Abstract
Background
The authors sought to monitor the impact of widespread varicella vaccination on the epidemiology of varicella and herpes zoster. While varicella incidence would be expected to decrease, mathematical models predict an initial increase in herpes zoster incidence if re-exposure to varicella protects against reactivation of the varicella zoster virus.
Methods
In 1998–2003, as varicella vaccine uptake increased, incidence of varicella and herpes zoster in Massachusetts was monitored using the random-digit-dial Behavioral Risk Factor Surveillance System.
Results
Between 1998 and 2003, varicella incidence declined from 16.5/1,000 to 3.5/1,000 (79%) overall with ≥66% decreases for all age groups except adults (27% decrease). Age-standardized estimates of overall herpes zoster occurrence increased from 2.77/1,000 to 5.25/1,000 (90%) in the period 1999–2003, and the trend in both crude and adjusted rates was highly significant (p < 0.001). Annual age-specific rates were somewhat unstable, but all increased, and the trend was significant for the 25–44 year and 65+ year age groups.
Conclusion
As varicella vaccine coverage in children increased, the incidence of varicella decreased and the occurrence of herpes zoster increased. If the observed increase in herpes zoster incidence is real, widespread vaccination of children is only one of several possible explanations. Further studies are needed to understand secular trends in herpes zoster before and after use of varicella vaccine in the United States and other countries.
Similar content being viewed by others
Background
Prior to the licensure of varicella vaccine in the U.S. in 1995, varicella (chickenpox) was a highly prevalent disease of childhood, resulting in approximately four million cases and an average of 10,630–13,500 hospitalizations and 90 deaths in the U.S. each year [1–4]. After primary infection, the varicella zoster virus (VZV) lies dormant in sensory ganglia and can reactivate later, producing herpes zoster (shingles) and sometimes persistent, debilitating pain and other serious complications [5]. Associated with a decline in specific cell-mediated immunity, herpes zoster affects some 600,000–900,000 mostly elderly or immunocompromised people in the U.S. each year, its incidence increasing with age [1, 6].
Establishing and/or enhancing varicella and herpes zoster surveillance has been essential in order to assess the impact of widespread varicella vaccination [7, 8]. Despite the fact that national vaccination coverage among children 19–35 months of age had risen to 85 percent by 2003 [9], varicella did not become a nationally notifiable disease in the U.S. until that year. Herpes zoster has never been a nationally notifiable disease, and no states require reporting of cases. Studies of herpes zoster using different methods have reported unadjusted incidence rates ranging from 1.3 to 4.8 per 1,000 population [10–13], but there is little documentation of secular trends over time. Some studies suggest that re-exposure to VZV after primary infection may decrease the risk of herpes zoster through immunologic boosting [14–16]. If this is the case, widespread use of varicella vaccine could, by reducing circulating VZV, increase the incidence of herpes zoster over the first few decades of universal childhood vaccination. On the other hand, studies in leukemic as well as healthy children have found a lower incidence and severity of herpes zoster among vaccinated than among comparable unvaccinated children [17–21], which would appear to bode well for the more distant future if vaccine coverage reaches high levels. Clearly, consistent and long-term surveillance for herpes zoster will be necessary in order to fully assess the impact of varicella vaccination on the epidemiology of zoster.
Starting in 1998, as uptake of varicella vaccine was increasing (due in part to immunization requirements for daycare and school entry), the Massachusetts Department of Public Health, in collaboration with the Centers for Disease Control and Prevention (CDC), enhanced varicella and herpes zoster surveillance by including questions in the Behavioral Risk Factor Surveillance System (BRFSS) about the occurrence of these diseases. Researchers in Kentucky had used both the BRFSS and a school-based cohort study to estimate age-specific varicella incidence in 1990–1992 and found generally close concordance between results of the two methods [22].
Herein, we analyze varicella and herpes zoster incidence as estimated by the Massachusetts BRFSS in the period 1998–2003.
Methods
Vaccine use and coverage
The Massachusetts Department of Public Health began to distribute varicella vaccine in September 1996 for use in 12–18-month-olds and susceptible sixth-graders. In October 1997, eligibility was expanded to include all children between 1 and 18 years of age. Beginning in August 1998, proof of immunity to varicella (written documentation of age-appropriate immunization or physician-certified reliable history of disease) was required for child-care center attendance for children ≥ 19 months of age who were born after 1996. Finally, in September 1999, proof of immunity became required for entry into kindergarten and seventh grade.
Vaccine coverage estimates were obtained from the National Immunization Survey. The CDC conducts this random-digit-dial telephone survey of households regarding the immunization status of children 19–35 months of age each year. National Immunization Survey methods have been published elsewhere [23].
Varicella and herpes zoster incidence
The BRFSS is an ongoing, random-digit-dial telephone survey of adults aged 18 years and older and gathers information on health characteristics, risks, and preventive behaviors. The survey is conducted in all U.S. states in collaboration with the CDC and state health departments. Once a household is contacted, one adult is randomly selected for interview. Characteristics of the BRFSS are described in more detail elsewhere [24].
The BRFSS includes a core set of questions asked by all states, as well as optional topics added by individual states. Massachusetts added questions about chickenpox and shingles among all household members in 1998–2000 and 2002–2003. Respondents were first asked to enumerate all household members and their ages. Trained interviewers then asked respondents whether any household members had chickenpox in the past 12 months and the current age(s) of the affected household member(s). Respondents were also asked whether any household members had ever had shingles, the current age(s) of affected household member(s), and the age(s) when they had shingles.
For these analyses, we calculated the annual age-specific incidence of varicella and herpes zoster per 1,000 population. In 1998 only, the response to the age-at-shingles question was recorded only in five-year age groups (0–4 years, 5–9 years, etc.), so annual incidence of herpes zoster could not be calculated for that year. We made some simplifying assumptions in estimating age-specific annual incidence of varicella and herpes zoster. For varicella, each case that was reported as occurring in the previous 12 months was assigned to the individual's current age. For herpes zoster, a case was counted as occurring within the previous year if the age at the time of the reported case was the same as or 1 year less than the current age. Our data on herpes zoster combine incident and recurrent cases, as no distinction was made between these in the interview. In presenting the varicella data, we used five standard child/adolescent age groups and grouped adults 20 years and older into a sixth. In the case of herpes zoster, we used four 20–25-year age intervals in order to increase the stability of the data.
BRFSS data were weighted to account for probability of selection and differential participation by age, sex, and race/ethnicity. All data were analyzed using appropriate procedures in SAS and SUDAAN, taking into account the survey sampling scheme, intra-class correlation among household members, and weighting of the data (SAS Institute, Cary, NC; SUDAAN, RTI, Research Triangle Park, NC). Tests for linear trend were done using logistic regression (with incidence as the outcome and year as the exposure variable), adjusting for age in comparisons of overall incidence. Age standardization was based on the standard 2000 U.S. population [25] in 14 mostly five-year age groups: 1–14, 15–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, and 75+. The oldest group was not further subdivided due to diminishing cell sizes.
Results
Response rate
The response rate for the Massachusetts BRFSS as a whole was 59% in 1998, 55% in 1999, 41% in 2000, 66% in 2002, and 65% in 2003. The large apparent increase in response rate in 2002 was mainly due to the fact that in that year BRFSS expanded the number of case disposition codes in order to more accurately differentiate between different types of response and non-response and to bring the BRFSS disposition codes more in line with those recommended by the American Association for Public Opinion Research (AAPOR). This broader array of codes allows for more accurate accounting of participation rates, such as response rates (pers. comm., Dr. Michael Link, BRFSS, CDC). The number of households responding to the varicella-herpes zoster module ranged between 4,200 and 4,900, representing between 11,000 and 14,000 members.
Vaccine coverage
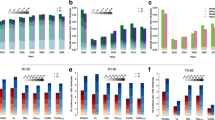
Using National Immunization Survey estimates for Massachusetts children 19–35 months of age [26], coverage increased from 23 percent in 1997 to 48 percent in 1998 and reached 89 percent in 2003 (Figure 1).
Varicella incidence
In 1998, the highest incidences of varicella were observed in children 1–4 years of age (83 per 1,000) and 5–9 years of age (76 per 1,000). Between 1998 and 2003, overall unadjusted varicella incidence declined by 13.0 cases per 1,000 (79%), from 16.5 to 3.5 per 1,000 (p < 0.0001; Table 1 and Figure 1). The overall incidence of varicella was higher in 2003 than in 2002 but not statistically significantly so. Over 1998–2003, incidence declined for all age groups, with the greatest decreases among infants, 1–4-, 5–9-, and 15–19-year olds (100%, 89%, 80%, and 92%, respectively) and the smallest among adults (27%). The trends were highly significant (p < 0.0001) for the 1–4- and 5–9-year olds and significant (p = 0.02) for the 10–14-year olds.
Herpes zoster incidence
Overall age-standardized (and crude) incidence per 1,000 of herpes zoster was 2.77 (2.23) in 1999, 3.54 (3.57) in 2000, 6.47 (6.51) in 2002, and 5.25 (5.38) in 2003 (Table 2), and the increasing trend was highly significant for both the age-adjusted (p < 0.0009) and crude (p < 0.0001) incidences. The increase in the age-standardized incidence was 90 percent over the five-year period. Over this period, age-specific incidences increased by 41–161 percent, although the annual age-specific incidence estimates were somewhat unstable (Table 2, Figure 2).
Discussion
The age-specific incidences of varicella in 1998 were generally within the range of estimates reported in studies conducted using a variety of methods in other parts of the U.S. prior to the introduction of the vaccine [1, 22, 27–29]. We noted a marked reduction in varicella incidence in all age groups in Massachusetts over the period 1998–2003; the decline was between 66 and 100 percent for all groups except adults, in whom incidence decreased by 27 percent. Vaccine coverage in Massachusetts 19–35-month-olds increased from 48 to 89 percent over the same 6-year period.
Seward et al. [30] observed a similarly consistent decline over the 6-year period of 1995–2000 in three areas of active surveillance, whose range of vaccine coverage as measured by the National Immunization Survey encompassed Massachusetts' coverage each year. Likewise, Jumaan et al. [31] documented a decline in age-adjusted incidence of varicella from 2.63 per 1,000 in 1995 to 0.92 per 1,000 in 2002. Decreases in varicella incidence after vaccine licensure have been noted in Illinois, Michigan, Texas, and West Virginia as well [32]. Similarly, large decreases in incidence among children in childcare centers and among U.S. Navy recruits have been associated with varicella vaccination [33, 34].
The overall incidence of varicella was higher in 2003 than in 2002, although the difference was not statistically significant. No such upturn was apparent in Massachusetts Department of Public Health surveillance data, which showed an overall decrease of 20 percent in cases and incidence between 2002 and 2003, in spite of increased efforts to stimulate varicella reporting. (Overall incidence of herpes zoster decreased from about 6 to about 5 per 1,000 from 2002 to 2003, but the change was not statistically significant for either the crude or the age-standardized estimates.)
Age-standardized overall annual herpes zoster incidence increased by 2.5 cases per 1,000 (90%) over the 1999–2003 period, to 5.25 per 1,000, higher than the age-standardized estimates derived using the same standard population from a study in the Netherlands in 1994–1999 (3.34 per 1,000) [35] and from a study in Seattle, Washington in 1992–2002 (range: 3.47–4.05 per 1,000) [31] but not substantially higher than the estimate obtained from a study in France in 1997–1998 (4.55 per 1,000) [13]. The age-specific incidences of herpes zoster found here were consistently higher than in a Minnesota study of 50 years ago [12], and more than half of the age-specific point estimates in four other studies [11–13, 6] fell below the 95 percent confidence intervals of our combined 2002–2003 estimates. (Confidence intervals for most of these previous studies were not available for comparison.) The four age groups showed increases of 41–161 percent, and the trend was significant for the 25–44 and 65+ age groups. In spite of the fact that the annual age-specific estimates of herpes zoster incidence were unstable, as can be seen particularly in the 65+ year-old age group (Figure 2), it does appear that there was a generally increasing trend in herpes zoster incidence for all age groups, based on these four years of data over a five-year period. This trend appears to conflict with findings from the Seattle study, a large longitudinal study in a health-maintenance organization in which age-adjusted as well as age-specific herpes zoster incidences remained relatively stable over the period 1992–2002, during the latter four years of which varicella incidence declined [31].
If in fact herpes zoster incidence is rising in the current period, there are several possible explanations, which may be operating in combination. One of these is varicella vaccination. Widespread varicella vaccination may end up increasing herpes zoster incidence among adults who have had varicella. Recent studies have suggested a protective effect against herpes zoster from exposure to varicella and/or children [14–16]. One mathematical model predicts a long-term (equilibrium state) elevated incidence of herpes zoster of 20 percent or more relative to pre-vaccination levels, under the assumption of immunologic boosting by exposure to VZV, if vaccine virus has a 75 percent or greater chance of reactivating to cause herpes zoster [36]. A later model by Brisson et al. [16], which was parameterized by means of a large, population-based survey and assumes that immunity from boosting lasts for 20 years, predicts a substantial increase in herpes zoster cases over the first 30–50 years after the initiation of mass vaccination, peaking about 20 years after the start of mass vaccination at an incidence of 51 percent over the pre-vaccination level and eventually falling below the initial incidence. Those aged 10–44 years at the start of mass vaccination will be most affected, according to the model, their lifetime risk exceeding 50 percent, compared to 33 percent in the pre-vaccination period – most will have been previously infected but will no longer experience boosting by exposure to children with varicella disease.
The Brisson model predicts the greatest rates of increase in herpes zoster incidence in the years just after the start of mass varicella vaccination, with a 40 percent increase over the pre-vaccination level expected by Year 10. Yet, over just a five-year period, we saw an increase of this magnitude in the 45–64-year age group and even larger increases in the other age groups, and it seems unlikely that varicella vaccination alone can explain our herpes zoster results. Other possible explanations include increases in the proportion of people with immunosuppressive conditions and therapies, in the duration of those conditions and treatments, and/or in the prevalence of other triggering factors. Some past studies have noted an increase in zoster incidence well before the availability of varicella vaccine, one finding 28- and 41-percent increases for Minnesota women and men, respectively, between the late 1940s and the late 1950s [12] and one finding a 35-percent increase between 1979 and 1997 in Canada [37]. Similarly, data from the National Health Interview Survey suggest that incidence of herpes zoster rose in the 60+ age groups between 1974 and 1994 [38].
Our study is subject to several limitations. Our method of estimating annual incidence of herpes zoster, by which a case was counted as occurring within the past year if the age at the time of the reported case was the same as or 1 year less than the age at interview, would tend to overestimate annual incidence because this encompasses a period greater than 12 months. The inclusion of recurrent cases with first-time cases would have the same effect. However, any overestimation due to these factors would be felt uniformly over age groups and time and would not be expected to influence year-to-year differences. Nevertheless, this would affect comparisons with other studies and likely contributes to our generally higher rates.
Secondly, varicella and herpes zoster incidences were based on respondent recall, both self- and proxy-report, and we did not validate the accuracy of the reporting. Proxy reporting may or may not introduce reporting error, depending on the health condition in question and the relationship of the respondent to the person about whom information is sought [39, 40]. Regarding varicella, in our combined data for 1999 and 2000, a parent or step-parent was the respondent in more than 90 percent of households with children, and restricting the varicella analysis to these parent-respondents did not appreciably change our estimates of annual age-specific incidence. Researchers using a telephone survey to investigate varicella incidence in a city in Minnesota estimated through medical chart abstraction that 13 percent of parental reports of varicella in the previous year were either not varicella or occurred more than 13 months before the interview [28]. Our data could reflect similar over-reporting; however, error of this nature would not likely change from year to year and thus the trends would not be affected. One study found shingles self-report among the elderly to be accurate, with few false-positive and false-negative reports when compared to physician diagnosis [41]. We found no significant difference between shingles incidence in respondents of age 50 and older and shingles incidence in the same age group as reported by proxies.
Also, in this study, herpes zoster was not likely often diagnosed by laboratory testing. If the public or physicians became more aware of zoster in the latter years of the study, there might have been more of a tendency to attribute rash illness to zoster with each successive annual survey, leading to an increase in observed incidence. However, we noted no increasing attention to zoster in the popular media nor a concern among physicians about a projected increase in incidence. Thus, we doubt that the observed trend is due to greater awareness.
We did not collect data on underlying immunosuppressive conditions, which would have permitted us to examine the extent to which changes in herpes zoster incidence were related to changes in the prevalence of such diseases or therapies. Although it is unlikely this prevalence changed much in the five-year study period, it is conceivable that the proportion of respondents with such conditions varied randomly from year to year, which could have affected the results.
Finally, the response rate for the BRFSS has been uneven, hitting a low of 41 percent in 2000. If the incidence of varicella or herpes zoster differs among responders and non-responders, this could introduce some bias in the estimates of both the absolute incidence and of trends over time. However, a comparison of the 2000 BRFSS sample to the 2000 U.S. Census data for Massachusetts concluded that the BRFSS sample reflected its target population in most demographic and socioeconomic variables despite its low response rate [42]. Any differences in characteristics that might be relevant to herpes zoster incidence, such as race/ethnicity and proportion of households with children, were slight. The proportion of households with children in the sample varied little between 2000 and 2003, from a low of 37.1 percent to a high of 38.2 percent. In 1999, it was 33.7 percent, but given that the effect of living with children is thought to be protective, this difference would not explain our results.
Conclusion
As varicella vaccine coverage in children increased, the incidence of varicella decreased and the occurrence of herpes zoster increased. If the observed increase is real, widespread vaccination of children is only one of several possible explanations.
Further research on herpes zoster is clearly warranted, particularly as a vaccine to prevent herpes zoster and its complications is likely to be available in the United States within the next few years [43–45]. A better understanding is needed of risk factors, the possible roles of internal (from subclinical reactivation) and external (from exposure to circulating VZV) boosting in preventing or delaying zoster, and temporal trends in incidence since mid-century. Additional methods for surveillance for herpes zoster should be considered to monitor the impact of widespread varicella vaccination of children as well as the impact of VZV vaccination of adults, should it become generally recommended.
References
Wharton M: The epidemiology of varicella-zoster virus infections. Infect Dis Clin North Am. 1996, 10: 571-581. 10.1016/S0891-5520(05)70313-5.
Galil K, C Brown, F Lin, J Seward: Hospitalizations for varicella in the United States, 1988 to 1999. Pediatr Infect Dis J. 2002, 21: 931-934. 10.1097/00006454-200210000-00009.
Meyer PA, Seward JF, Jumaan AO, Wharton M: Varicella mortality: trends before vaccine licensure in the United States, 1970–1994. J Infect Dis. 2000, 182: 383-390. 10.1086/315714.
Davis MM, Patel MS, Chem BS, Gebremariam A: Decline in varicella-related hospitalizations and expenditures for children and adults after introduction of varicella vaccine in the United States. Pediatrics. 2004, 114: 786-792. 10.1542/peds.2004-0012.
Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ: Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000, 342: 635-645. 10.1056/NEJM200003023420906.
Donahue JG, Choo PW, Manson JE, Platt R: The incidence of herpes zoster. Arch Intern Med. 1995, 155: 1605-1609. 10.1001/archinte.155.15.1605.
Centers for Disease Control and Prevention: Evaluation of varicella reporting to the National Notifiable Disease Surveillance System – United States, 1972–1997. Morb Mortal Wkly Rep. 1999, 48: 55-58.
Food and Drug Administration: Summary for Basis of Approval. Reference number: 93-0395, [http://www.fda.gov/cber/sba/varmer031795sba.pdf]
Centers for Disease Control and Prevention: National, state, and urban area vaccination levels among children aged 19–35 months – United States, 2003. Morb Mortal Wkly Rep. 2004, 53: 658-661.
McGregor RM: Herpes zoster, chicken-pox, and cancer in general practice. Br Med J. 1957, 32: 84-87.
Hope-Simpson RE: The nature of herpes zoster: A long-term study and a new hypothesis. Proc R Soc Med. 1965, 58: 9-20.
Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO: Population-based study of herpes zoster and its sequelae. Medicine (Baltimore). 1982, 61: 310-316.
Chidiac C, Bruxelle J, Daures JP, Hoang-Xuan T, Morel P, Lepiege A, El Hasnaoui A, de Labareyre C: Characteristics of patients with herpes zoster on presentation to practitioners in France. Clin Infect Dis. 2001, 33: 62-69. 10.1086/320884.
Gershon AA, LaRussa P, Steinberg S, Mervish N, Lo SH, Meier P: The protective effect of immunologic boosting against zoster: An analysis in leukemic children who were vaccinated against chickenpox. J Infect Dis. 1996, 173: 450-453.
Thomas SL, Wheeler JG, Hall AJ: Contacts with varicella or with children and protection against herpes zoster in adults: a case-control study. Lancet. 2002, 360: 678-682. 10.1016/S0140-6736(02)09837-9.
Brisson M, Gay NJ, Edmunds WJ, Andrews NJ: Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine. 2002, 20: 2500-2507. 10.1016/S0264-410X(02)00180-9.
Brunell PA, Taylor J, Geiser CF, Frierson L, Lydick E: Risk of herpes zoster in children with leukemia: varicella vaccine compared with history of chickenpox. Pediatrics. 1986, 77: 53-65.
Lawrence R, Gershon AA, Holzman R, Steinberg SP, the NIAID Varicella Vaccine Collaborative Study Group: The risk of zoster after varicella vaccination in children with leukemia. N Engl J Med. 1988, 318: 543-548.
Hardy I, Gershon AA, Steinberg SP, LaRussa PL, the Varicella Vaccine Collaborative Study Group: The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. N Engl J Med. 1991, 325: 1545-1550.
Plotkin SA, Starr SE, Connor K, Morton D: Zoster in normal children after varicella vaccine. J Infect Dis. 1989, 159: 1000-1001.
Gershon A, Silverstein S: Live attenuated varicella vaccine for prevention of herpes zoster. Biologicals. 1997, 25: 227-230. 10.1006/biol.1997.0089.
Finger R, Hughes JP, Meade BJ, Pelletier AR, Palmer CT: Age-specific incidence of chickenpox. Public Health Reports. 1994, 109: 750-755.
Smith PJ, Battaglia MP, Huggins VJ, Hoagland DC, Roden A, Khare M, Ezzati-Rice TM, Wright RA: Overview of the sampling design and statistical methods used in the National Immunization Survey. Am J Prev Med. 2001, 20: 61-69. 10.1016/S0749-3797(01)00285-9.
Powell-Griner E, Anderson JE, Murphy W: State and sex-specific prevalence of selected characteristics – Behavioral Risk Factor Surveillance System, 1994 and 1995. CDC Surveillance Summary, August 1, 1997. Morb Mortal Wkly Rep. 1997, 46: 1-31.
Klein RJ, Schoenborn CA: Age adjustment using the 2000 projected U.S. population. Statistical Notes. 20: 1-10. [http://www.cdc.gov/nchs/data/statnt/statnt20.pdf]
Centers for Disease Control and Prevention, National Immunization Program. [http://www.cdc.gov/nip/coverage/nis/03/tab03_antigen_state.xls]
Guess HA, Broughton DD, Melton LJ, Kurland LT: Population-based studies of varicella complications. Pediatrics. 1986, 78 (suppl): 723-727.
Yawn BP, Yawn RA, Lydick E: The community impact of childhood varicella infections. J Pediatrics. 1997, 130: 759-765.
Choo PW, Donahue JG, Manson JE, Platt R: The epidemiology of varicella and its complications. J Infect Dis. 1995, 172: 706-712.
Seward JF, Watson BM, Peterson CL, Mascola L, Pelosi JW, Zhang JX, Maupin TJ, Goldman GS, Tabony LJ, Brodovicz KG, Jumaan AO, Wharton M: Varicella disease after introduction of varicella vaccine in the United States, 1995–2000. JAMA. 2002, 287: 606-611. 10.1001/jama.287.5.606.
Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF: Herpes zoster incidence before and after declines in varicella incidence associated with varicella vaccination, 1992–2002. J Infect Dis.
Centers for Disease Control and Prevention: Decline in annual incidence of varicella – selected states, 1990–2001. Morb Mortal Wkly Rep. 2003, 52: 884-885.
Clements DA, Zaref JI, Bland CL, Walter EB, Coplan P: Partial uptake of varicella vaccine and the epidemiological effect on varicella disease in 11 day-care centers in North Carolina. Arch Pediatr Adolesc Med. 2001, 155: 433-461.
Ryan MA, Smith TC, Honner WK, Gray GC: Varicella susceptibility and vaccine use among young adults enlisting in the United States Navy. J Med Virol. 2003, S15-19. 10.1002/jmv.10314. Suppl 1
Opstelten W, Mauritz JW, de Wit NJ, van Wijck AJM, Stalman WAB, van Essen GA: Herpes zoster and postherpetic neuralgia: incidence and risk indicators using a general practice research database. Fam Pract. 2002, 19: 471-475. 10.1093/fampra/19.5.471.
Garnett GP, Grenfell BT: The epidemiology of varicella-zoster virus infection: The influence of varicella on the prevalence of herpes zoster. Epidemiol Infect. 1992, 108: 513-528. See pp. 521–522
Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M, Roos L, de Serres G: Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect. 2001, 127: 305-314.
Jumaan AO, Seward JF, Wooten K, Chen J, Singleton JA: Varicella and herpes zoster surveillance in the U.S., 1970–1994. Presented at 41st Annual Meeting of IDSA, October 9–12, 2003. San Diego, CA, Abstract # 899
Woolsey TD, Lawrence PS, Balamuth E: An evaluation of chronic disease prevalence data from the health interview survey. Am J Public Health. 1962, 52: 1631-1637.
Halabi S, Zurayk H, Awaida R, Darwish M, Saab B: Reliability and validity of self and proxy reporting of morbidity data: a case study from Beirut, Lebanon. Int J Epidemiol. 1992, 21: 607-12.
Schmader K, George LK, Newton R, Hamilton JD: The accuracy of self-report of herpes zoster. J Clin Epidemiol. 1994, 47: 1271-1276. 10.1016/0895-4356(94)90132-5.
Zhang Z, Cohen B: How representative is the Massachusetts BRFSS sample: a comparison to the current census data. 2003, Presented at BRFSS Annual Conference, St. Louis, MO
Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weiberg A, Boardman KD, et al: A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005, 352: 2271-2284. 10.1056/NEJMoa051016.
Gilden DH: Varicella-zoster virus vaccine – grown-ups need it, too. N Engl J Med. 2005, 352: 2344-2346. 10.1056/NEJMe058090.
Arvin A: Aging, immunity, and the varicella-zoster virus. N Engl J Med. 2005, 352: 2266-2267. 10.1056/NEJMp058091.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2458/5/68/prepub
Acknowledgements
Funding was provided by the National Immunization Program (grant number H23/CCH104486) and the Behavioral Risk Factor Surveillance System, Centers for Disease Control and Prevention. Dr. Yih receives support from the Vaccine Safety Datalink Project, National Immunization Program, Centers for Disease Control and Prevention. We gratefully acknowledge the bibliographic assistance of Jennifer Mann and critical readings by Paul Gargiullo.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
WKY helped design the questionnaire, interpreted the data, and drafted and revised the manuscript. DB helped design the questionnaire, oversaw implementation of the survey for several years, oversaw analysis of the data through 2002, interpreted the data, and critically reviewed all drafts of the manuscript, providing important insights. Recognizing the importance of carrying out the survey, SL secured funding to add the varicella and herpes zoster questions, and helped design the questionnaire, provided immunization data, and reviewed the manuscript. AJ contributed critical insights and raised questions in the interpretation of the data, provided comparative data and references, and critically reviewed all drafts of the manuscript. ZZ supported staff time for data analysis and himself re-analyzed the data through 2003; in addition, he helped interpret the data, ruling out certain artifactual explanations for the observations. KC analyzed the data through 2002 and provided much of the material for the methods section. JS funded the work, helped design the questionnaire, raised questions and contributed critical insights for the interpretation of the data, and critically reviewed the manuscript, supplying partial rewrites. All authors have approved the current version for publication.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yih, W.K., Brooks, D.R., Lett, S.M. et al. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998–2003. BMC Public Health 5, 68 (2005). https://doi.org/10.1186/1471-2458-5-68
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2458-5-68