Abstract
Background
Breathlessness is a debilitating and distressing symptom in a wide variety of diseases and still a difficult symptom to manage. An integrative review of systematic reviews of non-pharmacological and pharmacological interventions for breathlessness in non-malignant disease was undertaken to identify the current state of clinical understanding of the management of breathlessness and highlight promising interventions that merit further investigation.
Methods
Systematic reviews were identified via electronic databases between July 2007 and September 2009. Reviews were included within the study if they reported research on adult participants using either a measure of breathlessness or some other measure of respiratory symptoms.
Results
In total 219 systematic reviews were identified and 153 included within the final review, of these 59 addressed non-pharmacological interventions and 94 addressed pharmacological interventions. The reviews covered in excess of 2000 trials. The majority of systematic reviews were conducted on interventions for asthma and COPD, and mainly focussed upon a small number of pharmacological interventions such as corticosteroids and bronchodilators, including beta-agonists. In contrast, other conditions involving breathlessness have received little or no attention and studies continue to focus upon pharmacological approaches. Moreover, although there are a number of non-pharmacological studies that have shown some promise, particularly for COPD, their conclusions are limited by a lack of good quality evidence from RCTs, small sample sizes and limited replication.
Conclusions
More research should focus in the future on the management of breathlessness in respiratory diseases other than asthma and COPD. In addition, pharmacological treatments do not completely manage breathlessness and have an added burden of side effects. It is therefore important to focus more research on promising non-pharmacological interventions.
Similar content being viewed by others
Background
Breathlessness is a common symptom which occurs in a wide range of clinical conditions and affects many individuals [1]. Some two-thirds of patients experiencing breathlessness will have a respiratory or cardiac condition [2]. Breathlessness also often occurs in cancer, affecting 50-70% of patients [3]. Both frequency and severity of breathlessness increase towards the end of life [3–5].
The terms breathlessness and dyspnoea are often used interchangeably and definitions focus on sensations of difficulty, discomfort and distress in breathing experienced by those affected [1, 3, 6], with perhaps the most authoritative definition coming from the American Thoracic Society in 1999:
'...a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity. The experience derives from interactions among multiple physiological, psychological, social, and environmental factors, and may induce secondary physiological and behavioural responses' [1].
The subjective nature of breathlessness has led to comparisons with pain as a symptom [3] -and indeed breathlessness is the most common symptom after pain for which patients seek medical help [2].
Breathlessness can have a significant impact on daily living and health-related quality of life for patients and families [1, 3, 7–9]. The experience of breathlessness may markedly reduce patients' functional ability [1, 3, 8], and fear of this symptom can itself lead to activity avoidance [10, 11]. Strong associations between breathlessness and fatigue have also been noted [1, 12, 13], which can further reduce functional ability.
There is a strong relationship between breathlessness and anxiety or panic [7, 8, 10, 11, 14], with the latter often resulting in patients who experience breathlessness undergoing emergency hospital admissions. The nature of the relationship between breathlessness and anxiety or panic is complex [1, 10] and Bailey's [7] work in chronic obstructive pulmonary disease suggests that anxiety may be a consequence of breathlessness, rather than the reverse. Severe breathlessness may also be associated with depression, again the inter-relationship being complex [14].
Breathlessness is distressing for patients and their families [7, 9, 15] and may result in a heavy physical, social and emotional burden on carers [3, 7, 8]. Carers of breathless patients have reported feelings of helplessness, fear, isolation and frustration [3, 7, 8]. Social isolation is often reported in breathless individuals and their family members [3, 8].
Importantly, breathlessness may have prognostic significance. Abidov et al [16] identified increased mortality in patients with known or suspected cardiac disease in whom breathlessness was present, while others [13] comment on the relationship between breathlessness and poor prognosis in non-heart failure populations. Associations between breathlessness and mortality have also been reported in general and elderly population studies [17, 18].
Breathlessness is a common and distressing symptom in the advanced stages of both malignant and non-malignant disease. It is characteristic, for example, of chronic obstructive pulmonary disease (COPD), often causing marked impairment in activity [19] and confinement to the home [20]. COPD is a progressive, incurable illness and a leading cause of disability and mortality worldwide [21, 22]. Despite this, there is evidence that services for people with COPD are poor and needs (including palliative care needs) are unmet: indeed, breathlessness in COPD has been described as 'invisible', partly because of the lack of attention it receives from health services [23, 24]. Breathlessness is also a common and devastating symptom in advanced cancer, with prevalence increasing from referral to palliative care services (15-55.5%) to the last week of life (18-79%) and severity reported to be moderate-severe in 10-63% of cases [25]. It causes major impairment in function and quality of life (QoL) [26]. To date, however, there is limited evidence that palliative care interventions have improved breathlessness or that either medical or nursing care have provided effective assistance to individuals with this symptom [27].
Despite the wide range of conditions that cause distressing levels of breathlessness, including primary and secondary cancer, COPD, cystic fibrosis, interstitial lung disease (ILD) and chronic heart failure (CHF) it is still very difficult to manage [23, 28].
Method
Against this background we conducted an integrative review of pharmacological and non-pharmacological interventions for breathlessness within a wide range of cardiorespiratory diseases. For the purpose of this study, pharmacological interventions were defined as those classified as medicinal products under the EU Directive 2001/83/EEC, i.e. any substance or combination of substances which may be administered to human beings with a view to making a diagnosis or to restoring, correcting or modifying physiological function in human beings. Non-pharmacological interventions are those that are not classified as medicinal products.
This integrative review was undertaken both to provide a clear account of the current body of work on the subject and as a preliminary step in developing further interventions for the management of breathlessness and other respiratory symptoms. Given the large number of systematic reviews available, and the limited clinical utilisation of many of the interventions found (with the exception of a few pharmacological interventions such as steroids and beta-agonists), the decision was taken to focus and limit the integrative review to only systematic reviews.
We searched the following electronic databases for systematic reviews of randomised controlled trials, quasi-experimental trials, and uncontrolled trials where there were group comparisons of both pharmacological and non-pharmacological interventions for breathlessness in non-malignant disease:
-
Cochrane Library (Cochrane Airway Review Group)
-
AMED (1985-June 2007)
-
CINAHL (1982-June 2007)
-
EMBASE (1988-June 2007)
-
Ovid MEDLINE (1950-June 2007)
-
PsycINFO (1985-June 2007)
Searching was carried out with a combination of assigned index (MESH) terms and text words, with truncations where appropriate and different spellings (e.g. dyspnea AND dyspnoea). Between July 2007 and September 2009, while the data of the review was collated and analysed, regular further searches were carried out using the key words in order to identify any new published systematic reviews, adding them to the main body of the current review.
Databases were searched using the following search strategy
-
1.
asthma
-
2.
COPD
-
3.
Chronic AND obstruction AND pulmonary AND disease
-
4.
2 or 3
-
5.
Heart AND failure
-
6.
Cystic AND fibrosis
-
7.
interstitial MESH lung disease
-
8.
pulmonary AND arterial AND hypertension
-
9.
bronchiectasis
-
10.
1 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9
-
11.
dyspnea OR dyspnoea
-
12.
difficult AND breath
-
13.
breathlessness
-
14.
short AND breath
-
15.
short of breath
-
16.
11 OR 12 OR 13 OR 14 OR 15
-
17.
Systematic AND review
-
18.
10 AND 16 AND 17
Systematic reviews identified in this way were reviewed independently for inclusion by two of the authors (AM, RD). Any disagreements were resolved by discussion. Many reviews did not explicitly measure breathlessness as an outcome, and for others it was not the primary outcome. For this reason reviews were eligible for inclusion in our review if they reported research on adult participants using not only our primary outcome measure of breathlessness (e.g. Borg and/or modified Borg tests, verbal categorical scales, visual analogue scales), or some other measure of respiratory symptoms (e.g. including cough, wheeze in a composite score), but also if they reported other frequently used breathlessness related outcome measures such as lung function or physiological changes (e.g. spirometry, exercise tolerance, arterial blood gases, pulse oximetry). Although these secondary outcomes may act as proxies for breathlessness, they do have some limitations. For example, lung function only moderately relates to the symptom experience of patients, and exercise tolerance may be as much a consequence of fatigue as breathlessness. Moreover, many reviews also reported 'single symptom scores', which although included several symptoms, among them breathlessness too, yielded only a single composite score. Many reviews also reported upon improvements in other key outcomes (e.g. survival, health-related quality of life) that may be linked or influenced by levels of breathlessness, and these have been included within this review.
Trials of any non-pharmacological intervention were included, as well as any drug/pharmacological intervention (other than radiotherapy and anti-cancer chemotherapy), given by any route or in any dose. Reviews were excluded if they were not available in English, or were wholly or primarily concerned with the treatment of children (i.e. aged < 18 years), infections, sleep apnoea, or cough. They were also excluded if respiratory symptoms were not included as study outcomes.
Data were extracted by one of the authors (RD) using a data extraction form designed for the review and was independently verified by one other author (AM). A third author (AC) had the role of arbitrator in cases of disagreement. Data extracted included the nature of the intervention, the number of trials included in the review, the aggregated sample, primary and secondary outcome measures, the condition treated, and the main conclusions.
Following data extraction, systematic reviews identified were categorised by condition treated (asthma, COPD, bronchiectasis, interstitial lung disease, bronchitis, bronchoconstriction, pulmonary arterial hypertension, breathlessness (generic), and respiratory failure) and by treatment type (i.e. non-pharmacological/pharmacological).
Results
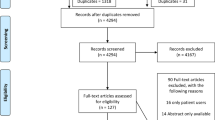
We initially identified 3359 citations, with an additional 436 resulting from the updated search, giving a total of 3,795 citations. Of these, 3576 were excluded based on the title/abstract as they were irrelevant studies, reviews but not systematic reviews, duplicates, laboratory studies or individual trials which were reported in an identified systematic review. From the 219 systematic reviews identified, 66 were further excluded primarily because they were either paediatric reviews or reviews of medical devices. Of the 153 reviews that met the inclusion criteria, 59 addressed non-pharmacological interventions and 94 addressed pharmacological interventions. A summary of key findings from the review is presented below; further details of individual systematic reviews are set out in the Tables presented in Additional Files 1, 2, 3.
Additional file 1 presents data from systematic reviews of interventions for asthma; Additional file 2 presents data from systematic reviews of interventions for chronic obstructive pulmonary disease (COPD), and Additional file 3 presents data from systematic reviews of interventions for other respiratory diseases.
Within this integrative review we have sought to draw together data from existing systematic reviews. However, few of the papers included meta-analyses. This was due to the quality of some of the studies included, variation of the interventions between and within categories, variability of the reported data and the provision of unreported data. It was therefore not possible, for the purposes of this integrative review, to summarise or draw conclusions regarding effect sizes and other statistical parameters.
Asthma (non-pharmacological treatments)
A total of 34 systematic reviews were identified. Six were excluded because they referred only to children. Of the remaining 28, 26 were published in the Cochrane Database of Systematic Reviews. Three included data on breathlessness [29–31], while all others assessed breathlessness as part of a composite score of 'asthma symptoms'. In some individual studies these symptom scores used lung function as a proxy for symptoms, others included the Asthma Quality of Life Questionnaire that included symptom categories, while others used the Asthma Symptom Checklist as an outcome. Furthermore, many of the original trials included a 'respiratory symptom' score, of which breathlessness/dyspnoea was only part.
Treatments assessed within the reviews included: acupuncture [32], breathing exercises/training [30, 33, 34]; dietary measures [35–39]; Heliox [29, 31]; homeopathy [40]; control of allergens [41, 42]; humidity control/ionisers [43, 44]; education programs [45–47]; manual therapy[48]; primary care clinics [49]; psychological interventions [50, 51]; individualised care plans [52]; and non-invasive positive pressure ventilation (NIPPV) [53], which enhances ventilation by compensating for fatigued ventilator muscles.
Of those reviews with positive findings, four reported a single study only [35, 49, 53, 54]. Holloway & Ram [34] identified seven randomised or quasi-randomised controlled trials of breathing exercises for asthma, concluding that trends for improvement in QOL were encouraging, although no firm conclusions could be drawn, as only 2 of the 7 studies reviewed showed positive results. Gibson et al [45] concluded from evidence from 12 RCTs of limited (information only) education programs that symptom perceptions may improve, although actual asthma symptoms showed no significant differences. Yorke et al's [50] review of 15 RCTs of psychological interventions was unable to draw firm conclusions, but following pooling of data, reported two trials as showing positive effects on QOL after cognitive behavioural therapy (CBT). Biofeedback also appeared to have a positive effect on peak expiratory flow (PEF) in two studies. Gibson et al's [47] large review of 36 randomised trials concluded that education in asthma self-management involving self-monitoring by PEF or symptoms, together with regular review and written action plans, does improve health outcomes in adults with asthma. While the systematic reviews show that the vast majority of non-pharmacological interventions tested to date have largely been ineffective in relation to asthma symptoms, some effect has been reported in trials of calorie controlled diet [35], inspiratory muscle training [30], manual therapy (particularly massage with relaxation) [48] and psychoeducational interventions [50]. The role of Heliox is unclear, as one review showed no effect on symptoms [29], while another showed that 3 of 15 trials reviewed had a positive effect [31]. Selenium supplementation has shown positive results, but there is only one trial available in the literature that included a small sample of 24 patients [54]. The conclusions of two trials was that there was no current evidence of the effectiveness of the 'Alexander technique' for chronic asthma [55] or that feather bedding and pillows reduced asthma symptoms compared with man-made fibres [56].
Asthma (pharmacological treatments)
In all, 69 systematic reviews were identified. Eighteen were excluded because they referred only to children, did not specify symptoms as outcomes, or were reviews of studies of vaccines or comparisons between devices. Of the 51 reviews included, 50 were accessed via the Cochrane Database of Systematic Reviews. Seven included data on breathlessness [57–63] while all others assessed breathlessness as part of a single score of 'asthma symptoms'. Again, there was heterogeneity between systematic reviews in the specific outcomes measured within these symptom scores.
Treatments included: anti-leukotrienes [64, 65]; beta-agonists (inhaled/non-inhaled) [61–63, 66–75]; allergen immunotherapy [76]; anti-IgE [77]; antibiotics [78]; anti-cholinergics [57]; azathioprine [79]; beclomethasone [80–82]; budesonide [83, 84]; caffeine [85]; beta-blockers [58]; chloroquine [86]; corticosteroids (inhaled/non-inhaled) [59, 60, 87–91]; cyclosporine [92]; fluticasone [93–96]; anti-gastro-oesophageal reflux treatment [97]; gold [98]; inhaled/non-inhaled magnesium sulphate [98, 100]; macrolides [101, 102]; methotrexate [103]; oxatomide [104]; and tailored interventions based on sputum eosinophils [105].
Many reviews report some level of benefit from or equivalence between treatments under review. Clearly steroids (both oral and intramuscular) improve lung function [60], while long-acting beta agonists (LABA) with inhaled corticosteroids (ICS) [68] demonstrate improved outcomes in lung function and symptoms compared to LABA and antileukotrienes, short-acting beta agonists (SABA) [75] or theophylline [74]. Treatments from large scale reviews that report positive effects most strongly include: allergen immunotherapy (57 trials) [76], beclomethasone (60 trials) [82], budesonide (43 trials) [84], fluticasone (75 trials) [96], ICS compared with sodium cromoglycate (25 trials) [88], LABA (67 trials) [62], and LABA vs. SABA (31 trials) [75].
Small reviews of caffeine (6 trials) [85], inhaled magnesium sulphate (6 trials) [99], intravenous magnesium sulphate (7 trials) [100], and tailored interventions based on sputum eosinophils, particularly those with severe asthma and frequent exacerbations (3 trials) [105] also suggested therapeutic benefits. Caffeine (taken orally) has demonstrated a modest, though short-term (up to 4 hours), effect as a bronchodilator.
A number of reviews identified the positive 'steroid sparing' or reduced rescue medication effect of treatments such as Anti-IgE (specifically omalizumab) (14 trials) [88] and LABA [82–84, 86] (combined total of 78 trials). One review (67 trials) [62], while reporting the positive steroid sparing effects of LABA, also raised some important safety concerns. These concerns related to a significant increase in asthma related deaths or life threatening experiences for patients treated with LABA, mainly among African-American patients and those not on inhaled corticosteroids. SABA, while generally less effective than LABA, showed in one review (49 trials) [70] that regular use of inhaled SABA can decrease symptoms and rescue medication. A modest effect has been shown with regards to antileukotrienes [64], allergen immunotherapy [76], cyclosporine [91], gold [98], and anticholinergics [57]. However, this modest effect in combination with serious adverse events and/or the need for close monitoring, make therapeutic options such as immunotherapy [74], cyclosporine [92] and gold [98], problematic and inappropriate for clinical use. Two trials found no evidence to indicate that either colchicines [106] or dapsone [107] were effective in the management of steroid dependent asthmatic patients.
COPD (non-pharmacological treatments)
A total of 28 systematic reviews were identified. One was excluded due to lack of focus on respiratory symptoms. Fifteen of the remaining reviews were published in the Cochrane Database of Systematic Reviews. Twenty also included specific data on breathlessness [108–123], while all others assessed breathlessness as part of a composite COPD 'symptom' score. Treatments included: action plans [124]; oxygen/Heliox [108, 110, 111, 113, 121, 125]; physical therapy [109]; home care/hospital at home [126, 127]; non-invasive positive pressure ventilation (NIPPV)/non-invasive ventilatory support (NIVS) during exercise [119, 128]; nutritional supplements [129]; inhalers [130]; rehabilitation [112, 131]; education/support [120, 132]; inspiratory muscle training [114–116]; physical activity [117]; psychological interventions [118]; and surgery [122].
The majority of reviews (n = 14) were based on fewer than 10 trials: six of these had aggregated samples of more than 150 participants [108–111, 119, 130]. Positive effects were reported for breathlessness with domiciliary oxygen (6 trials [125]), and short term ambulatory oxygen (31 trials [113]). People with COPD are prone to developing alveolar hypoventilation during exacerbations, which may be contributed to by the administration of high inspired oxygen concentrations. Nevertheless, there was no evidence to indicate whether different oxygen therapies in the pre-hospital setting have an effect on outcome for people with acute exacerbations of COPD [133].
Positive effects were also reported on varied outcomes, including breathlessness, for NIVS during exercise (7 trials) [119], and lung volume reduction surgery for end-stage COPD (19 trials) [122], and for outcomes other than breathlessness for oxygen during training (5 trials) [111] home care/hospital at home (4 trials [126], 7 trials [127]) and individualised action plans (3 trials) [124]. A large review of pulmonary rehabilitation (31 trials) [112] found that it relieves breathlessness and fatigue, improves emotional function, and enhances sense of control; a large review of the chronic care model in COPD (32 trials) [120] found reductions in hospitalization rates and emergency/unscheduled visits, and shorter length of hospital stay, although the result specifically for breathlessness/symptoms was unclear in most trials. Four reviews of inspiratory muscle training concluded that it can improve breathlessness and health-related quality of life in adults with COPD (19 trials [114], 25 trials [115], 15 trials [116], 15 studies [123]) and an important addition to pulmonary rehabilitation (15 trials) [116]. However, limitations have been identified within the literature and Shoemaker et al [123] found that it was unclear whether any improvements were mediated through improved muscle strength and endurance.
Ram et al [134] reviewed 14 trials concerning NIPPV for respiratory failure due to exacerbations of COPD. If applied intermittently and for short periods, NIPPV may be sufficient to reverse respiratory failure, and it was found to have a positive effect on a range of outcomes including mortality, need for intubation, and treatment failure. The authors conclude that trials demonstrate the benefits of NIPPV as first line intervention as an adjunct to usual medical care.
Bausewein et al [28] reviewed non-pharmacological interventions for breathlessness in COPD, cancer, chronic heart failure, interstitial lung disease, and motor neurone disease. Strong evidence was identified to show that neuro-electrical muscle stimulation (NMES) and chest wall vibration (CWV) could relieve breathlessness in COPD, and there was moderate evidence in favour of walking aids in COPD and breathing training in studies of mixed cardio-pulmonary disease and COPD. The authors found only low strength evidence of relief from breathlessness with acupuncture or acupressure, and no evidence for the effect of music. They noted that only a few studies included participants with conditions other than COPD. Insufficient data were available to evaluate evidence for a range of other interventions, including relaxation, counselling and support, case management and psychotherapy.
COPD (pharmacological treatments)
In all, 22 systematic reviews were identified. Three were excluded (lack of focus on respiratory symptoms/non-intervention studies). Two of the remaining 19 reviews were located from sources other than the Cochrane Database. Sixteen included data on breathlessness [135–149] while the others assessed breathlessness as part of a single symptom score. Treatments included: bronchodilators [135, 140, 141, 144, 147, 148]; LABA and LABA in addition to steroids [136, 142, 149]; vaccines [137, 139]; inhaled/non-inhaled corticosteroids [143, 146, 150]; SABA [138, 145]; methylxanthines [151]; and mucolytics [152].
Seven reviews were based on evidence from fewer than 10 trials [135, 136, 138–140, 147, 151]. One of these referred to an aggregated sample of fewer than 150 participants [138]. In one review the size of the aggregated sample was unclear [148]. Positive conclusions were drawn in respect of some aspect of intervention(s) for COPD in over half of the reviews, including: combined corticosteroid and LABA in a single inhaler (6 trials) [136]; influenza vaccine (6 trials) [137]; ICS (47 trials) [150]; ipratroprium vs. LABA (7 trials) [140]; LABA (23 trials) [142]; mucolytic agents (although their effect was small) (26 trials) [152]; oral corticosteroids (OCS) (24 trials) [143]; oral theophylline with a modest effect on lung function but largely ineffective in relation to breathlessness (20 trials) [144]; SABA (13 trials) [145]; systemic corticosteroids (10 trials) [146]; tiatropium (9 trials) [147]; bronchodilators, i.e. β-agonists, anti-cholinergics and theophyllines (33 trials) [148]; and LABA (12 trials) [149]. A large review of 47 trials (13,139 patients) [150] showed that ICS used for 2-6 months resulted in small symptom improvements, while use for more than 6 months had limited effect on symptoms.
Jennings et al [153] reviewed 18 trials concerning the use of opioids for dyspnoea in both cancer and non-malignant conditions (e.g. chronic heart failure, COPD, interstitial lung disease, pulmonary fibrosis), concluding that opioids have a positive effect on the sensation of breathlessness. Data did not support the use of nebulised morphine in the management of breathlessness.
Bronchiectasis (non-pharmacological treatments)
Searches identified three systematic reviews. One of these was excluded because it focussed on surgical intervention only. The remaining reviews (both from the Cochrane Database) featured nurse specialist care (1 trial) [154] and physical training (2 trials) [155]. Bradley et al [155] included data on breathlessness. Both reviews were based on aggregated samples of less than 80 participants. French et al [154] reviewed one trial of nurse-led care (no significant changes in outcome measures or significant difference compared to doctor-led care). Bradley et al [156] included two trials of physical therapy, concluding that inspiratory muscle training improved endurance exercise capacity.
Bronchiectasis (pharmacological treatments)
Searches identified four systematic reviews from the Cochrane database (2 trials [156]; 2 trials [157]; 3 trials [158]; 9 trials [159]) that found evidence of effectiveness. Two reviews [156, 157] were based on samples of more than 54; in a third review [158] it was not possible to determine the size of the aggregated sample. One review [156] included data on breathlessness. Wills & Greenstone [156] concluded that dry powder mannitol was shown to improve tracheobronchial clearance in bronchiectasis. Ram et al [157] found that ICS may improve lung function in bronchiectasis, but that larger trials were required to guide practice. Evans et al [159] found a small benefit from prolonged antibiotic use to interrupt the cycle of bacterial colonisation, inflammatory change and progressive lung damage, but also advocated further, more powerful trials. Crockett et al [158] concluded that there was insufficient evidence to evaluate mucolytics for bronchiectasis. There were also seven systematic reviews that found no evidence of effectiveness of pharmacological treatments in the management of bronchiectasis: for oral methylxanthines [160]; LABAs [161]; SABAs [162]; anticholinergic therapy [163]; leukotriene receptor antagonists [164]; oral steroids [165]; and pneumococcal vaccines [166].
Pulmonary sarcoidosis, pulmonary fibrosis, interstitial lung disease
Five systematic reviews (Cochrane Database) were considered, three of which included data on breathlessness [167–169]. Paramothayan et al [170] reviewed 13 trials of corticosteroids for pulmonary sarcoidosis, concluding that oral steroids may be of use in some circumstances. Paramothayan et al [168] further reviewed five trials and found limited evidence to support the use of immunosuppressive and cytotoxic therapy for this condition. Davis et al [167] reviewed three trials and found little to justify routine use of non-corticosteroid agents for idiopathic pulmonary fibrosis (IPF), and Richeldi et al [171] found no evidence for an effect of corticosteroid treatment in patients with IPF. Polosa et al [169] reviewed a single small trial of nebulised morphine in interstitial lung disease (six participants), which reported no improvement in maximal exercise performance or reduction in breathlessness during exercise.
Chronic bronchitis
Staykova et al [172] reviewed nine trials of prophylactic antibiotic use in chronic bronchitis/COPD, concluding that this treatment does significantly reduce days of illness due to exacerbations. Routine use is not recommended due to concerns about antibiotic resistance and it is noted that available data are over 30 years old.
Exercise-induced broncho-constriction
Three reviews were considered from the Cochrane Database [173–175]. Spooner et al [173] (24 trials) compared the effects of inhaled mast-cell stabilisers with single dose SABAs or anticholinergics prior to exercise, concluding that all provided relief against broncho-constriction. Overall, SABAs were more effective than mast-cell stabilisers, which were in turn more effective than anticholinergics. Spooner et al [174] (20 trials) concluded that a single dose of a mast-cell stabiliser (nedocromil sodium) before exercise reduces the severity and duration of exercise-induced bronchoconstriction. Kelly et al [175] (8 trials) found no difference between the effect of nedocromil sodium and sodium cromoglycate on lung function following exercise.
Pulmonary arterial hypertension (PAH)
Three reviews were considered from the Cochrane Database [176–178]. All included data on breathlessness. Liu & Chen [176] reviewed five trials that evaluated endothelin receptor antagonists (ERAs), finding that, in conjunction with conventional therapy, their use improved Borg dyspnoea scores and exercise capacity amongst patients with idiopathic PAH. Paramothayan et al [177] reviewed nine trials that considered the effects of prostacyclin with conventional therapy, identifying some short-term benefits (e.g. on exercise capacity). Kanthapillai et al [178] reviewed four trials that considered the effects of the vasodilator sildenafil, concluding that the benefits observed in small trials required further validation in more adequately-sized studies.
Cystic fibrosis
Reid et al [179] reviewed two trials concerning the effect of inspiratory muscle training (IMT) on patients with cystic fibrosis. Individual study results were found to be inconclusive for improvement in inspiratory muscle strength, though one study demonstrated improvement in inspiratory muscle strength endurance. The authors concluded that the benefit of IMT in cystic fibrosis for outcomes in inspiratory muscle strength was supported by weak evidence, but that its impact on exercise capacity, dyspnoea and quality of life was unclear.
Discussion
Improvements are needed in the management of breathlessness in both malignant and non-malignant conditions. Asthma affects an approximately 5.2 million people in the UK [180], with an estimated 42% of them facing significant challenges in their daily lives due to breathlessness [181]. COPD is projected to rank as the world's third leading cause of death by 2030 [182], while the reported frequency of breathlessness in cancer varies depending upon the stage of disease [183]. As disease becomes advanced, so the incidence of breathlessness tends to increase [3–5]. In advanced cardiorespiratory disease, for example, refractory breathlessness is described as a 'devastating' symptom with few effective palliative options [184]. Solano et al [185] found that breathlessness was one of three 'particularly universal' symptoms (with pain and fatigue) that affected more than 50% of patients with far advanced cancer, AIDS, heart disease, COPD and renal disease. Despite this high prevalence and level of severity, breathlessness and other related symptoms are under-assessed and under-treated [185].
Our review of systematic reviews suggests that while breathlessness in some non-malignant conditions has been intensively addressed, mostly through a small range of mainly pharmacological approaches, there are other conditions that have received little or no attention. The majority of systematic reviews we identified have been conducted on interventions for asthma and COPD. In comparison, no other category of disease produced more than six reviews for pharmacological and non-pharmacological approaches combined.
The emphasis in systematic reviews of pharmacological interventions for asthma is clearly on beta-agonists and corticosteroids, with the use of these for relevant asthma-related conditions and in combination with other appropriate pharmacological agents receiving wide support [59, 60, 62, 69, 71–75, 88, 91, 96], though with some safety concerns [62]. Reviews that address the 'steroid-sparing' or rescue medication reduction features of various approaches to the pharmacological treatment of asthma are an important sub-group of studies, given the known side-effect profile of this class of drugs [62, 64–66, 68, 70–73, 75, 77, 82–84, 87, 88, 92, 93, 96, 98, 103–105].
Systematic reviews of non-pharmacological interventions for asthma are limited by a lack of good quality evidence from randomised controlled trials. The largest review of non-pharmacological interventions, which assessed the effects of asthma self-management programmes with regular health practitioner review did, however, conclude that this approach, particularly when it includes training to use written action plans to adjust medication, can improve health outcomes in adults with asthma [47].
Bronchodilators, including beta-agonists, are the focus for the majority of reviews of pharmacological interventions for COPD. Positive results were identified among these, and for a number of other agents, including corticosteroids, vaccine, and mucolytics, although balancing potential benefits against known risks remains important. Non-pharmacological interventions with positive outcomes appear to be more established in COPD than in asthma, at least as far as number of trials is concerned. In a number of cases successful interventions were broadly rehabilitative in nature: home care from outreach nursing programmes was shown to have benefits in terms of survival and health related quality of life [127], hospital at home was an effective option for some patients attending emergency departments with an acute exacerbation of their condition [127], pulmonary rehabilitation was found to relieve breathlessness and fatigue, improve emotional function, and enhance sense of control [112], and use of two or more components of the chronic care model resulted in reduced hospitalisations and emergency visits and reduced length of hospital stay [120].
Benefits for a number of outcomes in COPD, including breathlessness, were also found to be provided by IMT within four studies [114–116, 124], though one study found that it was unclear whether any such improvement is mediated through improved muscle strength and endurance [123]. Inspiratory muscle training was also found to improve breathing, exercise capacity and quality of life in Bradley et al [155] review of physical training for bronchiectasis, although a similar review in patients with cystic fibrosis showed inconclusive results [179].
In contrast to reviews of asthma treatments, data on breathlessness were included in the majority of reviews of interventions for COPD, both pharmacological and non-pharmacological. Given the distressing nature of breathlessness from the point of view of those who experience it, it is surprising that more studies do not include a specific measure of this symptom. Breathlessness and dyspnoea characterise a range of qualitatively distinct experiences of breathing discomfort, and therefore, outcome measures should arguably take account of patients' perceptions and the language patients use to describe the symptom [186–188]. However, as Bausewein et al [189] point out, no one of the many assessment tools available is comprehensive enough as a single instrument to measure the sensation of breathlessness, its effect on quality of life, and response to treatment. Indeed, this review suggests that the research may emphasise physiological lung changes rather than the subjective experience of a very distressing symptom. It also suggests that findings may reflect lung function rather than symptom experience or symptom distress.
A considerable proportion of the original trials reported in the systematic reviews included either proxy measures for breathlessness (e.g. lung function), used unvalidated measures or used single composite scores for 'respiratory symptoms' that differed both within and between systematic reviews, so providing only indications for the level of improvement related to breathlessness per se. This is quite problematic as the 'validity' and sensitivity of such methods to accurately measure a complex, subjective symptom experience is questionable and can lead to biases, as there is a significant issue with the interpretation of the data, which clinicians often find confusing. Future research should address these measurement issues and use data from validated, patient-reported outcome measures (PROMS) of breathlessness as the primary outcome (e.g. the MRC dyspnoea scale [190] or Dyspnoea-12 [188]), in adequately powered trials.
With the exception of research into treatments for asthma and COPD, the majority of intervention studies, as reflected in our review, continue to focus on pharmacological approaches. We have included two reviews of non-pharmacological interventions for bronchiectasis, but in all other disease categories we found no further examples. More research should focus in the future on the management of breathlessness in respiratory diseases other than asthma and COPD.
As pharmacological treatments do not completely manage breathlessness and they have the added burden of side effects, it seems imperative that non-pharmacological interventions are seen as appropriate complementary interventions to standard pharmacological management, or even an alternative method in selected patients. Despite good evidence from the literature, many non-pharmacological interventions have not become mainstream and are not used in the rehabilitation of patients with respiratory diseases. New ways of thinking about evaluation research, and new combinations of pharmacological and non-pharmacological interventions may be needed for symptoms like breathlessness that continue to impose a heavy burden of distress across a wide range of disease types.
There are also a number of non-pharmacological studies that have shown some promise, although conclusions are difficult due to the small sample sizes they have used and limited replication. Such interventions include oxygen, self-management approaches, breathing exercises, use of fans, acupuncture/acupressure, calorie controlled diet, selenium supplementation, approaches to reduce anxiety and panic, or those interventions that may be theoretically useful but for which no studies have been carried out so far (e.g. Alexander technique or other complementary therapies). Future research should focus on such promising approaches and provide a good evidence-base for practice.
Conclusions
While the reviews have covered in excess of 2,000 trials, the management of breathlessness remains far from ideal and patients suffer unnecessarily, particularly in the advanced stages of their illness. The development of society guidelines and agreed standards of care should provide a good way for clinicians to navigate across the diversity of potential treatments for breathlessness. Such guidelines should incorporate both pharmacological and non-pharmacological interventions where appropriate. More research is needed in areas of potential benefit, with particular emphasis in the accurate and reliable assessment of breathlessness and in ways that could improve the quality of life as well as health care provision for patients experiencing this distressing symptom. The recent development of clinical guidelines for the management of breathlessness/dyspnea provides some direction for the clinicians in this complex area of symptom management [191].
References
American Thoracic Society: Dyspnea: mechanisms, assessment, and management: a consensus statement. Am J Respir Crit Care Med. 1999, 159: 321-340.
Karnani NG, Reisfield GM, Wilson GR: Evaluation of chronic dyspnea. Am Fam Physician. 2005, 71 (8): 1529-1537.
Cox C: Non-pharmacological treatment of breathlessness. Nurs Standard. 2002, 16 (24): 33-36.
Luddington L, Cox S, Higginson I, Livesley B: The need for palliative care for patients with non-cancer diseases: a review of the evidence. Int J Palliat Nurs. 2001, 7 (5): 221-226.
Rawlinson F: Dyspnoea and cough. Eur J Palliat Care. 2000, 7 (5): 161-164.
Cranston JM, Crockett A, Currow D: Oxygen therapy for dyspnoea in adults. Cochrane Database of Systematic Reviews. 2008, CD004769-
Bailey PH: The dyspnea-anxiety-dyspnea cycle-COPD patients' stories of breathlessness: "it's scary/when you can't breathe". Qual Health Res. 2004, 14 (6): 760-778. 10.1177/1049732304265973.
Harris S: COPD and coping with breathlessness at home: a review of the literature. Br J Community Nur. 2007, 12 (9): 411-415.
Seamark DA, Blake SD, Seamark CJ: Living with severe chronic obstructive pulmonary disease: perceptions of patients and their carers. Pallit Med. 2004, 18 (7): 619-625. 10.1191/0269216304pm928oa.
Simon NM, Weiss AM, Kradin R, Evans KC, Reese HE, Otto MW, Oppenheimer JE, Smoller JW, Zalta A, Worthington III JJ, Pollack MH: The relationship of anxiety disorders, anxiety sensitivity and pulmonary dysfunction with dyspnea-related distress and avoidance. J Nerv Ment Dis. 2006, 194 (12): 951-957. 10.1097/01.nmd.0000249062.25829.53.
Smoller JW, Pollack MH, Otto MW, Rosenbaum JF, Kradin RL: Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am J Respir Crit Care Med. 1996, 154 (1): 6-17.
Clark AL, Sparrow JL, Coats AJS: Muscle fatigue and dyspnoea in chronic heart failure: two sides of the same coin?. Eur Heart J. 1995, 16 (1): 49-52.
Witte KA, Clark AL: Dyspnoea versus fatigue: additional prognostic information from symptoms in chronic heart failure?. Eur J of Heart Failure. 2008, 10 (12): 1224-1228. 10.1016/j.ejheart.2008.09.017.
Maurer J, Rebbapragada V, Borson S, Goldstein R, Kunik ME, Yohannes AM, Hanania NA: Anxiety and depression in COPD. Chest. 2008, 134: 43S-56S. 10.1378/chest.08-0342.
Roberts DK, Thorne SE, Pearson C: The experience of dyspnoea in late stage cancer: patients' and nurse perspectives. Cancer Nurs. 1993, 16 (4): 310-320. 10.1097/00002820-199308000-00008.
Abidov A, Rozanski A Hachamovitch R, Hayes SW, Aboul-Enein F, Cohen I, Friedman JD Germano G, Berman DS: Prognostic significance of dyspnea in patients referred for cardiac stress testing. N Eng J Med. 2005, 353: 1889-1898. 10.1056/NEJMoa042741.
Frostad A, Soyseth V, Haldorsen T, Andersen A, Gulsvik A: Respiratory symptoms and long-term cardiovascular mortality. Respir Med. 2007, 101 (11): 2289-2296. 10.1016/j.rmed.2007.06.023.
Tessier JF, Nejjari C, Letenneur L, Filleul L, Marty ML, Barberger Gateau P, Dartigues JF: Dyspnea and 8-year mortality among elderly men and women: the PAQUID cohort study. Eur J of Epidemiology. 2001, 17: 223-229. 10.1023/A:1017977715073.
Jones PW: Activity limitation and quality of life in COPD. J COPD. 2007, 4: 273-278. 10.1080/15412550701480265.
Donaldson GC, Wilkinson TMA, Hurst JR, Perera WR, Wedzicha JA: Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005, 171: 446-452. 10.1164/rccm.200408-1054OC.
Murray CJL, Lopez AD: Alternative projections of mortality and disability by cause 1990-2020: global burden of disease study. Lancet. 1997, 349: 1498-1504. 10.1016/S0140-6736(96)07492-2.
Lopez AD, Murray CCJL: The global burden of disease, 1990-2020. Nat Med. 1998, 4 (11): 1241-1243. 10.1038/3218.
Gysels M, Higginson IJ: Access to Services for Patients with Chronic Obstructive Pulmonary Disease: The Invisibility of Breathlessness. J Pain & Symptom Manage. 2008, 36: 451-460.
Gore JM, Brophy CJ, Greenstone MA: How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000, 55: 1000-1006. 10.1136/thorax.55.12.1000.
Ripamonti C, Fusco F: Respiratory problems in advanced cancer. Support Care Cancer. 2001, 10: 204-216. 10.1007/s005200100296.
Bruera E, Schmitz B, Pither J, Neuman CM, Hanson J: The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2006, 19: 357-362. 10.1016/S0885-3924(00)00126-3.
Booth S, Moosavi SH, Higginson IJ: The etiology and management of intractable breathlessness in patients with advanced cancer: a systematic review of pharmacological therapy. Nature Clin Pract. 2008, 5 (2 February): 90-100. 10.1038/ncponc1034.
Bauswein C, Booth S, Gysels M, Higginson IJ: Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases (Review). Cochrane Database of Systematic Reviews. 2008, CD005623-
Rodrigo G, Pollack C, Rodrigo C, Rowe BH: Heliox for nonintubated acute asthma patients. Cochrane Database of Systematic Reviews. 2006, CD002884-
Ram FSF, Wellington SR, Barnes NC: Inspiratory muscle training for asthma. Cochrane Database of Systematic Reviews. 2003, CD003792-
Ho AM-H, Lee A, Karmaker MK, Dion PW, Chung DC, Contardi L-A H: Heliox vs air-oxygen mixtures for the treatment of patients with acute asthma - A systematic review. Chest. 2003, 123: 882-890. 10.1378/chest.123.3.882.
McCarney RW, Brinkhaus B, Lasserson TJ, Linde K: Acupuncture for chronic asthma. Cochrane Database of Systematic Reviews. 2003, CD000008-
Ram FSF, Robinson SM, Black PN, Picot J: Physical training for asthma. Cochrane Database of Systematic Reviews. 2005, CD001116-
Holloway E, Ram FSF: Breathing exercises for asthma. Cochrane Database of Systematic Reviews. 2004, CD001277-
Cheng J, Pan T, Ye GH, Liu Q: Calorie controlled diet for chronic asthma. Cochrane Database of Systematic Reviews. 2003, CD004674-
Thien FCK, De Luca S, Woods R, Abramson MJ: Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database of Systematic Reviews. 2002, CD001283-
Ram FSF, Ardern KD: Dietary salt reduction or exclusion for allergic asthma. Cochrane Database of Systematic Reviews. 2004, CD000436-
Ram FS, Ardern KD: Tartrazine exclusion for allergic asthma. Cochrane Database of Systematic Reviews. 2001, CD000460-
Ram FSF, Rowe BH, Kaur B: Vitamin C supplementation for asthma. Cochrane Database of Systematic Reviews. 2004, CD000993-
McCarney RW, Linde K, Lasserson TJ: Homeopathy for chronic asthma. Cochrane Database of Systematic Reviews. 2004, CD000353-
Gotzsche PC, Johansen HK, Schmidt LM, Burr ML: House dust mite control measures for asthma. Cochrane Database of Systematic Reviews. 2004, CD001187-
Kilburn S, Lasserson TJ, McKean M: Pet allergen control measures for allergic asthma in children and adults. Cochrane Database of Systematic Reviews. 2001, CD002989-
Singh M, Bara A, Gibson P: Humidity control for chronic asthma. Cochrane Database of Systematic Reviews. 2002, CD003563-
Blackhall K, Appleton S, Cates CJ: Ionisers for chronic asthma. Cochrane Database of Systematic Reviews. 2003, CD002986-
Gibson PG, Powell H, Coughlan J, Wilson AJ, Hensley MJ, Abramson M, Bauman A, Walters EH: Limited (information only) patient education programs for adults with asthma. Cochrane Database of Systematic Reviews. 2002, CD001005-
Powell H, Gibson PG: Options for self-management education for adults with asthma. Cochrane Database of Systematic Reviews. 2002, CD004107-
Gibson PG, Powell H, Coughlan J, Wilson AJ, Abramson M, Haywood P, Bauman A, Hensley MJ, Walters EH: Self-management education and regular practitioner review for adults with asthma. Cochrane Database of Systematic Reviews. 2002, CD001117-
Hondras MA, Linde K, Jones AP: Manual therapy for asthma. Cochrane Database of Systematic Reviews. 2005, CD001002-
Ram FSF, Jones A, Fay JK: Primary care based clinics for asthma. Cochrane Database of Systematic Reviews. 2002, CD003533-
Yorke J, Fleming SL, Shuldham CM: Psychological interventions for adults with asthma. Cochrane Database of Systematic Reviews. 2006, CD002982-
Smith J, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, Koutantji M, Upton C, Harvey I: A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma. Health Technol Assess. 2005, 9 (23): iii-iv, 1-167.
Toelle BG, Ram FSF: Written individualised management plans for asthma in children and adults. Cochrane Database of Systematic Reviews. 2004, CD002171-
Ram FSF, Wellington SR, Rowe B, Wedzicha JA: Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database of Systematic Reviews. 2005, CD004360-
Allam MF, Lucena RA: Selenium supplementation for asthma. Cochrane Database of Systematic Reviews. 2004, CD003538-
Dennis J, Cates CJ: Alexander technique for chronic asthma. Cochrane Database of Systematic Reviews. 2000, CD000995-
Campbell F, Jones K, Gibson P: Feather versus non-feather bedding for asthma. Cochrane Database of Systematic Reviews. 2000, CD002154-
Westby M, Benson M, Gibson P: Anticholinergic agents for chronic asthma in adults. Cochrane Database of Systematic Reviews. 2004, CD003269-
Salpeter S, Ormiston T, Salpeter E, Wood-Baker R: Cardioselective beta-blockers for reversible airway disease. Cochrane Database of Systematic Reviews 2002. 2002, CD002992-
Manser R, Reid D, Abramson M: Corticosteroids for acute severe asthma in hospitalised patients. Cochrane Database of Systematic Reviews. 2001, CD001740-
Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW: Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database of Systematic Reviews. 2001, CD000195-
Travers A, Jones AP, Kelly K, Barker SJ, Camargo CA, Rowe BH: Intravenous beta2-agonists for acute asthma in the emergency department. Cochrane Database of Systematic Reviews. 2001, CD002988-
Walters EH, Gibson PG, Lasserson TJ, Walters JAE: Long-acting beta2-agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid. Cochrane Database of Systematic Reviews. 2007, CD001385-
Richter B: Long- and Short-Acting Inhaled Beta~2-Agonists: Do Benefit-Risk-Ratios Differ in the Treatment of Bronchial Asthma and COPD?. Z Allg Med. 2006, 82 (6): 253-258. 10.1055/s-2006-933519.
Ducharme F, Schwartz Z, Kakuma R: Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database of Systematic Reviews 2004. 2004, CD003133-
Ducharme FM, Di Salvio F: Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database of Systematic Reviews. 2004, CD002314-
Ni Chroinin M, Greenstone IR, Ducharme FM: Addition of inhaled long-acting beta2-agonists to inhaled steroids as first line therapy for persistent asthma in steroid-naive adults. Cochrane Database of Systematic Reviews. 2004, CD005307-
Parameswaran K, Belda J, Rowe BH: Addition of intravenous aminophylline to beta2-agonists in adults with acute asthma. Cochrane Database of Systematic Reviews. 2000, CD002742-
Greenstone IR, Ni Chroinin MN, Masse V, Danish A, Magdalinos H, Zhang X, Ducharme FM: Combination of inhaled long-acting beta2-agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma. Cochrane Database of Systematic Reviews. 2005, CD005533-
Camargo CA, Spooner CH, Rowe BH: Continuous versus intermittent beta-agonists for acute asthma. Cochrane Database of Systematic Reviews. 2003, CD001115-
Walters EH, Walters J, Gibson P, Jones PW: Inhaled short acting beta2-agonist use in chronic asthma: regular versus as needed treatment. Cochrane Database of Systematic Reviews. 2003, CD001285-
Gibson PG, Powell H, Ducharme F: Long-acting beta2-agonists as an inhaled corticosteroid-sparing agent for chronic asthma in adults and children. Cochrane Database of Systematic Reviews. 2005, CD005076-
Ducharme FM, Lasserson TJ, Cates CJ: Long-acting beta2-agonists versus anti-leukotrienes as add-on therapy to inhaled corticosteroids for chronic asthma. Cochrane Database of Systematic Reviews. 2006, CD003137-
Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, Ducharme FM: Long-acting beta2-agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma. Cochrane Database of Systematic Reviews. 2005, CD005535-
Shah L, Wilson AJ, Gibson PG, Coughlan J: Long-acting beta2-agonists versus theophylline for maintenance treatment of asthma. Cochrane Database of Systematic Reviews. 2003, CD001281-
Walters EH, Walters JAE, Gibson PW: Regular treatment with long acting beta agonists versus daily regular treatment with short acting beta agonists in adults and children with stable asthma. Cochrane Database of Systematic Reviews. 2002, CD003901-
Abramson MJ, Puy RM, Weiner JM: Allergen immunotherapy for asthma. Cochrane Database of Systematic Reviews. 2003, CD001186-
Walker S, Monteil M, Phelan K, Lasserson TJ, Walters EH: Anti-IgE for chronic asthma in adults and children. Cochrane Database of Systematic Reviews. 2006, CD003559-
Graham V, Lasserson TJ, Rowe BH: Antibiotics for acute asthma. Cochrane Database of Systematic Reviews. 2001, CD002741-
Dean T, Dewey A, Bara A, Lasserson TJ, Walters EH: Azathioprine as an oral corticosteroid sparing agent for asthma. Cochrane Database of Systematic Reviews. 2003, CD003270-
Adams N, Bestall J, Jones P: Beclomethasone at different doses for chronic asthma. Cochrane Database of Systematic Reviews. 1999, CD002879-
Adams N, Bestall JM, Jones PW: Beclomethasone versus budesonide for chronic asthma. Cochrane Database of Systematic Reviews. 2000, CD003530-
Adams NP, Bestall JB, Malouf R, Lasserson TJ, Jones PW: Beclomethasone versus placebo for chronic asthma. Cochrane Database of Systematic Reviews. 2005, CD002738-
Adams N, Bestall J, Jones P: Budesonide at different doses for chronic asthma. Cochrane Database of Systematic Reviews. 2000, CD003271-
Adams N, Bestall J, Jones PW: Budesonide versus placebo for chronic asthma in children and adults. Cochrane Database of Systematic Reviews. 1999, CD003274-
Bara AI, Barley EA: Caffeine for asthma. Cochrane Database of Systematic Reviews. 2001, CD001112-
Dewey A, Dean T, Bara A, Lasserson TJ, Walters EH: Chloroquine as a steroid sparing agent for asthma. Cochrane Database of Systematic Reviews. 2003, CD003275-
Powell H, Gibson PG: High dose versus low dose inhaled corticosteroid as initial starting dose for asthma in adults and children. Cochrane Database of Systematic Reviews. 2003
Guevara JP, Ducharme FM, Keren R, Nihtianova S, Zorc J: Inhaled corticosteroids versus sodium cromoglycate in children and adults with asthma. Cochrane Database of Systematic Reviews. 2006, CD003558-
Edmonds ML, Camargo CA, Brenner BE, Rowe BH: Inhaled steroids for acute asthma following emergency department discharge. Cochrane Database of Systematic Reviews. 2000, CD002316-
Mash B, Bheekie A, Jones PW: Inhaled versus oral steroids for adults with chronic asthma. Cochrane Database of Systematic Reviews. 2001, CD002160-
Taramarcaz P, Gibson PG: Intranasal corticosteroids for asthma control in people with coexisting asthma and rhinitis. Cochrane Database of Systematic Reviews. 2003, CD003570-
Evans DJ, Cullinan P, Geddes DM, Walters EH, Milan SJ, Jones PW: Cyclosporin as an oral corticosteroid sparing agent in stable asthma. Cochrane Database of Systematic Reviews. 2000, CD002993-
Adams NP, Bestall JC, Jones PW, Lasserson TJ, Griffiths B, Cates CJ: Fluticasone at different doses for chronic asthma in adults and children. Cochrane Database of Systematic Reviews. 2005, CD003534-
Lasserson TJ, Cates CJ, Jones A-B, Steele EH, White J: Fluticasone versus HFA-beclomethasone dipropionate for chronic asthma in adults and children. Cochrane Database of Systematic Reviews. 2006, CD005309-
Adams N, Bestall JM, Lasserson TJ, Jones PW: Fluticasone versus beclomethasone or budesonide for chronic asthma in adults and children. Cochrane Database of Systematic Reviews. 2005, CD002310-
Adams NP, Bestall JC, Lasserson TJ, Jones PW, Cates CJ: Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews. 2005, CD003135-
Gibson PG, Henry RL, Coughlan JL: Gastro-oesophageal reux treatment for asthma in adults and children. Cochrane Database of Systematic Reviews. 2003, CD001496-
Evans DJ, Cullinan P, Geddes DM, Walters EH, Milan SJ, Jones PW: Gold as an oral corticosteroid sparing agent in stable asthma. Cochran Database of Systematic Reviews. 2000, CD002985-
Blitz M, Blitz S, Beasely R, Diner BM, Hughes R, Knopp JA, Rowe BH: Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database of Systematic Reviews. 2005, CD003898-
Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA: Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database of Systematic Reviews. 2000, CD001490-
Richeldi L, Ferrara G, Fabbri LM, Lasserson TJ, Gibson PG: Macrolides for chronic asthma. Cochrane Database of Systematic Reviews. 2005, CD002997-
Evans DJ, Cullinan P, Geddes DM, Walters EH, Milan SJ, Jones PW: Troleandomycin as an oral corticosteroid sparing agent in stable asthma. Cochrane Database of Systematic Reviews. 2000, CD002987-
Davies H, Olson L, Gibson P: Methotrexate as a steroid sparing agent for asthma in adults. Cochrane Database of Systematic Reviews. 1998, CD000391-
Hayashi K, Yanagi M, Wood-Baker R, Takamatsu I, Anami K: Oxatomide for stable asthma in adults and children. Cochrane Database of Systematic Reviews. 2003, CD002179-
Petsky HL, Kynaston JA, Turner C, Li AM, Cates CJ, Lasserson TJ, Chang AB: Tailored interventions based on sputum eosinophils versus clinical symptoms for asthma in children and adults. Cochrane Database of Systematic Reviews. 2007, CD005603-
Dewey A, Dean T, Bara A, Lasserson TJ, Walters EH: Colchicine as an oral corticosteroid sparing agent for asthma. Cochrane Database of Systematic Reviews. 2003, CD003273-
Dewey A, Bara A, Dean T, Walters EH: Dapsone as an oral corticosteroid sparing agent for asthma. Cochrane Database of Systematic Reviews. 2002, CD003268-
Ram FSF, Wedzicha JA: Ambulatory oxygen for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2008, CD000238-
Jones AP, Rowe BH: Bronchopulmonary hygiene physical therapy for chronic obstructive pulmonary disease and bronchiectasis. Cochrane Database of Systematic Reviews. 1998, CD000045-
Rodrigo G, Pollack C, Rodrigo C, Rowe B, Walters EH: Heliox for treatment of exacerbations of chronic obstructive pulmonarydisease. Cochrane Database of Systematic Reviews. 2001, CD003571-
Nonoyama ML, Brooks D, Lacasse Y, Guyatt GH, Goldstein RS: Oxygen therapy during exercise training in chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2007, CD005372-
Lacasse Y, Goldstein R, Lasserson TJ, Martin S: Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2006, CD003793-
Bradley JM, O'Neill B: Short-term ambulatory oxygen for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2005, CD004356-
Geddes EL, Reidb WD, Crowe J, O'Brien K, Brooks D: Inspiratory muscle training in adults with chronic obstructive pulmonary disease: A systematic review. Respir Med. 2005, 99: 1440-1458. 10.1016/j.rmed.2005.03.006.
Geddes EL, O'Brien K, Reid WD, Brooks D, Crowe J: Inspiratory muscle training in adults with chronic obstructive pulmonary disease: an update of a systematic review. Respir Med. 2008, 102: 1715-1729. 10.1016/j.rmed.2008.07.005.
Lotters FB, van Tol G, Kwakkel R: Gosselink: Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur Respir J. 2002, 20: 570-576. 10.1183/09031936.02.00237402.
Chavannes N, Vollenberg JJ, van Schayck CP, Wouters EFM: Effects of physical activity in mild to moderate COPD: a systematic review. Br J Gen Pract. 2002, 52: 574-578.
Rose C, Wallace L, Dickson R, Ayres J, Lehman R, Searle Y, Burge PS: The most effective psychologically-based treatments to reduce anxiety and panic in patients with chronic obstructive pulmonary disease (COPD): a systematic review. Patient Education and Counselling. 2002, 47 (4): 311-318. 10.1016/S0738-3991(02)00004-6.
van 't Hul A, Kwakkel G, Gosselink R: The Acute Effects of Noninvasive Ventilatory Support During Exercise on Exercise Endurance and Dyspnea in Patients With Chronic Obstructive Pulmonary Disease: A Systematic Review. J Cardiopulm Rehabil. 2002, 22: 290-297.
Adams SG, Smith PK, Allan PF, Anzueto A, Pugh JA, Cornell JE: Systematic review of the chronic care model in chronic obstructive pulmonary disease prevention and management. Arch Intern Med. 2007, 167 (6): 551-561. 10.1001/archinte.167.6.551.
O'Neill B, MacMahon J, Bradley J: Short-burst oxygen therapy in chronic obstructive pulmonary disease: Evidence-based review. Respir Med. 2006, 100: 1129-1138.
Young J, Fry-Smith A, Hyde C: Lung volume reduction surgery (LVRS) for chronic obstructive pulmonary disease (COPD) with underlying severe emphysema. Thorax. 1999, 54: 779-789. 10.1136/thx.54.9.779.
Shoemaker MJ, Donker S, LaPoe A: Inspiratory muscle training in patients with chronic obstructive pulmonary disease: the state of the evidence. Cardiopulmon Phy Ther J. 2009, 20 (3): 5-15.
Turnock AC, Walters EH, Walters JAE, Wood-Baker R: Action plans for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2005, CD005074-
Cranston JM, Crockett AJ, Moss JR, Alpers JH: Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2005, CD001744-
Smith B, Appleton S, Adams R, Southcott A, Ruf R: Home care by outreach nursing for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2001, CD000994-
Ram FSF, Wedzicha JA, Wright J, Greenstone M: Hospital at home for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2003, CD003573-
Wijkstra PJ, Lacasse Y, Guyatt GH, Goldstein RS: Nocturnal non-invasive positive pressure ventilation for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2002, CD002878-
Ferreira IM, Brooks D, Lacasse Y, Goldstein RS, White J: Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2005, CD000998-
Ram FSF, Brocklebank DM, Muers M, Wright J, Jones PW: Pressurised metered-dose inhalers versus all other hand-held inhalers devices to deliver bronchodilators for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2002, CD002170-
Salman GF, Mosier MC, Beasley BW, Calkins DR: Rehabilitation for patients with chronic obstructive pulmonary disease: meta-analysis of randomised controlled trials. J Gen Intern Med. 2003, 18 (3): 213-221. 10.1046/j.1525-1497.2003.20221.x.
Monninkhof EM, van der Valk PDLPM, van der Palen J, van Herwaarden CLA, Partidge MR, Walters EH, Zielhuis GA: Self management education for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2002, CD002990-
Austin M, Wood-Baker R: Oxygen therapy in the pre-hospital setting for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2006, CD005534-
Ram FSF, Picot J, Lightowler J, Wedzicha JA: Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2004, CD004104-
McCrory DC, Brown CD: Anticholinergic bronchodilators versus beta2-sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2003, CD003900-
Nannini L, Cates CJ, Lasserson TJ, Poole P: Combined corticosteroid and longacting beta-agonist in one inhaler for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2004, CD003794-
Poole PJ, Chacko E, Wood-Baker RWB, Cates CJ: Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2006, CD002733-
Brown CD, McCrory D, White J: Inhaled short-acting beta2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2001, CD002984-
Granger R, Walters J, Poole PJ, Lasserson TJ, Mangtani P, Cates CJ, Wood-Baker R: Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2006, CD001390-
Appleton S, Jones T, Poole P, Pilotto L, Adams R, Lasserson TJ, Smith B, Muhammad J: Ipratropium bromide versus long acting beta-2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2006, CD006101-
Appleton S, Jones T, Poole P, Pilotto L, Adams R, Lasserson TJ, Smith B, Muhammad J: Ipratropium bromide versus short acting beta-2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2006, CD001387-
Appleton S, Poole P, Smith B, Veale A, Lasserson TJ, Chan MM, Cates CJ: Long-acting beta2-agonists for poorly reversible chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2006, CD001104-
Walters JAE, Walters EH, Wood-Baker R: Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2005, CD005374-
Ram FSF, Jones PW, Castro AA, de Brito Jardim JR, Atallah AN, Lacasse Y, Mazzini R, Goldstein R, Cendon S: Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2002, CD003902-
Sestini P, Renzoni E, Robinson S, Poole P, Ram FSF: Short-acting beta2-agonists for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2002, CD001495-
Wood-Baker RR, Gibson PG, Hannay M, Walters EH, Walters JAE: Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2005, CD001288-
Barr RG, Bourbeau J, Camargo CA, Ram FSF: Tiotropium for stable chronic obstructive pulmonary disease: a meta-analysis. Thorax. 2006, 61: 854-862, July 2006. 10.1136/thx.2006.063271.
Liesker JJW, Wijkstra PJ, Ten Hacken NHT, Keoter GH, Postma DS, Kerstjens HAM: A systematic review of the effects of bronchodilators on exercise capacity in patients with COPD. Chest. 2002, 121: 597-608. 10.1378/chest.121.2.597.
Husereau D, Shukla V, Boucher M, Mensinkai S, Dales R: Long acting β2 agonists for stable chronic obstructive pulmonary disease with poor reversibility: a systematic review of randomised controlled trials. BMC Pulm Med. 2004, 4: 7-10.1186/1471-2466-4-7.
Yang IA, Fong KM, Sim EHA, Black PN, Lasserson TJ: Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2007, CD002991-
Barr RG, Rowe BH, Camargo CA: Methylxanthines for exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2003, CD002168-Art. No CD002168, 2
Poole PJ, Black PN: Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2006, CD001287-
Jennings A-L, Davies , Higgins JPT, Gibbs JSR, Broadley KE: A systematic review of the use of opioids in the management of dyspnoea. Thorax. 2002, 57: 939-944. 10.1136/thorax.57.11.939.
French J, Bilton D, Campbell F: Nurse specialist care for Bronchiectasis. Cochrane Database of Systematic Reviews. 2003, CD004359-
Bradley J, Moran F, Greenstone M: Physical training for bronchiectasis. Cochrane Database of Systematic Reviews. 2002, CD002166-
Wills P, Greenstone M: Inhaled hyperosmolar agents for bronchiectasis (Review). Cochrane Database of Systematic Reviews. 2006
Ram FSF, Wells A, Kolbe J: Inhaled steroids for bronchiectasis. Cochrane Database of Systematic Reviews. 2000, CD000996-
Crockett AJ, Cranston JM, Latimer KM, Alpers JH: Mucolytics for bronchiectasis. Cochrane Database of Systematic Reviews. 2001, CD001289-
Evans DJ, Bara AI, Greenstone M: Prolonged antibiotics for purulent bronchiectasis in children and adults. Cochrane Database of Systematic Reviews. 2007, CD001392-
Steele K, Lasserson JA, Greenstone M: Oral methylxanthines for bronchiectasis. Cochrane Database of Systematic Reviews. 2000, CD002734-
Sheikh A, Nolan D, Greenstone M: Long-acting beta2-agonists for bronchiectasis. Cochrane Database of Systematic Reviews. 2001, CD002155-
Franco F, Sheikh A, Greenstone M: Short acting beta2-agonists for bronchiectasis. Cochrane Database of Systematic Reviews. 2003, CD003572-
Lasserson TJ, Holt K, Evans D, Milan SJ, Greenstone M: Anticholinergic therapy for bronchiectasis. Cochrane Database of Systematic Reviews. 2001, CD002163-
Corless JA, Warburton CJ: Leukotriene receptor antagonists for non-cystic fibrosis bronchiectasis. Cochrane Database of Systematic Reviews. 2000, CD002174-
Lasserson TJ, Holt K, Milan SJ, Greenstone M: Oral steroids for bronchiectasis (stable and acute exacerbations). Cochrane Database of Systematic Reviews. 2001, CD002162-
Chang CC, Singleton RJ, Morris PS, Chang AB: Pneumococcal vaccines for children and adults with bronchiectasis. Cochrane Database of Systematic Reviews. 2007, CD006316-
Davies HR, Richeldi L, Walters EH: Immunomodulatory agents for idiopathic pulmonary fibrosis. Cochrane Database of Systematic Reviews, D003134. 2003, CD003134-
Paramothayan S, Lasserson TJ, Walters EH: Immunosuppressive and cytotoxic therapy for pulmonary sarcoidosis. Cochrane Database of Systematic Reviews. 2006, CD003536-
Polosa R, Simidchiev A, Walters EH: Nebulised morphine for severe interstitial lung disease. Cochrane Database of Systematic Reviews. 2002, CD002872-
Paramothayan NS, Lasserson TJ, Jones PW: Corticosteroids for pulmonary sarcoidosis. Cochrane Database of Systematic Reviews. 2005, CD001114-
Richeldi L, Davies HR, Ferrara G, Franco F: Corticosteroids for idiopathic pulmonary fibrosis. Cochrane Database of Systematic Reviews. 2003, CD002880-
Staykova T, Black P, Chacko E, Ram FSF, Poole P: Prophylactic antibiotic therapy for chronic bronchitis. Cochrane Database of Systematic Reviews. 2001, CD004105-
Spooner CH, Spooner GR, Rowe BH: Mast-cell stabilising agents to prevent exercise-induced broncho-constriction. Cochrane Database of Systematic Reviews. 2003, CD002307-
Spooner CH, Saunders LD, Rowe BH: Nedocromil sodium for preventing exercise-induced broncho-constriction. Cochrane Database of Systematic Reviews. 2002, CD001183-
Kelly K, Spooner CH, Rowe BH: Nedocromil sodium versus sodium cromoglycate for preventing exercise-induced broncho-constriction in asthmatics. Cochrane Database of Systematic Reviews. 2000, CD002731-
Liu C, Chen J: Endothelin receptor antagonists for pulmonary arterial hypertension. Cochrane Database of Systematic Reviews. 2006, CD004434-
Paramothayan NS, Lasserson TJ, Wells AU, Walters EH: Prostacyclin for pulmonary hypertension in adults. Cochrane Database of Systematic Reviews. 2005, CD002994-
Kanthapillai P, Lasserson TJ, Walters EH, Sildena l: Sildenafil for pulmonary hypertension. Cochrane Database of Systematic Reviews. 2004, CD003562-
Reid WD, Geddes EL, O'Brien K, Brooks D, Crowe J: Effects of inspiratory muscle training in cystic fibrosis. Clin Rehabil. 2008, 22: 1003-1013. 10.1177/0269215508090619.
Asthma UK: Where do we stand? Asthma in the UK today. London, Asthma UK. 2004
Smith NM: The 'needs of people with asthma' survey and initial presentation of the data. Asthma Journal. 2000, 5: 133-7.
World Health Organisation: World Health Statistics. 2008, [http://www.who.int/whosis/whostat/EN_WHS08_Full.pdf]
Lutz S, Norell R, Bertucio C, Kachnic L, Johnson C, Arthur D: Symptom frequency and severity in patients with metastatic or locally recurrent lung cancer: a prospective study using the lung cancer symptom scale in a community hospital. Journal of Palliative Medicine. 2001, 4 (2): 157-65. 10.1089/109662101750290191.
Booth S, Bausewein C, Higginson I, Moosavi SH: Pharmacological treatment of refractory breathlessness. Expert Rev Resp Med. 2009, 3 (1): 21-36. 10.1586/17476348.3.1.21.
Solano JP, Gomes B, Higginson IJ: A Comparison of Symptom Prevalence in Far Advanced Cancer, AIDS, Heart Disease, Chronic Obstructive Pulmonary Disease and Renal Disease. J Pain Symptom Manage. 2006, 31 (1): 58-69. 10.1016/j.jpainsymman.2005.06.007.
Scano G, Stendardi L, Grazzini M: Understanding Dyspnoea by its language. Eur Respir J. 2005, 25: 380-385. 10.1183/09031936.05.00059404.
Coli C, Picariello M, Stendardi L Grazzini M, Binazzi B, Duranti R, Scano G: Is there a link between the qualitative descriptors and the quantitative perception of dyspnoea in asthma?. Chest. 2002, 130: 1072-1081.
Yorke J, Moosavi SH, Shuldham C, Jones PW: Quantification of dyspnoea using descriptors: development and initial testing of the Dyspnoea-12. Thorax. 2010, 65: 21-26. 10.1136/thx.2009.118521.
Bausewein C, Farquhar M, Booth S, Gysels , Higgins IJ: Measurement of breathlessness in advanced disease: a systematic review. Resp Med. 2007, 101: 339-410. 10.1016/j.rmed.2006.07.003.
Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA: Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999, 54: 581-586. 10.1136/thx.54.7.581.
Mahler DA, Selecky PA, Harrod CG, Benditt JO, Carrieri-Kohlman V, Curtis JR, Manning HL, Mularski RA, Varkey B, Campbell M, Carter ER, Chiong JR, Ely EW, Hansen-Flaschen J, O'Donnell DE, Waller A: Consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010, 137: 674-691. 10.1378/chest.09-1543.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2466/10/63/prepub
Acknowledgements
This review was partly funded by The Breathlessness Research Charitable Trust, UK and the National Cancer Research Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
Conception of study, development of protocol, study coordination: AM; Searches: RD, RW; Data Extraction, Data Verification, Arbitration: AM, RD, RW, AC, JS; Data Synthesis/Analysis: CB, RW, AM, AC, JS; Data discussions: All authors; Drafting paper: AM, RW, CB. All authors read and approved the final manuscript.
Electronic supplementary material
12890_2010_233_MOESM1_ESM.DOC
Additional File 1: Table 1. Systematic reviews of interventions for asthma. List of all the studies reviewed under asthma and the data reported in the reviews. (DOC 176 KB)
12890_2010_233_MOESM2_ESM.DOC
Additional File 2: Table 2. Systematic reviews of interventions for chronic obstructive pulmonary disease (COPD). List of all the studies reviewed under COPD and the data reported in the reviews. (DOC 130 KB)
12890_2010_233_MOESM3_ESM.DOC
Additional File 3: Table 3. Systematic reviews of interventions for other respiratory diseases. List of all the studies reviewed under other respiratory diseases and the data reported in the reviews. (DOC 66 KB)
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bailey, C.D., Wagland, R., Dabbour, R. et al. An integrative review of systematic reviews related to the management of breathlessness in respiratory illnesses. BMC Pulm Med 10, 63 (2010). https://doi.org/10.1186/1471-2466-10-63
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2466-10-63