposed as mediators of cancer development [28].
With respect to colorectal liver metastasis, Stephenson et al. [29] reported that patients who received more than 11 units of blood had significantly shorter disease-free intervals and worse survival than those who received 3–10 units of blood after surgery. Of the included studies in the current analysis, Pastorino, Keller, Little, Nosotti and their colleagues noted that the number of units transfused did not affect the survival or recurrence-free survival [5–7, 14]. In contrast, Cata et al. [23] found that the number of units transfused was a factor associated with worse RFS and OS. We were unable to examine whether there was a dose-dependent effect of transfusion on survival because the stratification for the amount of transfused blood was not always the same between these studies.
Several weaknesses of the present study should be taken into consideration in interpreting our results. First, all the included studies were retrospective and are therefore subject to inherent biases, although the results of pooled data of multivariate RRs are similar to the findings from overall analysis. Second, funnel plot analysis revealed the sign of publication bias, which may relate to only published studies included. Third, significantly heterogeneity was detected within primay outcomes. There are considerable disparities between the studies that might introduce heterogeneity, including variation in the preoperative status (such as the American Society of Anesthesiologist physical status, body mass index, comorbidities and hemoglobin level), disease stage, the extent of resection and transfusion policies. In addition, some patients received preoperative or postoperative chemotherapy, which might have influenced the outcome. Also, it should be noted that these studies were conducted over a 20-year period, improvements in operative techniques and anesthesiological management as well as perioperative care are strongly linked to the outcome after lung cancer surgery. In order to minimize this effect, the RR was calculated by a random-effects model. Finally, it has been suggested that pre-, intra-, and postoperative administration of blood would increase the likelihood of colorectal cancer recurrences by 50, 74 and 36%, respectively [30]. Unfortunately, no study available has reported the effect of the timing of transfusion on long-term survival or tumor recurrence after lung cancer resection.
Given a negative effect of transfusion on lung cancer survival, both surgeons and anesthesiologists should be more prudent in using perioperative blood transfusion. Cata et al.[31] proposed an patient blood management protocol that comprises three main components: (i) evaluating high-risk patients and optimizing erythrocyte mass and function for such patients; (ii) minimizing perioperative erythrocyte loss through blood-sparing surgical techniques, maintenance of normothermia, intraoperative cell salvage techniques when appropriate, use of antifibrinolytics when indicated, and optimized fluid therapy and haemodynamic control; and (iii) using patient-specific transfusion triggers to decide when administration of blood products is warranted.
Conclusions
The current literature review suggests that perioperative blood transfusion appears to be associated with a worse prognosis in patients undergoing lung cancer resection, which highlights the importance of avoiding or minimizing blood transfusion.
Abbreviations
- PRISMA:
-
Preferred reporting items for systematic reviews and meta- analyses
- OS:
-
Overall survival
- RFS:
-
Recurrence-free survival
- RR:
-
Relative risk ratio
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- SEs:
-
Standard errors.
References
Gascon P, Zoumbos NC, Young NS: Immunological abnormalities in patients receiving multiple blood transfusions. Ann Intern Med. 1984, 100: 173-177. 10.7326/0003-4819-100-2-173.
Tartter PI, Burrows L, Kirschner P: Perioperative blood transfusion adversely affects prognosis after resection of stage I (subset NO) non-oat cell lung cancer. J Thorac Cardiovasc Surg. 1984, 88: 659-662.
Hyman NH, Foster RS, DeMeules JE, Costanza MC: Blood transfusions and survival after lung cancer resection. Am J Surg. 1985, 149: 502-507. 10.1016/S0002-9610(85)80047-7.
Moores DW, Piantadosi S, McKneally MF: Effect of perioperative blood transfusion on outcome in patients with surgically resected lung cancer. Ann Thorac Surg. 1989, 47: 346-351. 10.1016/0003-4975(89)90371-8.
Little AG, Wu HS, Ferguson MK, Ho CH, Bowers VD, Segalin A, Staszek VM: Perioperative blood transfusion adversely affects prognosis of patients with stage I non-small-cell lung cancer. Am J Surg. 1990, 160: 630-632. 10.1016/S0002-9610(05)80762-7.
Pastorino U, Valente M, Cataldo I, Lequaglie C, Ravasi G: Perioperative blood transfusion and prognosis of resected state Ia lung cancer. Eur J Cancer Clin Oncol. 1986, 22: 1375-1378. 10.1016/0277-5379(86)90148-3.
Keller SM, Groshen S, Martini N, Kaiser LR: Blood transfusion and lung cancer recurrence. Cancer. 1988, 62: 606-610. 10.1002/1097-0142(19880801)62:3<606::AID-CNCR2820620327>3.0.CO;2-Q.
Pena CM, Rice TW, Ahmad M, Medendorp SV: The significance of perioperative blood transfusions in patients undergoing resection of stage I and II non-small cell lung cancers. Chest. 1992, 102: 84-87. 10.1378/chest.102.1.84.
Rainio P, Bloigu R, Satta J, Pokela R, Paakko P: Ten-year survival after resection for lung carcinoma. effect of blood transfusion and tumour stage on outcome. Scand J Thor CardioVasc Surg. 1996, 30: 87-91. 10.3109/14017439609107248.
Moher D, Liberati A, Tetzlaff J, Altman DG: PRISMA group: preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009, 6: e1000097-10.1371/journal.pmed.1000097.
Simillis C, Constantinides VA, Tekkis PP, Darzi A, Lovegrove R, Jiao L, Antoniou A: Laparoscopic versus open hepatic resections for benign and malignant neoplasms–a meta-analysis. Surgery. 2007, 141: 203-211. 10.1016/j.surg.2006.06.035.
Parmar MK, Torri V, Stewart L: Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998, 17: 2815-2834. 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8.
Piantadosi S, Moores DW, McKneally MF: The adverse effect of perioperative blood transfusion in lung cancer. Chest. 1994, 106: 382S-384S. 10.1378/chest.106.6_Supplement.382S.
Nosotti M, Rebulla P, Riccardi D, Baisi A, Bellaviti N, Rosso L, Santambrogio L: Correlation between perioperative blood transfusion and prognosis of patients subjected to surgery for stage I lung cancer. Chest. 2003, 124: 102-107. 10.1378/chest.124.1.102.
Rzyman W, Dziadziuszko R, Skokowski J, Wilimski R, Raiter A, Szymanowska A, Jassem J: The influence of blood transfusion on survival in operated non-small cell lung cancer patients. J Thorac Cardiovasc Surg. 2003, 126: 755-760. 10.1016/S0022-5223(03)00217-4.
Ghosh S, Ahmed K, Hopkinson DN, Vaughan R: Pulmonary adenocarcinoma is associated with poor long-term survival after surgical resection. effect of allogeneic blood transfusion. Cancer. 2004, 101: 2058-2066. 10.1002/cncr.20590.
Berardi R, Brunelli A, Tamburrano T, Verdecchia L, Onofri A, Zuccatosta L, Gasparini S, Santinelli A, Scartozzi M, Valeri G, Giovagnoni A, Giuseppetti GM, Fabris G, Marmorale C, Fianchini A, Cascinu S: Perioperative anemia and blood transfusions as prognostic factors in patients undergoing resection for non-small cell lung cancers. Lung Cancer. 2005, 49: 371-376. 10.1016/j.lungcan.2005.04.011.
Peñalver JC, Padilla J, Jordá C, Escrivá J, Cerón J, Calvo V, García A, Pastor J, Blasco E: Use of blood products in patients treated surgically for stage I non-small cell lung cancer. Arch Bronconeumol. 2005, 41: 484-488.
Chen J, Jiao S: The relationship between prognosis and per ioper ative allogenenic blood transfusions in 280 non- small cell lung cancer patients. Chin J Clin Oncol. 2007, 34: 632-635.
Thomas P, Michelet P, Barlesi F, Thirion X, Doddoli C, Giudicelli R, Fuentes P: Impact of blood transfusions on outcome after pneumonectomy for thoracic malignancies. Eur Respir J. 2007, 29: 565-570. 10.1183/09031936.00059506.
Panagopoulos ND, Karakantza M, Koletsis E, Apostolakis E, Sakellaropoulos GC, Filos KS, Eleni T, Dougenis D: Influence of blood transfusions and preoperative anemia on long-term survival in patients operated for nonsmall cell lung cancer. Lung Cancer. 2008, 62: 273-280. 10.1016/j.lungcan.2008.02.025.
Ng T, Ryder BA, Chern H, Sellke FW, Machan JT, Harrington DT, Cioffi WG: Leukocyte-depleted blood transfusion is associated with decreased survival in resected early-stage lung cancer. J Thorac Cardiovasc Surg. 2012, 143: 815-819. 10.1016/j.jtcvs.2011.12.031.
Cata JP, Chukka V, Wang H, Feng L, Gottumukkala V, Martinez F, Vaporciyan AA: Perioperative blood transfusions and survival in patients with non-small cell lung cancer: a retrospective study. BMC Anesthesiol. 2013, 13: 42-10.1186/1471-2253-13-42.
Burrows L, Tartter P: Effect of blood transfusions on colonic malignancy recurrent rate. Lancet. 1982, 2: 662-
Chung M, Steinmetz OK, Gordon PH: Perioperative blood transfusion and outcome after resection for colorectal carcinoma. Br J Surg. 1993, 80: 427-432. 10.1002/bjs.1800800407.
Liu L, Wang Z, Jiang S, Shao B, Liu J, Zhang S, Zhou Y, Zhou Y, Zhang Y: Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: a meta-analysis. PLoS One. 2013, 8: e64261-10.1371/journal.pone.0064261.
Blajchman MA, Bordin JO: The tumor growth-promoting effect of allogeneic blood transfusions. Immunol Invest. 1995, 24: 311-317. 10.3109/08820139509062781.
Coussens LM, Werb Z: Inflammation and cancer. Nature. 2002, 420: 860-867. 10.1038/nature01322.
Stephenson KR1, Steinberg SM, Hughes KS, Vetto JT, Sugarbaker PH, Chang AE: Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resection of colorectal liver metastases. Ann Surg. 1988, 208: 679-687. 10.1097/00000658-198812000-00002.
Amato A, Pescatori M: Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006, 1: CD005033
Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI: Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. British J Anaesth. 2013, 110: 690-701. 10.1093/bja/aet068.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2482/14/34/prepub
Acknowledgements
We thank Doctor Yanfang Zhao (Department of Health Statistics, Second Military Medical University, Shanghai, China) for her critical revision of the meta-analysis section.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YZ participated in the design and coordination of the study, carried out the critical appraisal of studies and wrote the manuscript. HL, LW, JJ, and FY developed the literature search, carried out the extraction of data, assisted in the critical appraisal of included studies and assisted in writing up. YZ carried out the statistical analysis of studies. All authors read and approved the final manuscript.
Haixing Luan, Feng Ye contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Luan, H., Ye, F., Wu, L. et al. Perioperative blood transfusion adversely affects prognosis after resection of lung cancer: a systematic review and a meta-analysis. BMC Surg 14, 34 (2014). https://doi.org/10.1186/1471-2482-14-34
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2482-14-34