Abstract
Background
Food borne disease are major health problems in developing countries like Ethiopia. Food handlers with poor personal hygiene working in food establishments could be potential sources of disease due to pathogenic organisms. However; information on disease prevalence among food handlers working in University of Gondar cafeterias are very scarce. The aim of this study is to assess the prevalence of nasal carriage of Staphylococcus aureus, their drug resistance pattern and prevalence of intestinal parasites among food handlers working in University of Gondar student’s cafeterias.
Method
A cross sectional study was conducted among food handlers working in University of Gondar student’s cafeterias. A pretested structured questionnaire was used for collecting data. Nasal swab and stool were investigated for S. aureus and intestinal parasites; respectively as per the standard of the laboratory methods.
Results
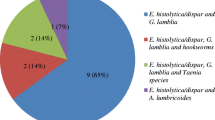
Among 200 food handlers, females comprised 171(85.5%). The majority (67.5%) of the food-handlers were young adults aged 18–39 years. One hundred ninety four (97%) of the food handlers were not certified as a food handler. Forty one (20.5%) food handlers were positive for nasal carriage of S. aureus, of these 4(9.8%) was resistant for methicilin. Giardia lamblia was the most prevalent parasites 22 (11%), followed by Ascaris lumbricoides 13(6.5%), Entamoeba histolytica 12 (6%), Strongyloides stercolaris (0.5), Taenia species 1(0.5%) and Schistosoma mansoni 1(0.5%).
Conclusion
The finding stressed that food handlers with different pathogenic micro organisms may pose significant risk on the consumers. Higher officials should implement food handler’s training on food safety, periodic medical checkup and continuous monitoring of personal hygiene of food handlers.
Similar content being viewed by others
Background
Food borne diseases are major health problems in developed and developing countries. The World Health Organization (WHO) estimated that in developed countries, up to 30% of the population suffer from food borne diseases each year, whereas in developing countries up to 2 million deaths are estimated per year [1, 2].
The spread of food borne diseases via food handlers are a common and persistent problem worldwide [3, 4]. Many diseases are communicable and caused by micro-organisms that enter into the body via food [5]. Numerous outbreaks of gastroenteritis have been associated with ingestion of raw foods, foods incorporating raw ingredients or foods obtained from unsafe sources [6, 7].
Food poisoning has been reported to be a result of infection with enterotoxigenic strains of staphylococcus aureus[8–13]. It accounts for 14–20% of outbreaks involving contaminated food in the USA [14], and in the United Kingdom restaurants are the second most important place for acquiring staphylococcal food poisoning [15]. This organism may exist on food handler’s nose or skin, from which it may be transmitted to cooked moist protein-rich foods, and become intoxication agent, if these foods are then kept for several hours without refrigeration or stored in containers.
Antibiotic resistant staphylococci are major public health concern since the bacteria can be easily circulated in the environment. Infections due to methicilin-resistant S. aureus (MRSA) have increased world-wide during the past twenty years [16, 17]. Multiple drug-resistant S. aureus have been frequently recovered from foodstuffs [18], nasal mucosa of humans [19].
Likewise intestinal parasitic infections remain important public health problems in developing countries. Infection of intestinal parasites usually occurs primarily by ingestion of eggs and cysts via a fecal-oral route or directly from human to human through poor personal hygiene [20, 21]. In Ethiopia amoebiasis and giardiasis are common causes of intestinal protozoa infections throughout the nation. The prevalence of amoebiasis ranges from 0–4% and that of giardiasis is 3–23% [22]. Food-handlers with poor personal hygiene working in food-serving establishments could be potential sources of infections of many intestinal helminthes, protozoa, and entero pathogenic bacteria [23]. Food-handlers who harbour and excrete intestinal parasites may contaminate foods from their faeces via their fingers, then to food processing, and finally to healthy individuals [21].
Though there are no or few indicative studies in hospital and university food catering service regarding food safety in the study area. There is no doubt food borne illnesses resulted from improper food handling. Therefore; this study aimed at assessing prevalence of nasal carriage of S. aureus, its drug resistance pattern and prevalence of intestinal parasites among food handlers.
Methods
Study design and area
A cross sectional study was conducted among food handlers working in University of Gondar students cafeterias from January 1, 2011 to June 30, 2011. Gondar town is one of the tourist destinations in Northwest Ethiopia 739 km away from Addis Ababa.
Study population
All food handlers working in University of Gondar student cafeterias.
Inclusion and exclusion criteria
Inclusion
Food handlers working in the University of Gondar student cafeterias and given informed consent were included in the study.
Exclusion criteria
Food handlers who had taken antibiotics and antihelminthics within the three weeks prior to the study were excluded.
Sample size and sampling procedure
All food handlers working in University of Gondar student cafeterias namely in Gondar College of Medicine and Health Sciences, Maraki campus and Tewodros campus cafeteria. Two hundred food handlers were included in the study.
Data collection procedure and sample collection
A pretested structured questionnaire was used for collecting information on age, sex, marital status, service years, educational status, status of training and habits of hand washing of each food-handler. Nasal swab was collected aseptically from food handlers’ nostrils rolling six times by applicator stick tipped with cotton and moistened with normal saline. Stool specimen was collected from food handlers by leak proof plastic stool cup.
Culture and identification
A single nasal swab was obtained from each food handler inoculated onto Manitol salt agar (MSA) and Blood agar plate (BAP) incubated for 24 hours in 35–37°C in incubator. Isolates were identified as S. aureus by growth characteristics on blood agar plate, MSA, Gram stain and biochemical test such as catalase test and slide coagulase were done following standard procedures [24].
Antimicrobial susceptibility testing
Susceptibility testing was performed on Muller Hinton agar (Oxoid, Hampshire, UK ) using agar disc diffusion technique recommended by Bauer et al.[25]. The drugs that were tested include methicilin (10 μg), penicillin (10 μg), erythromycin (15 μg), ampicilin (30 μg), ciprofloxacin (10 μg), tetracycline (30 μg), cotrimoxazole (25 μg), and vancomycin (30 μg) (Oxoid, UK). Staphylococcus aureus ATCC 25922 was used as a quality control organism for the antimicrobial susceptibility test. The resistance and sensitivity were interpreted according to the National Committee for Clinical Laboratory Standards [24].
Microscopic examination of stool
Intestinal parasites were investigated microscopically from each stool samples using both direct smears mount in saline and formol-ether concentration sedimentation procedures as per the standards [24].
Data processing and analysis
Statistical analysis was done using SPSS version 16.00 soft ware. The chi-square test was employed to assess the association between variables. A p-value of less than 0.05 was considered to indicate statistical significance.
Ethical consideration
The data were collected after written informed consent obtained from all study participants, and the study was approved by the Research Ethics Committee of the University of Gondar. Study participants found positive for intestinal parasites were treated and MRSA carriers were decolonized.
Results
Sociodemographic characterstics
A total of two hundred food-handlers, (171 of females and 29 males) were included in the study. Their mean age were 34.54 years, ranging from 18–64 years. The majority 135 (67.5%) of the food handlers were young adults aged 18–39 years. Only 105 (52.5%) of the food-handlers had education above primary school. The educational levels, age category, sex and work experiences were shown in (Table 1).
In hand washing practices, 179 (89.5%) food handlers had a habit of hand washing after toilet while 21(10.5%) of food handlers had no habit of hand washing after toilet. While 148 (74%) of food handlers had the habit of hand washing with soap and water, the rest 52(26% ) did not use soap for their hand after toilet. However, 92(46%) food handlers had a habit of hand washing after touching nose between handling of food items. Almost half of food handlers 93(46.5%) had no medical check-up previously including stool examination. Only 6(3%) the 200 of food handlers were certified for training in food handling and preparation (Table 2).
Sociodemographic in relation to carriage of S. aureusand intestinal parasites
In this study the rate of colonization of S. aureus related to age greater than 60 was 100%. However, infection to parasite age greater than 60 was 0%. The lowest rate of colonization of S. aureus was 15.8% in educational status of certificate. The amount of service years <1 yrs observed the lowest rate of colonization by S. aureus which was 8.3%. Though there were no significance association between sociodemographic variables and carriage of S. aureus and intestinal parasite infection (Table 3).
There is no significance association between certified in food preparation training and the presence of intestinal parasites (P = 0.810). However; there is significance association between poor hand washing practice after toilet with soap and water and the presence of intestinal parasites (P = 0.001) (Table 4).
Nasal carriage of S. aureus
Among the 200 healthy food handlers, the overall prevalence of nasal carriage of S. aureus was 41(20.5%). Considering the drug susceptibility pattern, all isolates of S. aureus were sensitive to vancomycin. However, half of the isolates of S. aureus 21(51.2%) and 19(46.3%) were resistant to penicillin and ampicillin; respectively. Sixteen (39%) of the isolates were resistant to amoxicillin. Thirteen (31.7%) and 11 (26.8%) of the isolates were resistant to tetracycline and cotrimoxazole; respectively. Six (14.6%) of the isolates were resistant to erythromycin, whilst 4 (9.8%) of the isolates were resistant to methicillin and ciprofloxacin; respectively (Table 5).
Intestinal parasites
Direct microscopic and concentration techniques were used for identifying intestinal parasites from 200 stool specimens. The consistency of stool was 168(84%) formed, 18(9%) semi formed, 12(6%) diarrhea and 2(1%) dysentery. Only the formed stool was done by sedimentation concentration techniques. Fifty (25%) stool specimens were positive for different intestinal parasites. Giardia lamblia was the most prevalent parasites 22(11%), followed by Ascaris lumbricoides 13(6.5%) and Entamoeba histolytica/dispar 12(6%). In our study trophozoites of G. lamblia, E. histolytica and larvae of S. stercolaris were found in diarrhea stool. As noted in the (Table 6), G. lamblia and E. histolytica, cyst forms of the parasites are higher than the trophozoite form.
Discussion
In this study, nasal swab culture and stool microscopic examination of 200 food handlers had been investigated for the presence of bacteria and intestinal parasites. The rate of isolation of S. aureus from the nasal cultures in our study 41 (20.5%) was found to be similar to those reported by several researchers as 26.6%, 23.1% and 21.6% [26–29]. However, our finding was found to be higher than the rate 69(0.77%) obtained from a study conducted in Turkey [30] and much lower than the findings reported in Brazil and Botswana as 30%, and 44.6%; respectively [31, 32]. Nasal carriage rates reported by several workers vary and the variation has been attributed to the ecological differences of the study population.
It is very important to note that although S. aureus causes severe infections it may also be as a member of the normal flora of the nasal cavity [33]. If by chance, a food handlers carries, an enterotoxin producer S. aureus he/she may contaminate the food and causes staphylococcal food poisoning outbreak in the students population. However, in our nasal carriage strains isolated from food handlers, we were not able to identify the presence or the absence enterotoxin producer strains because of lack of reagent enterotoxin kit, Phage typing and PCR techniques.
Our study demonstrated that 4(9.8%) strains of S. aureus were resistant to methicillin. It is important to note that the emergence and dissemination of MRSA (Methicillin Resistant Staphylococcus aureus) is an increasing global health problem that complicates the therapeutic management of staphylococcal infections. However, the rate of resistance in our study was much lower than that reported as 3(20%) the study done in Gondar from nasal swab isolate of health professionals (unpublished). The possible explanation for the higher rate of MRSA in the previous study may be due to cross transmission with hospital strains. In this study isolates of S. aureus resistant to ampicilin was 19(46.3%) in line with reported as 45% in Brazil [27]. However, the resistance of S. aureus to penicillin in our study was lower than from the reported 70% in Brazil [31]. In our study all isolates were sensitive to vancomycin in line with a finding by Acco et al.[31]. However, a study conducted in Botswana showed that 9(27.3%) of the isolates were resistant to vancomycin [32].
In this study, the overall prevalence of intestinal parasite among food handlers were 50(25%) consistent with the study done in Gondar town (29%) and in Sudan 23.1% [29, 34]. However, this prevalence was much lower compared to previous study done at Bahir Dar town [34], reported as 158(41.1%) and in Jimma which was 59(58.4%) [35]. The possible explanation may be more than half of the food handlers in this study had taken medical examination and might be treated for intestinal parasites or this study did not use sensitive techniques like Kato-thick smear for most of intestinal helminthes especially for Schistosoma mansoni, water emergency technique for Strongloides stercolaris and the adhesive scotch tape for E. vermicularis.
A. lumbricoides, S. mansoni, Taenia species and S. stercolaris were reported in this study, note that this parasites are not food borne pathogens. However, the presence of such pathogens may indicate low personal hygiene in food handlers and as the same time these pathogens must be treated.
It was noted that 12 (6%) and 2(1%) of food handlers working in the kitchens were suffering from diarrhea and dysentery; respectively. Active trophozoites forms of E. histolytica, G. lamblia and larva of S. stercolaris were associated with diarrheic food handlers. Infections with the protozoan parasites like E. histolytica and G. lamblia are common causes of diarrhoea worldwide [35]. G. lamblia and E. histolytica infected food handlers can directly transmit to consumers if ingested via contaminated food and water because G. lamblia cysts and E. histolytica cyst do not need environmental maturation. Thus, food handlers should be in a good health and those suffering from diarrhea and dysentery must be excluded from work until they have been completely free of symptoms and must get rest.
In this study, majority of food handlers working in the cafeterias were young adults 135 (67.5%) but which was older than study done in Bahir Dar 371(96.6%) [37]. More than half of (53.5%) the food handlers had medical check-up in the past. However, none of the food handlers had medical check-up in the past in Bahir Dar study [34].
Conclusion
Multiple antimicrobial resistant strains of S. aureus were isolated and protozoan cysts were detected from food handlers working at University of Gondar students’ cafeterias. These findings indicate that the food handlers may be potential source of food borne disease for the students’ population being served in three cafeterias.
Abbreviations
- BAP:
-
Blood agar plate
- FEC:
-
Formol ether concentration
- MRSA:
-
Methicillin resistant Staphylococcus aureus
- MSA:
-
Manitol salt agar
- PCR:
-
Polymerase chain reaction
- SOPs:
-
Standard operating procedures
- WHO:
-
World Health Organization.
References
World Health Organization: Food safety and food borne illness. 2007, Geneva: WHO
World Health Organization: “Forging links between Agriculture and Health” CGIAR on Agriculture and Health Meeting in WHO/HQ. Food Safety – Food borne diseases and value chain management for food safety. 2007
Zain M, Naing N: Sociodemographic characteristics of food handlers and their knowledge, attitude and practice towards food sanitation: a preliminary report. Southeast Asian J Trop Med Public Health. 2002, 33: 410-417.
Scott E: Food safety and food borne diseases in 21st century homes. Can J Infect Dis. 2003, 14: 277-280.
Essex-caster AJ: A synopsis of public health and social medicine. 1987, Bristol: Sohn Wright, Sons Ltd, 2
Lengerich EJ, Addis DG, Juranek DD: Severe giardiasis in the United States. J Clin Dis. 1994, 18: 760-763. 10.1093/clinids/18.5.760.
Hopkins RS, Juranek DD: Acute giardiasis: an improved clinical case definition for epidemiologic studies. Am J Epidemiol. 1991, 133: 402-407.
Carmo LS, Dias RS, Linardi VR, Sena MJ, Dos Santos DA: An outbreak of staphylococcal food poisoning in the municipality of Passos, Minas Gerais, Brazil. Braz Arch Biol Technol. 2003, 46: 581-586.
Arbuthnott JP, Coleman DC, Azavedo JC: Staphylococcal toxins in human disease. J Appl Bacteriol. 1990, 69: 101-107. 10.1111/j.1365-2672.1990.tb02917.x.
Buckle KA, Davey JA, Eyles MJ, Hocking AD, Newton KG, Stuttard EJ: Food borne microorganisms of public health Significance. 1993, Sydney: JM Sydney Executive Printing Service Pty Ltd, 271-284.
Mossel DA, Netten P: Staphylococcus aureus and related staphylococci in foods: ecology, proliferation, toxinogenesis, control and monitoring. J Appl Bacteriol. 1990, 19: 123-145.
Tranter HS, Brehm RD: The detection and aetiological significance of staphylococcal enterotoxins. J Rev Med Microbiol. 1994, 5: 56-64. 10.1097/00013542-199401000-00008.
Todd ECD: Food borne disease in six countries. A comparison. J Food Protect. 1998, 4: 559-565.
Ughes JM, Tauxe RV: Food borne diseases. Principles and Practice of Infectious Diseases. Edited by: Mandell GL, Douglas RG, Bennett JE. 1990, New York: Churchill Livingstone, 879-3
Wieneke AA, Roberts D, Gilbert RJ: Staphylococcal food poisoning in the United Kingdom, 1969–90. J Epidemiol Infect Dis. 1993, 110: 519-531. 10.1017/S0950268800050949.
Ippolito G, Leone S, Lauria FN FN, Nicastri E, Wenzel RP: Methicillin-resistant Staphylococcus aureus: The superbug. Int J Infect Dis. 2010, 14 (4): S7-S11.
Deresinski S: Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic and therapeutic odyssey. Clin Infect Dis. 2005, 40: 562-573. 10.1086/427701.
Abulreesh HH, Organji SR: The prevalence of multidrug-resistant staphylococci in food and the environment of Makkah, Saudi Arabia. Res J Microbiol. 2011, 6 (6): 510-523. 10.3923/jm.2011.510.523.
Acco M, Ferreira FS, Henriques JAP, Tondo EC: Identification of multiple strains of Staphylococcus aureus colonizing nasal mucosa of food handlers. Food Microbiol. 2003, 20: 489-493. 10.1016/S0740-0020(03)00049-2.
Ukoli FMA: Introduction to parasitology in tropical Africa. 1990, New York: John Wiley and Sons, 201-315.
Kaferstein F, Abdussalam M: Food safety in the 21st century. B World Health Organ. 1999, 77 (4): 347-351.
Haile G, Jirra C, Mola T: Intestinal parasitism among Jiren elementary and junior secondary school students, southwest Ethiopia. Ethiop J Heal Dev. 1994, 8: 37-41.
World Health Organization: Health surveillance and management procedures of food-handling personnel. 1999, Geneva: World Health Organization, 7-36. Technical report series no. 785
National Committee for Clinical Laboratory Standards: Methods for determining bactericidal activity of antimicrobial agents. 1993, Villanova, PA: Tentative Guidelines, M26-TNCCLS
Bauer AW, Kirby WM, Sherris JC, Turch M: Antibiotic susceptibility testing by standard single disk method. Am J Clin Pathol. 1966, 45: 493-496.
Bustan MA, Udo EE, Chugh TD: Nasal carriage of enterotoxin-producing Staphylococcus aureus among restaurant workers in Kuwait City. J Epidemiol Infect. 1996, 116: 319-322. 10.1017/S0950268800052638.
Simsek Z, Koruk I, Copur A, Cicek G, Gulcan MD: The prevalence of Staphylococcus aureus and intestinal parasites among food handlers in Saniurfa, Southeastern Anatolia. J Public Health Manag Pract. 2009, 15 (6): 518-523.
Andargie G, Kassu A, Moges F, Tiruneh M, Huruy K: Prevalence of bacteria and intestinal parasites among food-handlers in Gondar Town, Northwest Ethiopia. J Health Popul Nutr. 2008, 26 (4): 445-451.
Ahmed H, Hassan H: Bacteriological and parasitological assessment of food handlers in the Omdurman area of Sudan. J Microbiol Immunol. 2010, 43 (1): 70-73.
Gunduz T, Limoncu ME, Cumen S, Ari A, Etiz S, Tay Z: Prevalence of intestinal parasites and nasal carriage of Staphylococcus areus among food handlers in Manisa Turky. J Environ Health. 2008, 18 (5): 230-235.
Acco M, Ferreira FS, Henriques JAP, Tondo EC: Identification of multiple strains of Staphylococcus aureus colonizing the nasal mucosa of food handlers. J Food Microbiol. 2003, 20 (5): 489-493. 10.1016/S0740-0020(03)00049-2.
Loeto D, Matsheka ML, Gashe BA: Enterotoxigenic and antibiotic resistance determination of Staphylococcus aureus strains isolated from food handlers in Gaborone, Botswana. J Food Protect. 2007, 70 (12): 2764-2768.
Williams REO: Healthy carriage of staphylococcus aureus: its prevalence and importance. J Bacteriol Rev. 1993, 27: 56-71.
Abera B, Biadegelgen F, Bezabih B: Prevalence ofSalmonella typhiand intestinal parasites among food handlers in Bahir Dar Town, Northwest Ethiopia. Ethiop J Heal Dev. 2010, 24 (1): 46-50.
Sahlemariam Z, Mekete G: Examination of fingernail contents and stool for ova, cyst and larva of intestinal parasites from food handlers working in student cafeterias in three Higher Institutions in Jimma. Ethiopian J Health Sci. 2001, 11 (2): 131-137.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2458/12/837/prepub
Acknowledgements
We acknowledge University of Gondar for funding this study. We greatly appreciate University of Gondar Hospital Laboratory for cooperation during the study. We are also grateful to the food handlers who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MD was the primary researcher, conceived the study, designed, participated in data collection, conducted data analysis, drafted and finalized the manuscript for publication. MT and FM assisted in data collection and reviewed the initial and final drafts of the manuscript. MD, MT, FM and ZT interpreted the results, and reviewed the initial and final drafts of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Dagnew, M., Tiruneh, M., Moges, F. et al. Survey of nasal carriage of Staphylococcus aureus and intestinal parasites among food handlers working at Gondar University, Northwest Ethiopia. BMC Public Health 12, 837 (2012). https://doi.org/10.1186/1471-2458-12-837
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2458-12-837