Abstract
Background
The clinical syndrome of joint pain and stiffness in older people is the commonest cause of disability and health care consultation in this age group. Yet there have been few prospective studies of its course over time and its impact on personal and social life. We plan a cohort study in the general population aged 50 years and over to determine the course and prognosis of hand, hip, knee and foot pain, and the impact of these syndromes on participation levels and health care use.
Methods
All patients aged 50 years and over registered with 3 local general practices are to be recruited to a population-based cohort study through the use of a two-stage mailing process. Participants will initially complete a "Health Survey" questionnaire. This will collect information on several areas of life including socio-demographics, general health, physical function, participation, and bodily pain. Those who state that they have experienced any hand problem or any pain in their hands, hips, knees, or feet in the previous 12 months, and also give permission to be re-contacted, will be mailed a "Regional Pains Survey" questionnaire which collects detailed information on the four selected body regions (hand, hips, knees, feet). Follow-up data for the three-year period subsequent to cohort recruitment will be collected through two sources: i) general practice medical records and ii) repeat mailed survey.
Similar content being viewed by others
Background
Osteoarthritis is the commonest cause of chronic pain in older people, and the single most frequent reason for restricted activity in this age-group [1]. Osteoarthritis of the knee and hip have the greatest impact on individuals; the hand and foot are also commonly affected.
The term "osteoarthritis" refers to two different but overlapping syndromes. First is the disease of synovial joints in which cartilage is lost, subchondral bone alters, and new bone is formed around the joint. The typical radiographic features of joint space narrowing, bony sclerosis and osteophytosis are considered to reflect this dynamic process of degeneration and repair. Second is the clinical syndrome of joint pain and stiffness in older people.
Population studies have established that these two syndromes are not equivalent. Although people with severe radiographic osteoarthritis of the hip or knee, for example, are more likely to have hip or knee pain, a substantial proportion do not have symptoms [2]. By contrast, only a minority of older people with knee pain, for example, have definite radiographic changes of osteoarthritis, and the severity of their restricted lower limb function is more closely related to quadriceps strength and depression than to the severity of the radiographic changes [3].
Such observations suggest that clinical osteoarthritis should be regarded as a syndrome of chronic pain in older people, in much the same way as chronic back pain is now regarded as a condition in its own right, with the presence of radiographic changes defining a subgroup [4]. It is this symptomatic syndrome that is common in the general population and which is presented to primary care. Indeed joint pain is the commonest chronic condition in the elderly for which primary care is sought and provided [5]. Yet there is remarkably little evidence about the occurrence, natural history, and prognostic characteristics of joint pain in older people as it occurs in the community and presents to primary care.
Chronic joint pain occurs in the context of the everyday lives of older people, and its impact is not only to be measured in terms of pain experience or restricted function of the affected joints, but the influence which it has on the life that the sufferer wishes to live. The World Health Organisation has identified the term "participation" to represent this level of social functioning [6]. The extent to which chronic joint pain in older people might affect participation, and the way in which this may change over time and influence consultation in primary care, has not been investigated.
We are initiating a three-year cohort study of the clinical syndrome of osteoarthritis in a general population sample of older people. The areas under scrutiny are the hand, knee, hip and foot, and the general aims are (i) to determine the impact these syndromes have on activity and participation levels in older people, (ii) to determine factors which predict prognosis over time with respect to change in pain, activity and participation, and (iii) to determine frequency and predictors of health care use by sufferers of these syndromes through prospective linkage of self-completion survey instruments with primary care records.
Methods
Design
A population-based cohort study in three phases in a sample of patients, aged 50 years and over, registered with three local general practices. Ethical approval for all phases of the study has been obtained from the North Staffordshire Local Research Ethics Committee.
Phase 1: Baseline two-stage mailed survey to recruit the cohort for the study
Phase 2: Three-year prospective follow-up of general practice medical records
Phase 3: Repeat mailed survey at three years
Phase 1 – Cohort recruitment
Sampling frame
Three practices from the North Staffordshire Primary Care Research Consortium are to be recruited to the study and contact details of all adults aged 50 years and over will be taken from each practice list (n = 11309). In the United Kingdom (UK), about 98% of the population are registered with a general practitioner (GP) and so practice registers provide a convenient frame for sampling a local population [7]. The samples obtained will then be checked by the GPs for exclusions (for example, severe psychiatric or terminal illness) prior to the mailing procedure.
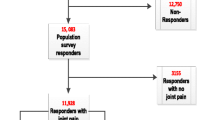
The baseline phase of the study is a two-stage mailed survey. Firstly, the study population will be mailed a "Health Survey" questionnaire which collects information on several areas of life including socio-demographics, general health, physical function, participation, and bodily pain. Responders to this questionnaire who give permission to be re-contacted and who report experiencing, in the previous 12 months, a hand problem or pain in either their hands, hips, knees, or feet will be mailed a "Regional Pains Survey" questionnaire which focuses on these four regional sites. At each stage, questionnaires will be mailed with a letter from the GP practice and a study information leaflet, with reminders to be sent to non-responders after two and four weeks.
"Health Survey" questionnaire
Details of the information to be collected by the "Health Survey" questionnaire are presented in Table 1. On receipt by the Research Centre, the date of birth and gender information given on the questionnaire will be compared with that obtained from the general practice lists to ensure that the respondent is the intended participant.
Participants to this first stage (Phase 1) will be eligible to take part in the second mailing stage if they:
a) Consent to further contact by the Research Centre
b) Respond positively to at least one of the questions relating to regional sites (hands, hips, knees, or feet)
If respondents satisfy both of these criteria, they will be mailed the "Regional Pains Survey" questionnaire.
"Regional Pains Survey" questionnaire
Details of the information collected by the "Regional Pains Survey" questionnaire are presented in Table 2.
Phase 2: Follow-up using general practice medical records
All participants to Phase 1 who give permission for their GP records to be accessed will have their computerised medical records tagged by a member of the Centre's Health Informatics Specialist team. All consultations for the 12-month period prior to cohort recruitment and all consultations for the three-year period following recruitment will be identified. The three practices participating in this research are fully computerised and all practice staff enter a code for each contact. These codes are part of the READ classification of morbidity and event coding which is widely used in the National Health Service in the UK and can be mapped to the International Classification of Diseases. The practices undergo annual audits completed by the Centre's Health Informatics team to assess the quality and completeness of the data entry at the practices.
The practice-held information will provide the basic data for analysis of health care utilisation. This will include all consultations, medications, and referrals to other health care professionals both within primary care and on to secondary care. All sensitive data (name, contact details) will be removed from the medical records data and the consultation data will be linked to the survey data through the unique survey identifier.
Phase 3: Follow-up using repeat mailed survey
A follow-up survey, at three-years post-cohort recruitment, will be mailed to all those participants in the baseline phase who gave permission for re-contact. This questionnaire will focus on current problems and changes occurring since recruitment. Details of the information to be collected by this questionnaire are presented in Table 3.
Sample size
The size of the sample chosen for this study reflects the need to ensure that a cohort of sufficient size is identified in Phase 1 to enable follow-up analysis to be adequately powered. The three participating practices have a total of 11309 registered patients who are suitable to take part in the study. From pilot work we have estimated that the response to the "Health Survey" questionnaire would be approximately 73% (n = 8300) and 70% of those completing this questionnaire (n = 5800) would give permission for re-contact and access to medical records. Moreover, of those who give permission for re-contact, 71% (n = 4100) will have hand problems or pain in at least one of the four specified pain regions and be mailed the "Regional Pains Survey" questionnaire, with an estimated 90% (n = 3700) who will complete it.
Three-year follow-up, in terms of survey and general practice data, will then be collected on those responders to the "Health Survey" who are alive and registered at the study practices at the time of follow-up and who gave permission for re-contact and access to medical records. Based on data from a recently conducted three-year follow-up survey in an older population (Jinks, personal communication) it is anticipated that 90% (n = 5220) of those who complete the "Health Questionnaire" will be alive and registered at the same study practices at the time of follow-up. Moreover, it is estimated that approximately 77% (n = 4000) will complete the follow-up survey.
Statistical analysis
The data collected in Phase 1 will be examined for cross-sectional relationships. The data collected in Phase 2 will be linked to Phase 1 data to determine predictors of consultation for joint pain sufferers. The data collected at Phase 3 will be linked to Phase 1 data to determine changes occurring between the two surveys.
Conclusions
This protocol describes a prospective observational cohort study which aims to determine the frequency and natural history of joint pain at four common sites in the general population aged over 50 years, and the patterns and predictors of outcome as measured by participation levels and primary health care use. The results of this study, along with other work in this research programme, will form the basis for the development and piloting of randomised controlled trials of primary care management of osteoarthritis symptoms in primary care. The results of this study will be published as soon as they are available.
References
Bradley EM, Tennant A: Changing profile of joint disorders with age: findings from a postal survey of the population of Calderdale, West Yorkshire, United Kingdom. Ann Rheum Dis. 1992, 51: 366-371.
Peat G, McCarney R, Croft P: Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001, 60: 91-97. 10.1136/ard.60.2.91.
O'Reilly SC, Jones A, Doherty M: Quadriceps weakness in knee osteoarthritis: the effect on pain and disability. Ann Rheum Dis. 1998, 57: 588-594.
McAlindon TE: The knee. Baillière's Best Pract Res Clin Rheumatol. 1999, 13: 329-344. 10.1053/berh.1999.0023.
McCormick A, Fleming D, Charlton J: Morbidity statistics from general practice. Fourth national study 1991–1992. Office of Population Censuses and Surveys. Series MB5 No. 3. London: HMSO. 1995
World Health Organisation: International classification of functioning, disability and health. [http://www3.who.int/icf/icftemplate.cfm]
Bowling A: Research methods in health. 1997, Buckingham: Open University Press
Ware JE, Kosinski M, Keller SD: A 12 item Short Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996, 34: 220-233. 10.1097/00005650-199603000-00003.
Wilkie R, Peat G, Croft P: Keele Assessment of Participation (KAP): A new participation instrument to measure the impact of osteoarthritis on the individual [abstract]. Rheumatology. 2002, 41 (Suppl 2): 22-
Berkman LF, Syme SL: Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979, 109: 186-204.
Michael YL, Berkman LF, Colditz GA, Kawachi I: Living arrangements, social integration, and change in functional health status. Am J Epidemiol. 2001, 153: 123-131. 10.1093/aje/153.2.123.
Zigmond AS, Snaith RP: The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983, 67: 361-370.
Weinman J, Petrie KJ, Moss-Morris R, Horne R: The illness perceptions questionnaire: a new method for assessing the cognitive representation of illness. Psychol Health. 1996, 11: 431-445.
Office for National Statistics:. Standard occupational classification 2000. 2000, The coding index. London, 2:
Office for National Statistics:. The National Statistics Socio-economic classification user manual. Version 1. London. 2002
Bergner M, Bobbitt RA, Carter WB, Gilson BS: The Sickness Impact Profile: Development and final revision of a health status measure. Med Care. 1981, 19: 787-805.
Jenkins CD, Stanton A-B, Niemcryk SJ, Rose RM: A scale for the estimation of sleep problems in clinical reseach. J Clin Epidemiol. 1988, 41: 313-321.
Ware JE, Sherbourne CD: The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992, 30: 473-483.
Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE: AIMS2: the content and properties of a revised and expanded Arthritis Impact Measurement Scales health questionnaire. Arthritis Rheum. 1992, 35: 1-10.
Chung KC, Pillsbury MS, Walters MR, Hayward RA: Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. J Hand Surg [Am]. 1998, 23: 575-587.
Bellamy N, Campbell J, Haraoui B, Buchbinder R, Hobby K, Roth JH, MacDermid JC: Dimensionality and clinical importance of pain and disability in hand osteoarthritis: Development of the Australian/Canadian (AUSCAN) Osteoarthritis Hand Index. Osteoarthritis Cartilage. 2002, 10: 855-862. 10.1053/joca.2002.0837.
Jinks C, Lewis M, Ong BN, Croft P: A brief screening tool for knee pain in primary care. 1. Validity and reliability. Rheumatology. 2001, 40: 528-536. 10.1093/rheumatology/40.5.528.
Bellamy N: WOMAC osteoarthritis index. A user's guide. 1996, Ontarion: London Health Services Centre, McMasters University
Garrow AP, Papageorgiou AC, Silman AJ, Thomas E, Jayson MI, Macfarlane GJ: Development and validation of a questionnaire to assess disabling foot pain. Pain. 2000, 85: 107-113. 10.1016/S0304-3959(99)00263-8.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2474/5/2/prepub
Acknowledgements
This study is supported financially by a Programme Grant awarded by the Medical Research Council, UK (grant code: G9900220) and Support for Science funding secured by the North Staffordshire Primary Care Research Consortium for NHS service support costs. KD is supported by a grant from the Arthritis Research Campaign.
The authors would like to thank the administrative and health informatics staff at Keele University's Primary Care Sciences Research Centre and the staff of the participating general practices.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Authors' contributions
All authors participated in the design of the study, baseline data collection, and drafting the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Thomas, E., Wilkie, R., Peat, G. et al. The North Staffordshire Osteoarthritis Project – NorStOP: Prospective, 3-year study of the epidemiology and management of clinical osteoarthritis in a general population of older adults. BMC Musculoskelet Disord 5, 2 (2004). https://doi.org/10.1186/1471-2474-5-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2474-5-2