Abstract
Background
Micronutrient deficiency is a common public health problem in developing countries, especially for infants and children in the first two years of life. As this is an important time window for child development, micronutrient fortified complementary feeding after 6 months of age, for example with milk or cereals products, in combination with continued breastfeeding, is recommended. The overall effect of this approach is unclear.
Methods
We performed a Systematic Review and Meta-analysis to assess the impact of micronutrient fortified milk and cereal food on the health of infants and little children (aged 6 months to 5 years) compared to non-fortified food. We reviewed randomized controlled trials using electronic databases (MEDLINE and Cochrane library searches through FEB 2011), reference list screening and hand searches. Three reviewers assessed 1153 studies for eligibility and extracted data. One reviewer assessed risk of bias using predefined forms.
Results
We included 18 trials in our analysis (n = 5’468 children; range of mean hemoglobin values: 9.0 to 12.6 g/dl). Iron plus multi micronutrient fortification is more effective than single iron fortification for hematologic outcomes. Compared to non-fortified food, iron multi micronutrient fortification increases hemoglobin levels by 0.87 g/dl (95%-CI: 0.57 to 1.16; 8 studies) and reduces risk of anemia by 57% (relative risk 0.43; 95%-CI 0.26 to 0.71; absolute risk reduction 22%; number needed to treat 5 [95%-CI: 4 to 6]; 6 Studies). Compared to non-fortified food, fortification increases serum levels of vitamin A but not of zinc. Information about functional health outcomes (e.g. weight gain) and morbidity was scarce and evidence is inconclusive. Risk of bias is unclear due to underreporting, but high quality studies lead to similar results in a sensitivity analysis.
Conclusions
Multi micronutrient fortified milk and cereal products can be an effective option to reduce anemia of children up to three years of age in developing countries. On the basis of our data the evidence for functional health outcomes is still inconclusive.
Similar content being viewed by others
Background
Micronutrient (MN) deficiency is a common public health problem, specifically for infants and children, in many low and middle income countries. For example, anemia (caused by iron deficiency) or increased infection rates and mortality (exacerbated by vitamin A and zinc deficiency) are serious threats for child development [1]. The first two years of life represent a narrow time window, which is of outstanding importance for child development [2]. During this time period future growth and vulnerable physiological capacities, such as cognitive function and motor development, are determined. Even with optimum breastfeeding, these steps depend on a an adequate quantity and quality of complementary feeding, leading to an adequate MN supply [2]. Negative health consequences resulting from suboptimal feeding, such as stunting (i.e. low height-for-age), are associated with higher morbidity and decreased function in later life [3].
Several strategies have been shown to be effective in resolving MN deficiencies for different target groups and are proposed in recommendations and guidelines [4–6]: Food based approaches (e.g. spreads to increase energy-density and MN content of food; MN powders for home fortification with sprinkles) and MN supplementation (e.g. vitamin A capsules administered at defined intervals). In addition, fortification of staple food (e.g. fortified salt, flour or oil) is widely used to resolve MN deficiencies of general populations.
Fortified complementary feeding after 6 months of age, in combination with continued breastfeeding [7], typically comprises milk or cereals products (e.g. porridge or gruel) for infants. This type of food, however, is often not covered by programs that provide fortified staple food for the general population. Primary studies have assessed the effects of fortified milk or cereals for infants and children [8, 9] and some countries, such as Mexico, have introduced country wide food programs, where fortified milk is one component [10]. However, the overall evidence of the effect of fortified milk and cereals on children has not been systematically assessed.
Thus, we performed a Systematic Review to specifically assess the impact of micronutrient fortified milk and cereal food on the health of infants and children compared to non-fortified food in randomized controlled trials.
Methods
We performed our review in accordance with current guidelines for performing [11, 12] and reporting of systematic reviews [13] and established a scientific advisory board (see Acknowledgments for participating experts). A review study protocol was developed in advance, though not published.
According to our research question we defined the following inclusion criteria: Population: Infants and children from 6 months to 5 years of age. While our primary focus was on age groups up to 2 years, we decided to set an upper age limit at 5 years, in order not to miss suitable studies with mixed age groups. Intervention: Micronutrient fortified milk or cereal food. Control intervention: Non-fortified food; additional other nutritional approaches, if such approaches were applied in the intervention and control group. Outcome: At least one of the following health related outcomes: surrogate measures (such as MN serum levels, hematological parameters), functional outcome (e.g. motor development), measures of morbidity (such as disease rates) or mortality. Study designs: Randomized controlled trials of any follow-up time.
We excluded studies with infants and toddlers younger than 6 months [14] or applying infant formula [15], studies addressing adolescents or adult women, interventions based on supplementation, home fortification, bare food based approaches, fortification with components other than micronutrients, and studies testing absorption of MN. A priori, we also excluded studies with fortification of staple food as provided for larger population groups to isolate the effect of fortified milk and cereals.
We systematically searched for studies using electronic databases (Medline [search strategy Table 1, Cochrane library; from 1966 to February 2011; no language restriction). As this review was part of a larger project, that evaluates the economic effects of MN fortification as well, we also included search terms such as “cost” and “economics”. We screened reference lists of included papers and contacted experts in the field for additional references. In addition, we screened homepages of relevant organizations (e.g. WHO, United Nations [World Food Programme, Unicef, Millennium Development Goals], The World Bank, Pakistan National Nutrition Survey; International Clinical Epidemiology Network [16]; Global Alliance for Improved Nutrition, GAIN [17]; The Micronutrient Initiative [18]; Bill & Melinda Gates Foundation [19]). We also contacted a manufacturer (Nestlé) for further material and performed hand searches in relevant journals with developing countries issues (such as The Lancet). All references were stored in an EndNote X4 database (Thomson/ISI ResearchSoft Berkeley, CA, USA).
Study selection and data extraction
Three reviewers screened titles and abstracts for relevance and assessed potentially relevant studies for inclusion by full text. Teaching sessions were held in advance to improve conceptual consistency between reviewers. Disagreements were resolved by consensus meetings. If data of a specific population were published in several papers or if follow-up data were presented, we included each population only once. Using a predefined form, data were extracted by one reviewer in an Excel database and checked independently by a second reviewer.
We extracted data on general study information (e.g. study region; length and completeness of follow up), study setting (e.g. level of population recruitment), population details, intervention (e.g. daily amount of fortified MN, determined as daily difference between intervention and control group; composition of MN; comparator food) and outcome (e.g. morbidity rates; hemoglobin levels [g/dl; conversion to g/L with factor 10]).
One reviewer assessed risk of bias in individual studies with a component approach exploring methodological quality on the study level (adequate generation of random sequence, concealment of allocation, blinding) as well as on the outcome level (incomplete outcome data due to attrition; selective outcome reporting) [12].
Statistical analysis
First, we calculated pooled estimates. For continuous variables we computed weighted mean differences (WMD) and 95%-confidence intervals (CI). For example, for analysis of hemoglobin change we used the mean change in the intervention and in the control group and their pooled standard deviation (SD). If the sample size decreased during the study, we used the lower sample size at the end of the study. If mean hemoglobin change per group and SD were not reported, we calculated change as the difference of baseline and final values for intervention and control group and applied the SD of final values [20]. If 95%-CI of mean values were reported we converted them to SD assuming normal distribution [21]. To check results for robustness, we also calculated WMD for final hemoglobin values of both study groups, as this data was reported more often. Due to considerable heterogeneity between trials, we applied a random effects model [22]. When authors reported only medians for continuous data (e.g. for ferritin levels), we did not include those data in the meta-analysis. For binary data, we calculated risk ratios and 95%-CI. Heterogeneity between trials was calculated with I2, that is the percentage of the total variation in estimated effects that is due to heterogeneity rather than chance (where values of 25% are assigned low, 50% moderate and 75% high) [23].
Second, we divided our dataset into pre-specified subgroups to explore the influence of possible modifying factors on the outcome (fortified milk vs. cereal food; high vs. low/middle-income countries; single- vs. dual/multi-micronutrient fortification strategy).
Third, we performed a meta-regression analysis weighted for the inverse of the variance of the outcome [12]. With this approach we evaluated the unique contribution of other a priori chosen independent factors on the most often reported outcome (dependent variable: hemoglobin level; independent variables: hemoglobin levels before intervention; daily amount of fortified MN; length of follow-up; completeness of follow-up).
For parametric and non-parametric tests P-values <0.05 were considered significant. Analyses were performed using the STATA SE 9 software package (StataCorp. 2007. Stata Statistical Software, College Station, Texas, USA).
Results
Description of included studies
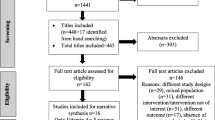
Our searches retrieved 1153 potentially relevant studies (Figure 1). Eighteen RCT [8–10, 24–38] (n = 10 fortified milk; n = 8 fortified cereals) fulfilled inclusion criteria and were included for our main analysis (Table 2).
These 18 trials comprised 5468 infants and children from different regions (2 studies from Asia [8, 37], 5 studies from Africa [9, 33–36]; 5 Studies from South- and Middle-America [10, 27, 29, 30, 38]; 6 Studies from Europe [24–26, 28, 31, 32]).
Study population sizes varied from n = 33 to n = 1120 participants (median 166; IQR 92 to 361). Most participants belonged to vulnerable groups and had been recruited from different settings (8 studies: medical or community care centers:, 7 studies: low income risk groups; 2 studies: general population of peri-urban and rural areas; 1 study: no information given). The most frequent exclusion criteria were chronic diseases, severe anemia, severe mal-/under-nutrition, and low birth weight. Mean age of participants ranged from 6 to 23 months at inclusion (upper age limit was 3 years in one study [8]) and the sex ratio was well balanced. Mean hemoglobin values of children at baseline varied between studies from 9.0 g/dl to 12.6 g/dl (median of study values: 11.1 g/dl). Follow up periods were generally short and did not exceed one year (mean follow up: 8.2 months; range: 2.3 to 12).
Fortified milk was prepared with centrally processed fortified milk powder in most of the studies. Fortified cereals comprised centrally processed weaning or complementary food, such as fortified porridge, gruel or weaning rusk to prepare a pap. Iron was the most frequently used MN for fortification (15 of 18 trials), followed by zinc (9 trials) and vitamin A (6 trials). Seven studies used a single-MN fortification strategy (6 studies with iron only; 1 study with zinc only), two studies a dual- and 9 studies a multi-micronutrient (MMN) strategy (i.e. 3 or more MN, for example additional fortification with vitamin C and E, selenium, copper).
Effect on hemoglobin levels
Hemoglobin blood level was the most frequently reported outcome parameter. Across 13 studies that tested iron fortification irrespective of other added MN, the mean increase of hemoglobin compared to the control group was 0.62 g/dl (95%-CI: 0.34 to 0.89) for children fed with fortified milk or cereals (Figure 2). Heterogeneity was high (I2 = 86%). Comparison of different subgroups showed a stronger effect of the iron MMN fortification approach (n = 8 studies; hemoglobin increase 0.87 g/dl (95%-CI: 0.57 to 1.16; I2 = 82%) compared to the iron single-fortification strategy (n = 5 studies; hemoglobin increase 0.20 g/dl (95%-CI: -0.05 to 0.45; I2 = 43%). The daily applied iron dosage was similar for the single-iron approach (median: 6.5 mg) and the MMN-approach (median 6.7 mg).
Effect of iron fortification of milk and cereals on hemoglobin (Hb) levels compared to non-fortified food. Only studies with iron fortification included (n = 13 RCT). Results are provided as weighted mean difference in hemoglobin (WMD: g/dl with 95%-CI; conversion to g/L with factor 10) between intervention and control group (iron single-fortification (1); iron multi micronutrient fortification (3); overall effect).
Effect on anemia prevalence
Eleven trials provided data for anemia rates, all of them using iron as a single- or a MMN-fortification strategy. Applied thresholds for anemia varied between 10.5 g/dl and 11 g/dl and the median of anemia rates at baseline was 36% (IQR: 15% to 40%; 9 studies with data). Fortified milk or cereals reduced the risk of suffering from anemia by 50% (risk ratio 0.50, 95%-CI: 0.33 to 0.75; I2 = 71%; Figure 3). Again, a stronger effect of the MMN fortification approach emerged (n = 7 studies; risk ratio 0.43 (95%-CI: 0.26 to 0.71; I2 = 81%) compared to the iron single-fortification strategy (n = 4 studies; risk ratio 0.76 (95%-CI: 0.45 to 1.28; I2 = 0%). Overall, the absolute risk reduction (ARR) of suffering from anemia was 14% (un-weighted data of 11 trials), translating into a number needed to treat (NNT) with fortified milk or cereals of 7 (95%-CI: 6 to 9) participants over a period of 8 months (i.e. the mean follow-up time) to avoid one case of anemia. For the MMN approach these results are even more favorable (un-weighted data of 7 trials: ARR 22%; NNT 5 [95%-CI: 4 to 6]).
Effect of iron fortification of milk and cereals on anemia compared to non-fortified food. Only studies with iron fortification included (n = 11 RCT). Results are provided as risk ratio (RR, 95%-CI) of suffering from anemia in the intervention group compared to the control group (iron single-fortification (1); iron multi micronutrient fortification (3); overall effect).
Effect on ferritin levels
Ferritin is the most direct measure to conclude if iron stores increase by iron-fortified food consumption. Eleven trials provided data for ferritin serum levels. Ferritin levels were not adjusted for subclinical infections. Given the skewed distribution of ferritin values, authors often reported median estimates. Medians were significantly higher in the intervention groups (ranges of ferritin medians at end of study [micro-g/l]: intervention: 15.8 to 44.6; control: 6.5 to 28; P < 0.01). Only three studies provided mean values to be included in a meta-analysis, which showed an effect in the same direction. The mean ferritin increase with iron fortification was 11.3 micro-g/l (95%-CI: 3.3 to 19.2; I2 = 79%) compared to control groups.
Effects on serum zinc and vitamin A levels
Five studies provided data for change in serum zinc levels. MN fortification with zinc led to no relevant change in zinc serum levels (0.4 micro-g/dl (95%-CI: -1.7 to 2.6; I2 = 0%) compared to control groups. However, fortification increased vitamin A serum levels compared to control groups (four studies with data: Retinol increase by 3.7 micro-g/dl [95%-CI: 1.3 to 6.1; I2 = 37%]).
Effects on growth, functional measures and morbidity
For three European studies, no relevant effect of fortification on height and weight was seen and morbidities were not an issue in this population.
All other results relate to non-European low-/middle income countries. Due to the short follow-up period in most of the studies, no meaningful conclusion can be drawn for possible effects of fortification on height or weight gain or z-scores (weight-for-age; height-for-age; weight-for-height). Of 9 studies with data, 7 trials reported no relevant differences between intervention and control group at the end of the study. In one study [36], more weight gain was seen in the intervention group after one year (4.6 kg vs. 2.0 kg; P < 0.05), in another study [8] children consuming fortified milk showed improvement in weight gain compared to control group (difference 0.21 kg/year [95%-CI 0.12 to 0.31) and height gain (difference 0.51 cm/year [95%-CI 0.27 to 0.75).
Of three studies with data for psychomotor development of children, two trials reported no relevant difference between groups [9, 35] and one study [33] found slight improvements compared to the control group.
Of four studies with morbidity data of children, three trials reported no relevant differences between groups for infections [29], for diarrhea, fever and respiratory illness [34] and for referral to hospital or death in partly HIV exposed children [35]. In one study [8] fortified milk significantly reduced the probability of days with severe illness (by 15%), and the relative risk of diarrhea (by 18%) and lower respiratory illness (by 26%).
Exploring heterogeneity
In our pre-specified subgroup analyses no relevant influence on the outcome was detected for the mode of fortified food (fortified milk vs. cereals). Hemoglobin change was somewhat higher in studies from low/middle income countries (0.78 g/dl (95%-CI: 0.41 to 1.15) compared to high income countries (0.42 g/dl (95%-CI: 0.10 to 0.73), but the difference was not statistically significant. The dual-/multi-micronutrient approach led to a significantly stronger effect of iron fortification on hemoglobin increase than the iron single-fortification strategy (Figure 3).
In our multivariable meta-regression analysis, none of the tested independent variables (mean hemoglobin level before intervention; daily amount of consumed iron, length of follow-up, completeness of follow up) was significantly associated with the change in hemoglobin.
Summary assessment of risk of bias
Only two [8, 25] of 18 trials provided enough information to conclude that both random sequence generation and allocation concealment was adequately performed (Table 3). For 11 trials this was unclear and inadequate procedures had been applied in 5 trials. Other criteria were fulfilled more often: Blinding was reported in 13 of 18 studies, incomplete outcome data were addressed in 8 of 18 trials and 12 of 18 studies showed no selective outcome reporting (i.e. besides serum markers also height/weight, functional measures or morbidities were reported).
In summary, the risk of bias for the most often reported outcomes hemoglobin change and anemia rates is unclear. However, a sensitivity analysis including only studies with low risk of bias led to similar results (three studies that fulfilled 4 of 5 quality criteria [8, 25, 26]: hemoglobin increase 0.87 g/dl (95%-CI: 0.09 to 1.65; I2 = 92%). Another sensitivity analysis showed that the result pattern remained basically unchanged after performing analyses using mean values of groups at the end of the study, instead of mean changes of groups.
Discussion
To our knowledge, this is the first systematic review, that has applied a meta-analysis to specifically weigh the overall evidence for the effects of fortified milk and cereal food suitable for complementary feeding of children. The evidence relates to study populations between 6 month and three years of age. Iron fortification leads to a clinically relevant increase in hemoglobin levels and reduction of anemia rates. For zinc and vitamin A fortification only surrogate parameters are reported, but the combination with iron (MMN approach) leads to a more pronounced effect on hemoglobin levels compared to an iron single-fortification strategy. The evidence for functional health outcomes is inconclusive.
Strengths and limitations
We applied a thorough search strategy with a stepwise retrieval of studies using electronic databases and additional sources. We cannot exclude having missed references but we believe that we found a near complete sample of relevant papers for our specific research question.
Some limitations have to be mentioned. First, included studies showed short follow-up periods, thus the impact of fortified milk or cereal food on functional health outcomes (such as sustainable height and weight gain or mental and motor development) could not be assessed thoroughly. Second, for zinc and vitamin A fortification only surrogate outcomes as serum levels are available. However, the presence of additional fortified micronutrients seems to be important for the effects of iron on hemoglobin levels. The MMN approach is more effective than iron single fortification in our review, reflecting that complex micronutrient deficiencies are responsible for health problems [20]. Third, risk of bias is unclear mainly due to underreporting of the randomization procedure and incomplete outcome data. Finally, pooled estimates have to be interpreted cautiously as statistical heterogeneity between studies was considerable and meta-regression did not reveal significant associations of pre-specified study characteristics with study results. Possible sources for unexplained heterogeneity might be underreporting for co-interventions (e.g. public supplementation or food programs) or the diversity of applied MN preparations that have influence on MN absorption. For example, five different iron compounds were used (12 studies with data: six times FeSulfate; twice FeGluconate; one time, each, FePyrophosphate; FeFumarate; Ferric ammonium citrate; electrolytic iron). In addition, the difference in daily consumed iron between intervention and control group varied between 1.8 mg and 14.3 mg. Furthermore, molar ratios, a determinant for MN absorption, also showed variation (ranges of molar ratios: ascorbic acid/iron: 0.68 to 30; phytic acid/iron: 1.7 to 2.2; calcium/iron: 40 to 134).
Existing systematic reviews and research needs
Important contributions have been made in the recent years with other systematic reviews to evaluate the health effects of MN interventions. These reviews differ from ours: Dewey and Adu-Afarwuah gave a broad systematic overview of studies and programs aimed at improving biochemical and functional outcomes with complementary foods [43]. However, they did not perform a meta-analysis and presented results in a tabulated form or as averaged effect sizes. Some reviews have concentrated on MN supplementation only [44–48] or home fortification [49], other reviews have combined supplementation and fortification strategies for analysis [20, 50], included children as well as adolescents or adult women [6, 51, 52], or included fortified staple food interventions [6, 52].
The health effects found in our review are in line with effect sizes shown in some similar reviews above [20, 47, 52]. This underpins the validity of our findings and supports a strategy to intervene with fortified milk and cereal food for infants and children. Supplementation trials, for example with vitamin A, have been shown to reduce mortality and morbidity via improved nutritional status [47, 48], even though serum level increases were small [53], similar to our review. Thus, some authors conclude that fortification would also have an impact on morbidity and mortality, although a conclusive answer cannot yet be given [52]. On the other hand, negative aspects of iron supplementation have been reported, such as increased morbidity and mortality in regions where malaria transmission is intense [54]. Thus, recommendations concerning iron supplementation have been formulated [55]. These adverse effects, however, may not be that relevant for fortified foods. Daily micronutrient dosages of fortified foods are much lower as compared to supplementation. Furthermore, children stop eating once they get saturated, which may also not be the case for high dosage sprinkles, that can be seen as a specific application of supplementation. Nevertheless, long term data concerning negative effects of iron fortified foods in regions with high prevalence of malaria and infectious diseases are lacking.
Further compiled evidence is needed to agree on the optimal MN preparation for fortified milk and cereals (such as composition of MN; suitable compounds; molar ratios for additives) to fully exhaust the potential of this approach. Future studies should also focus on health outcomes of MN fortification beyond the effect of iron on hematological results, for example via long-term follow-up of study populations.
Implications for decision makers
There are multiple delivery mechanisms for fortified milk and cereal food. Production and distribution via government programs and local public agencies would be an obvious option to strengthen local structures. Implementation of effective strategies, however, does not always work well in the field due to logistical problems or inappropriate priority setting [56]. Thus, some have discussed the role of the business community in improving nutrition in developing countries [56–58]. Commercially distributed fortified foods (e.g. with iron) are already available in many markets, even in low-income countries. In a public private partnership, business partners can provide their professional knowledge and experience concerning technical problems with processing and fortification, supply and transport, or refrigeration and conservation issues (specifically important for milk) to get interventions more efficient.
A limitation of the market approach is that it may not reach the poorest of the poor. Thus, a combination of different delivery channels, as well as affordable prices, may be needed. Children with severe anemia, who may be overrepresented in very poor groups, are often excluded from trials due to ethical reasons. One may assume, that the positive effects on the hemoglobin levels may be even stronger for such children. Additional economic analyses are necessary to contribute to a deeper understanding of the health economic effects of such a strategy and to inform the priority setting of decision makers.
Conclusions
Multi micronutrient fortified milk and cereal products can be an effective option to reduce anemia of children up to three years of age in developing countries. On the basis of our data the evidence for functional health outcomes is still inconclusive.
Expert and Affiliation
Lindsay H. Allen - Western Human Nutrition Research Center, USA; Narendra K. Arora - International Clinical Epidemiology Network, INCLEN, India; Zulfiqar A. Bhutta - Aga Khan University, Pakistan; Rodolfo F. Florentino - Nutrition Foundation of the Philippines; Guillermo Meléndez - Mexican Health Foundation, Mexico; Noel W. Solomons - Program Director for Central America, International Nutrition Foundation, Guatemala; Edgar Vasquez-Garibay - University of Guadalajara, Mexico.
Abbreviations
- Fe:
-
Iron
- MN:
-
Micro nutrients
- MMN:
-
Multi micro nutrients
- RCT:
-
Randomized controlled trial
- WMD:
-
Weighted mean difference.
References
Horton S: Alderman H. 2008, Rivera JA: Hunger and Malnutrition. Copenhagen Consensus Challenge Paper. Copenhagen Consensus Center
Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J: Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008, 371 (9608): 243-260. 10.1016/S0140-6736(07)61690-0.
Dewey KG, Begum K: Long-term consequences of stunting in early life. Matern Child Nutr. 2011, 7 (Suppl 3): 5-18.
Word Health Organisation: Conclusions and recommendations of the WHO consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food and Nutrition Bulletin. 2007, 28 (4): S621-S631.
Allen L, de Benoist B: Dary O. 2006, Hurrel R: Guidelines on food fortification with micronutritients. Edited by WHO Food and Agricultural Organization of the United Nations. Geneva
Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, Haider BA, Kirkwood B, Morris SS, Sachdev HP, et al: What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008, 371 (9610): 417-440. 10.1016/S0140-6736(07)61693-6.
World Health Assembly Resolution: Infant and young child nutrition. In WHA Resolution. 2001, 542:
Sazawal S, Dhingra U, Dhingra P, Hiremath G, Sarkar A, Dutta A, Menon VP, Black RE: Micronutrient fortified milk improves iron status, anemia and growth among children 1–4 years: a double masked, randomized, controlled trial. PLoS One. 2010, 5 (8): e12167-10.1371/journal.pone.0012167.
Oelofse A, Van Raaij JM, Benade AJ, Dhansay MA, Tolboom JJ, Hautvast JG: The effect of a micronutrient-fortified complementary food on micronutrient status, growth and development of 6- to 12-month-old disadvantaged urban South African infants. Int J Food Sci Nutr. 2003, 54 (5): 399-407. 10.1080/0963748031000092161.
Rivera JA, Shamah T, Villalpando S, Monterrubio E: Effectiveness of a large-scale iron-fortified milk distribution program on anemia and iron deficiency in low-income young children in Mexico. Am J Clin Nutr. 2010, 91 (2): 431-439. 10.3945/ajcn.2009.28104.
NHS Centre for Reviews and Dissemination: CRD’s guidance for undertaking reviews in health care. 2008, York: University of York
Higgins JP, Green S: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011
Moher D, Liberati A, Tetzlaff J, Altman DG: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009, 151 (4): 264-269.
Heird WC: Progress in promoting breast-feeding, combating malnutrition, and composition and use of infant formula, 1981–2006. J Nutr. 2007, 137 (2): 499S-502S.
Food and Agricultural Organization of the United Nations: Standard for infant formula and formulas for special medical purposes intended for infants. In CODEX STAN. 2007, 72-1981.
International Clinical Epidemiology Network. http://www.inclentrust.org/,
Global Alliance for Improved Nutrition. http://www.gainhealth.org/,
The Micronutrient Initiative. http://www.micronutrient.org/english/view.asp?x=1,
Bill & Melinda Gates Foundation: http://www.gatesfoundation.org/Pages/home.aspx,
Allen LH, Peerson JM, Olney DK: Provision of multiple rather than two or fewer micronutrients more effectively improves growth and other outcomes in micronutrient-deficient children and adults. J Nutr. 2009, 139 (5): 1022-1030. 10.3945/jn.107.086199.
Altman DG, Machin D, Bryant TN, Gardner JM: Statistics with confidence. 2001, Bristol: BMJ Books
Khan KS, Kunz R, Kleijnen J, Antes G: Systematic reviews to support evidence-based medicine. 2003, London: Royal Society of Medicine Press
Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ. 2003, 327 (7414): 557-560. 10.1136/bmj.327.7414.557.
Gill DG, Vincent S, Segal DS: Follow-on formula in the prevention of iron deficiency: a multicentre study. Acta Paediatr. 1997, 86 (7): 683-689. 10.1111/j.1651-2227.1997.tb08568.x.
Maldonado Lozano J, Baro L, Ramirez-Tortosa MC, Gil F, Linde J, Lopez-Huertas E, Boza JJ, Gil A: Intake of an iron-supplemented milk formula as a preventive measure to avoid low iron status in 1–3 year-olds. An Pediatr (Barc). 2007, 66 (6): 591-596. 10.1157/13107394.
Morley R, Abbott R, Fairweather-Tait S, MacFadyen U, Stephenson T, Lucas A: Iron fortified follow on formula from 9 to 18 months improves iron status but not development or growth: a randomised trial. Arch Dis Child. 1999, 81 (3): 247-252. 10.1136/adc.81.3.247.
Villalpando S, Shamah T, Rivera JA, Lara Y, Monterrubio E: Fortifying milk with ferrous gluconate and zinc oxide in a public nutrition program reduced the prevalence of anemia in toddlers. J Nutr. 2006, 136 (10): 2633-2637.
Virtanen MA, Svahn CJ, Viinikka LU, Raiha NC, Siimes MA, Axelsson IE: Iron-fortified and unfortified cow's milk: effects on iron intakes and iron status in young children. Acta Paediatr. 2001, 90 (7): 724-731.
Brown KH, López de Romaña D, Arsenault JE, Peerson JM, Penny ME: Comparison of the effects of zinc delivered in a fortified food or a liquid supplement on the growth, morbidity, and plasma zinc concentrations of young Peruvian children. Am J Clin Nutr. 2007, 2: 538-547.
Walter T, Pino P, Pizarro F, Lozoff B: Prevention of iron-deficiency anemia: comparison of high- and low-iron formulas in term healthy infants after six months of life. J Pediatr. 1998, 4: 635-640.
Daly A, MacDonald A, Aukett A, Williams J, Wolf A, Davidson J, Booth IW: Prevention of anaemia in inner city toddlers by an iron supplemented cows' milk formula. Arch Dis Child. 1996, 1: 9-16.
Stevens D, Nelson A: The effect of iron in formula milk after 6 months of age. Arch Dis Child. 1995, 3: 216-220.
Faber M, Kvalsvig JD, Lombard CJ, Benadé AJ: Effect of a fortified maize-meal porridge on anemia, micronutrient status, and motor development of infants. Am J Clin Nutr. 2005, 5: 1032-1039.
Lartey A, Manu A, Brown KH, Peerson JM, Dewey KG: A randomized, community-based trial of the effects of improved, centrally processed complementary foods on growth and micronutrient status of Ghanaian infants from 6 to 12 mo of age. Am J Clin Nutr. 1999, 70 (3): 391-404.
Gibson RS, Kafwembe E, Mwanza S, Gosset L, Bailey KB, Mullen A, Baisley K, Filteau S: A Micronutrient-Fortified Food Enhances Iron and Selenium Status of Zambian Infants but Has Limited Efficacy on Zinc. J Nutr. 2011, 141: 935-943. 10.3945/jn.110.135228.
Nesamvuni AE, Vorster HH, Margetts BM, Kruger A: Fortification of maize meal improved the nutritional status of 1-3-year-old African children. Public Health Nutr. 2005, 8 (5): 461-467.
Liu DS, Bates CJ, Yin TA, Wang XB, Lu CQ: Nutritional efficacy of a fortified weaning rusk in a rural area near Beijing. Am J Clin Nutr. 1993, 57 (4): 506-511.
Schumann K, Romero-Abal ME, Maurer A, Luck T, Beard J, Murray-Kolb L, Bulux J, Mena I, Solomons NW: Haematological response to haem iron or ferrous sulphate mixed with refried black beans in moderately anaemic Guatemalan pre-school children. Public Health Nutr. 2005, 8 (6): 572-581.
Williams J, Wolff A, Daly A, MacDonald A, Aukett A, Booth IW: Iron supplemented formula milk related to reduction in psychomotor decline in infants from inner city areas: randomised study. BMJ. 1999, 318 (7185): 693-697. 10.1136/bmj.318.7185.693.
Chilenje Infant Growth Nutrition Infection Study Team: Micronutrient fortification to improve growth and health of maternally HIV-unexposed and exposed Zambian infants: a randomised controlled trial. PloS one. 2010, 6: e11165-
Manno D, Kowa PK, Bwalya HK, Siame J, Grantham-McGregor S, Baisley K, De Stavola BL, Jaffar S, Filteau S: Rich micronutrient fortification of locally produced infant food does not improve mental and motor development of Zambian infants: a randomised controlled trial. Br J Nutr. 2011, 1-11. 10.1017/S0007114511003217.
Sazawal S, Dhingra U, Dhingra P, Hiremath G, Kumar J, Sarkar A, Menon VP, Black RE: Effects of fortified milk on morbidity in young children in north India: community based, randomised, double masked placebo controlled trial. BMJ. 2007, 334 (7585): 140-10.1136/bmj.39035.482396.55.
Dewey KG, Adu-Afarwuah S: Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008, 4 (Suppl 1): 24-85.
Gera T, Sachdev HP, Nestel P, Sachdev SS: Effect of iron supplementation on haemoglobin response in children: systematic review of randomised controlled trials. J Pediatr Gastroenterol Nutr. 2007, 44 (4): 468-486. 10.1097/01.mpg.0000243440.85452.38.
Ojukwu JU, Okebe JU, Yahav D, Paul M: Oral iron supplementation for preventing or treating anaemia among children in malaria-endemic areas. Cochrane Database Syst Rev. 2009, 3: CD006589
Bhutta ZA, Black RE, Brown KH, Gardner JM, Gore S, Hidayat A, Khatun F, Martorell R, Ninh NX, Penny ME, et al: Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. Zinc Investigators' Collaborative Group. J Pediatr. 1999, 135 (6): 689-697.
Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA: Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ. 2011, 343: d5094-10.1136/bmj.d5094.
Haider Batool A, Bhutta Zulfiqar A: Neonatal vitamin A supplementation for the prevention of mortality and morbidity in term neonates in developing countries (Review). Cochrane Library. 2011, 10: CD006980-
De-Regil LM, Suchdev PS, Vist GE, Walleser S, Pena-Rosas JP: Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database Syst Rev. 2010, 12: CD008524-CD006980
Ramakrishnan U, Aburto N, McCabe G, Martorell R: Multimicronutrient interventions but not vitamin a or iron interventions alone improve child growth: results of 3 meta-analyses. J Nutr. 2004, 134 (10): 2592-2602.
Hess SY, Brown KH: Impact of zinc fortification on zinc nutrition. Food Nutr Bull. 2009, 30 (1 ): 79-107.
Bhutta Zulfiqar A, Das JK, Dean SV, Salam RA: Effectiveness of Food Fortification with Micronutrients. A Review. 2012, Geneva: Nestle
Imdad A, Herzer K, Mayo-Wilson E, Yakoob M, Bhutta Z: Vitamin A supplementation for preventiing morbidity and mortality in children six months to five years of age. Cochrane Database of Systematic Reviews. 2010, 5:
Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, et al: Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006, 367 (9505): 133-143. 10.1016/S0140-6736(06)67962-2.
World Health Organisation: Iron supplementation of young children in regions where malaria transmission is intense and infectious disease higly prevalent. 2007, Geneva: World Health Organisation
Bryce J, Coitinho D, Darnton-Hill I, Pelletier D, Pinstrup-Andersen P: Maternal and child undernutrition: effective action at national level. Lancet. 2008, 371 (9611): 510-526. 10.1016/S0140-6736(07)61694-8.
Darnton-Hill I, Nalubola R: Fortification strategies to meet micronutrient needs: successes and failures. Proc Nutr Soc. 2002, 61 (2): 231-241. 10.1079/PNS2002150.
Scaling up Nutrition: Road Map Implementation. http://www.unscn.org/sacling_up_nutrition_sun/,
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2458/12/506/prepub
Acknowledgements
Thanks also to Richard Hurrell (Swiss Federal Institute of Technology, Switzerland) and Monika Potter (Dietitian) who provided valuable advice. Thanks to Paul Kelly for English language editing.
The study was supported by the Nestlé Nutrition Institute. The supporting source had no influence on study design; in the collection, analysis, and interpretation of the data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KE designed and conducted research, analyzed data and drafted the manuscript. SW helped to design research, conducted research, helped to draft the manuscript. IR conducted research, helped to draft the manuscript. UB helped to design research, helped to draft the manuscript. All authors read and approved the final manuscript.
Isabelle Rüthemann and Urs Brügger contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Eichler, K., Wieser, S., Rüthemann, I. et al. Effects of micronutrient fortified milk and cereal food for infants and children: a systematic review. BMC Public Health 12, 506 (2012). https://doi.org/10.1186/1471-2458-12-506
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2458-12-506