Abstract
Background
Evidence suggests that white matter integrity may play an underlying pathophysiological role in schizophrenia. N-acetylaspartate (NAA), as measured by Magnetic Resonance Spectroscopy (MRS), is a neuronal marker and is decreased in white matter lesions and regions of axonal loss. It has also been found to be reduced in the prefrontal and temporal regions in patients with schizophrenia. Diffusion Tensor Imaging (DTI) allows one to measure the orientations of axonal tracts as well as the coherence of axonal bundles. DTI is thus sensitive to demyelination and other structural abnormalities. DTI has also shown abnormalities in these regions.
Methods
MRS and DTI were obtained on 42 healthy subjects and 40 subjects with schizophrenia. The data was analyzed using regions of interests in the Dorso-Lateral Prefrontal white matter, Medial Temporal white matter and Occipital white matter using both imaging modalities.
Results
NAA was significantly reduced in the patient population in the Medial Temporal regions. DTI anisotropy indices were also reduced in the same Medial Temporal regions. NAA and DTI-anisotropy indices were also correlated in the left medial temporal region.
Conclusion
Our results implicate defects in the medial temporal white matter in patients with schizophrenia. Moreover, MRS and DTI are complementary modalities for the study of white matter disruptions in patients with schizophrenia.
Similar content being viewed by others
Background
Alterations in connectivity between brain regions including the frontal lobe, basal forebrain and limbic system, have been proposed as network deficits in schizophrenia [1–6]. Connections between the prefrontal cortex (PFC) and other cortical and subcortical regions implicated in the pathophysiology of schizophrenia suggest that a white matter abnormality in this region could have widespread consequences for neural connectivity in brain regions that are critical to the core symptoms of schizophrenia. The finding of increased neuronal density [7–13] is consistent with the notion that the integrity of white matter tracts is compromised. In addition, MRI evidence for decreased global [14–16], prefrontal [15, 17–21] and temporal [15, 21] white matter in schizophrenia is quite convincing. A meta-analysis of studies of white matter volumes in schizophrenia reveals a reduction of approximately 2%, with the greatest differences of approximately 5% in medial temporal lobe structures [14]. Indeed, regional rather than global reductions are far more relevant to the question of the origins of disconnectivity and hence of particular interest in the presentation of symptoms. For example, volume reductions in the white matter of PFC, repeatedly found in schizophrenia [17–20], are associated with the presentation of negative symptoms [18, 20].
Both MRS [22, 39, 41, 42, 44, 46, 23–27] and DTI [28–34] have been used extensively to study white matter abnormalities in patients with schizophrenia. The results of these two techniques may reflect different mechanisms of abnormal pathologies. MRS measurements are determined by biochemical profiles of the underlying pathologies, whereas DTI is more sensitive to structural differences.
Magnetic resonance spectroscopy allows one to quantify the concentrations of various metabolites in the brain. N-acetylaspartate (NAA) is the most prominent metabolite detected in the normal human brain. NAA serves as a neuronal marker and is only present in mature neurons. Some studies have also shown the presence of NAA in oligodendrocyte-2A progenitor cells [35]. NAA has been shown to be decreased in well-known demyelinating diseases such as multiple sclerosis (MS) and progressive multifocal leukoencephalopathy (PML) and it is thought to be associated with axonal injury [36]. Decreased NAA in patients with schizophrenia has been found in the cerebellum [37], thalamus [23, 38–44], dorso-lateral prefrontal cortex (DLPFC) [23, 42–44], anterior cingulate [45], and medial temporal lobe [24–27, 46, 47]. Reduction in NAA may signify structural abnormality or reduced viability of the underlying neurons [35, 48]. Given these results we have acquired MRS data on the DLPFC white matter, medial temporal (MT) white matter and on the occipital (OC) white matter as a reference.
Diffusion tensor imaging allows one to quantify the integrity of densely packed fiber bundles such as axonal tracts and to measure the orientation of such bundles [49–53]. DTI probes the microstructure of white matter by measuring the anisotropy of self-diffusion of water molecules in the restricted compartments of axonal tracts. Two quantitative measures can be obtained from DTI: anisotropy indices and fiber tract orientations. Anisotropy indices such as relative anisotropy (RA) and fractional anisotropy (FA) [49, 53] measure the amount of coherence of water diffusion which putatively reflects the amount of myelination in axonal bundles or the coherence of fiber tracts. The same dataset also provides information on the three-dimensional orientation of the anisotropy and can be used to study fiber tract connectivity [54–56]. Previous DTI studies have shown reduced anisotropy in patients with schizophrenia in frontal white matter [28, 29, 57, 58], the cingulum bundle [31, 59], the temporal gyrus [32, 60], and the corpus callosum [32, 34, 61].
Given that MRS and DTI can provide complementary imaging data on white matter changes in brain we sought to investigate the white matter brain changes associated with schizophrenia by simultaneously acquiring DTI and MRS data in a cohort of schizophrenic patients and a group of matched healthy control subjects. MRS was acquired targeting three regions of interests: frontal white matter, occipital white matter and medial temporal white matter. Since the DTI scans are much less time-consuming than MRS scans, we acquired whole brain DTI and extracted matching voxels for correlation analysis with the MRS voxels.
Methods
Subjects
Schizophrenic subjects were recruited from inpatient, outpatient, day treatment and vocational rehabilitation services at Mount Sinai Hospital (New York, N.Y.), Pilgrim Psychiatric Center (W. Brentwood, N.Y.), Bronx VA Medical Center (Bronx, N.Y.), Hudson Valley Veterans Affairs Medical Center (Montrose, N.Y.), and Queens Hospital Center (Jamaica, N.Y.) following approvals by each institutional review board. Informed consent was obtained on each subject following an assessment of capacity to provide informed consent by a psychiatrist independent of the study. The inclusion criteria were 1) a DSMIV diagnosis of schizophrenia or schizoaffective disorder based on the Comprehensive Assessment of Symptoms And History (CASH) [62], and 2) aged 18–80. Healthy comparison subjects who were without any DSMIV axis I disorder (by CASH interview) were recruited from the New York area and were matched for age and gender to the schizophrenic subjects, they also provided informed consent. Subjects were excluded if they had 1) a positive urine drugs of abuse screen, 2) a medical diagnosis which may produce white matter changes (i.e. HIV, MS), 3) a history of brain disorder which may produce cognitive impairment or behavioral symptoms (i.e. head injury, cerebrovascular disease), or 4) had an unstable medical condition (i.e. poorly controlled diabetes or hypertension, symptomatic coronary artery disease). Each subject was carefully screened and evaluated with a neuropsychological evaluation, structured assessment of symptoms, screening medical history, physical examination and laboratory studies (including complete blood count, routine chemistry, liver enzymes, and thyroid function tests) and urine toxicology screen, to ensure they fulfilled the inclusion criteria.
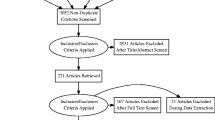
42 schizophrenic subjects and 40 healthy comparison subjects were recruited, assessed and scanned with both DTI and MRS modalities. Mean age of the schizophrenic group did not differ significantly from the healthy comparison group (schizophrenic mean = 38.69 years [sd = 11.42]), healthy comparison mean = 43.3 years [sd = 20.18], t = 1.28, p = .20), nor did the gender distribution (healthy comparison males = 57.5%, schizophrenic males = 69%, X2 = 1.18, p = .28). However, schizophrenic subjects attained significantly lower levels of education than the healthy comparison subjects (schizophrenic mean = 12.21 years [sd = 2.07]), healthy comparison mean = 15.3 years [sd = 2.24], t = 6.48, p < .001). The average age of illness onset for the schizophrenic group was 23.53 years (sd = 7.09), 5% of the schizophrenic sample was not receiving treatment with any antipsychotic at the time of scanning while 19% were receiving first generation antipsychotics, 17% clozapine, and 59% were receiving other second generation antipsychotics (risperidone, olanzapine, ziprasidone, quetiapine, aipiprazole).
Data Acquisition
All imaging studies were performed on an Allegra 3T head-dedicated system (Siemens, Ehrlangen, Germany) with a gradient strength of 40 mT/m and slew rate of 400~900 mT/m/s, allowing EPI acquisitions with minimal susceptibility distortions. Patient head motion was minimized by inflatable pillows inserted between the patients' head and the head coil. Subjects were recruited to receive two scans, one MRS and one structural. Most subjects received both scans on the same day, but there were some that preferred to return on a second day for the other scan. The duration between the two scans was at most 2 months apart. During this time there was no change in treatment that the patients received.
MRS
Localizer MR images for prescribing the MRS volumes consisted of a T1 sagittal with the following parameters: TR = 500 ms, TE = 10 ms, FOV = 18 cm × 14 cm, matrix size = 512 × 384, 4.3 mm thick with 1.1 mm spacing. 25 slices were obtained to cover the whole brain. From these sagittal images, two T1-weighted transverse slices (TR = 500 ms, TE = 10 ms, Thickness = 10 mm, FOV = 16.5 cm × 22 cm with matrix 512 × 384) were identified for MRS acquisition: the first slice covered the DLPFC white matter and the OC white matter. A nearly axial plane was chosen for the plane going through the striatum. It is chosen to be parallel to the AC-PC line as identified on the sagittal planes (Fig 1). The second slice was prescribed to be co-axial with the Sylvian fissure such that it contained the medial temporal lobe white matter bilaterally (Fig 2). 1H spectroscopic imaging (SI) data of the left and right medial temporal lobes were obtained in two sequential scans using the phase-encoded version of the standard PRESS volume localization sequence, with TR = 2000 ms, TE = 30 ms, 24 × 24 phase-encoding steps over a field-of-view of 16 cm (zerofilled to 32 × 32 phase-encoding steps before 3D Fourier transformation), a slice thickness of 10 cm slice, 1 average per phase-encoding step and circular k-space sampling, to obtain voxels having a nominal size of 0.25 cm3 (1.0 × 0.5 × 0.5 cm3). Outer volume saturation bands were prescribed to coincide with all 8 sides of the PRESS box. Water suppression and magnet shimming were automatically performed and adjusted by the host computer. The 1H SI data for the DLPFC were acquired with the same protocol except that the FOV changed to 30 cm. The resulting interpolated pixel size was (1.0 × 0.9 × 09 cm3). One slice was acquired for the DLPFC and OC white matter tracts. Total imaging time for all three slices averaged about 75 minutes.
The raw SI data were processed and fitted in the frequency-domain to obtain metabolite peak areas using manufacturer-supplied MRS data processing software. Individual CSI images were reconstructed and overlaid onto the T1 anatomical images. Automatic phase correction was applied, voxels of interests were identified, and the metabolite levels were derived from the spectral fits. For the DLPFC white matter and OC white matter ROIs, three voxels were identified visually (Fig 1) for each hemisphere. For the MT white matter four voxels were identified per hemisphere because of the higher CSI resolution (Fig 2). MRS metabolites (NAA, Cho, Cr, Ins1 & Ins2) were obtained from these regions of interests (ROIs) (Fig 3). The number of voxels per ROI was variable ranging between 1 and 3. Voxels with poor spectral data quality, defined asunresolved Cr and Cho resonances, were excluded from analysis. The metabolites from the selected voxels in each of the ROIs were averaged and transferred to Statistica v6 (StatSoft Inc., Tulsa, Ok) for statistical analysis.
DTI
DTI data was acquired in a separate scanning session that included other structural scans for morphological analysis. The protocol for the structural scans consisted of a three-plane sagittal localizer from which all other structural scans were prescribed. The following structural scans were acquired: Axial 3D-MPRage (TR = 2500 ms, TE = 4.4 ms, FOV = 21 cm, matrix size = 256 × 256, 208 slices with thickness = 0.82 mm); Turbo spin echo T2-weighted Axial (TR = 5380 ms, TE = 99 ms, FOV = 18.3 cm × 21 cm, matrix = 512 × 448, Turbo factor = 11, 28 slices, thickness = 3 mm skip 1 mm); DTI using a pulsed-gradient spin-echo sequence with EPI-acquisition (TR = 4100 ms, TE = 80 ms, FOV = 21 cm, matrix = 128 × 128, 28 slices, thickness = 3 mm skip 1 mm, b-factor = 1250 s/mm2, 12 gradient directions, 5 averages); and magnetization transfer imaging (MTI) using a turbo spin echo sequence (TR = 700 ms, TE = 12 ms, FOV = 21 cm, Matrix = 256 × 256, 28 slices, thickness = 3 mm skip 1 mm). Total imaging time for the structural scans averaged about 50 minutes.
Raw DTI data were transferred to an off-line workstation for post-processing. In-house software written in Matlab v6.5 (The Mathworks Inc. Natick, MA) was used to compute the anisotropy and vector maps. The Fractional Anisotropy images were then converted to analyze format (Fig 4). MEDx v3.4.3 software (Medical Numerics Inc, Sterling, VA) was used to inspect and define ROIs on the FA images. Two adjacent axial slices (4 mm) were selected from the FA dataset for each matching CSI image (10 mm). The planes were selected to contain the same CSI voxel locations (Fig 5). The voxels for the FA images measured 1.6 × 1.6 × 3 mm3. ROI dimensions for the FA images were set to 5 × 5 and 3 × 3 for the DLPFC/OC and MT respectively. These settings gave us the closest match to the MRSI data in terms of actual volumes sampled; ROI dimensions for DLPFC and OC were 8.2 mm (FA) vs. 9 mm (MRSI), ROI dimensions for the MT were 4.9 mm (FA) vs. 5 mm (MRSI). For all structures 2 FA slices were sampled corresponding to a thickness of 8 mm (FA) vs. 10 mm (MRSI). The FA voxel statistics for each ROI were extracted and transferred to Statistica and merged with MRS data for t-tests and correlation analysis.
In order to ensure reliability of our region of interest procedures inter-rater reliability measures were calculated for the two raters for each white matter region in 20 subjects. The intraclass correlation coefficients on the mean values of the ROIs as obtained by the two raters were as follows; optic radiations (right = .83, left = .83), MT (right = .88, left = .91), DLPF WM (right = .94, left = .84).
Results
Statistical tests between the average ROI values were performed on the fractional anisotropy as well as the MRSI data for the three regions of interests in both hemispheres. Table 1 shows the mean fractional anisotropy and metabolite ratio values for the schizophrenic and healthy comparison groups in each medial temporal regions of interest. Independent T-tests on the metabolite ratios demonstrated significantly reduced NAA in the left medial temporal white matter regions in patients with schizophrenic subjects (mean = 1.17) compared to healthy control subjects (mean = 1.31) (t = 2.35, df = 75, p < .021). Similar significances were found in the right medial temporal white matter regions with schizophrenic subjects (mean = 1.07) and healthy control subjects (mean = 1.21) (t = 2.29, df = 75, p < .025). We also computed the significances for NAA/Cr ratios in the medial temporal white matter region. Significant differences were found in the left with schizophrenic subjects (mean = 1.96) and healthy controls (mean = 2.37) (t = 2.58, df = 75, p < 0.012) as well as on the right with schizophrenic subjects (mean = 1.94) and healthy controls (mean = 2.25) (t = 2.17, df = 75, p < 0.033). Other areas and metabolite concentrations were not significantly different between groups.
T-tests on the matching voxels of the FA data showed significantly reduced FA values in the same right medial temporal white matter voxels as the MRS in patients with schizophrenia compared to normal control subjects (0.307 vs. 0.342) (t = 3.02, df = 79, p < .003). FA was also significantly reduced in the left medial temporal white matter region of schizophrenic subjects compared to healthy control subjects (0.339 vs. 0.370) (t = 2.31, df = 79, p < .023). Other areas surveyed were not significant. Correlation analysis on combined patient and control subjects showed a significant correlation between FA and NAA in the left medial temporal (r = 0.210, p = .050) as well as FA and NAA/Cr (r = 0.230, p = 0.040). No significant correlation was found in the right medial temporal side (r = 0.210, p = 0.090) and (r = 0.140, p = 0.220). There have been several reports suggesting that additional neuro-degenerative factors such as age might have effects on FA [63, 64]. NAA in our patient sample as well as some other studies [65] did not show any age effect. We have also tested partial correlations with the FA values corrected for age: a slight increase in significances were found in the same regions, correlations between FA and NAA, NAA/Cr in the left medial temporal white matter were (r = 0.332., p = .010) and (r = 0.308, p = 0.017) respectively. The correlations in all other ROIs surveyed were not significant: right medial temporal side (r = 0.20, p = 0.07) and (r = 0.16, p = 0.22); left DLPFC (r = 0.03, p = 0.80) and (r = -0.07, p = 0.56), right DLPFC (r = 0.07, p = 0.56) and (r = 0.23, p = 0.06), left occipital (r = 0.13, p = 0.10) and (r = 0.15, p = 0.21), right occipital (r = 0.10, p = 0.39) and (r = 0.18, p = 0.13) respectively.
Discussion
The regions sampled in the current study shows that NAA/Cr and fractional anisotropy are reduced in the medial temporal white matter in patients with schizophrenia. Reduced anisotropy was detected in the areas surveyed despite normal appearing white matter on conventional T1 and T2 weighted images which suggest microscopic damage to these fiber tracts.
NAA is thought to be present almost exclusively in neurons and their dendritic and axonal extensions but not in glia [66–68]. The NAA signal provides a marker of the number of viable neurons [69]. NAA is an intracellular amino acid derivative produced in the mitochondria [70], but it is also found in large quantities in O-2A progenitor cells [35]. O2-A progenitor cells are putatively involved with glia repair processes[71]. MS studies have shown that it is the failure of these repair processes that is the main cause of the disease [72]. A reduction in NAA may reflect both a volume loss as well as a defect in the myelin maintenance infrastructure. FA indices from DTI measurements reflect the amount of coherently restricted diffusion (imposed by the presence of myelin) of free water. These coherently restricted diffusion pathways are most prominent in axonal bundles. DTI voxels are several orders of magnitude larger than cellular dimensions, so that the computed anisotropy indices reflect the cumulative effect of the underlying microstructure. While there is still controversy regarding the source of the anisotropy such as the contribution of the intracellular versus extracellular water to the diffusion signal [73–76], one can argue from a physical point of view that a reduced anisotropy can be the result of one of the following phenomenon: loss of myelin leading to reduced restricted diffusion, intact fibers but not coherently oriented and loss of fibers. Measurements using multiple b-values have shown a dependence of the diffusion signal on fast and slow diffusion as well as a deviation from mono-exponential signal decay with higher b-values. With b-values below 2000 s/mm2 the signal decays mono-exponentially and is most sensitive to fast diffusion [74]. Our DTI data was acquired with a b-value of 1250 s/mm2 and is thus sensitized to fast diffusing water protons (probably in the extra-cellular space).
In a systematic review of proton MRS investigations measuring NAA differences between healthy controls and patients with schizophrenia [77] levels of NAA do appear to be substantially reduced in frontal WM and temporal WM of schizophrenic patients. Although studies of temporal WM NAA changes are less well corroborated than frontal WM changes in schizophrenia the mean reduction of FA in temporal WM across studies was estimated at 12.7% compared to 5.2% for frontal WM [77]. Moreover, antipsychotic treatment of the patients in our study may have confounded the measurements of NAA in the prefrontal white matter. In a longitudinal evaluation chronic schizophrenic patients first scanned off medication then after treatment was started NAA levels increased significantly and selectively in the dorsolateral prefrontal cortex within 4 weeks but not in temporal lobe or other areas assessed [42]. The fact that we only found significant differences in the medial temporal white matter may support these findings.
Our group has previously published a report on the DTI findings of the same subject population [78]. SPM analysis showed that there were significant differences in the medial temporal white matter as well as frontal white matter regions. The areas of significance were in the same locations as our ROI placements. The reason that we did not find significant FA differences in the frontal white matter is partly due to the size of the ROIs. The ROI dimensions and locations chosen for the current analysis was based on the need to match the voxels obtained from the MRS data. These results suggest that axonal disruptions in the frontal white matter areas might be limited to specific tracts within the frontal white matter. The subjects used for the current analysis is a subset of the subjects used in our previous report (N = 55, S = 63) versus (N42, S = 40) because some scans had to be eliminated due to poorly resolved spectra.
A similar study was performed by Steel et. al. [79], but no significant differences were found in NAA or FA. This might have been due to several reasons. Single voxel MRS is prone to partial volume contamination. Our technique used multivoxel spectroscopic imaging with much smaller voxel size; we only sampled the voxels which are well contained within the white matter regions. The second reason for the difference in findings may be due to our much larger sample of subjects (n = 40/42 vs. n = 10/10) and potential mean age differences (Steel's group = 35/34, this study = 43/39). The Steel's group did not survey the medial temporal lobe which was the location where we found significances for both NAA and FA.
A more recent study has been performed by Irwan et. al. [80] using DTI and CSI acquired in a supraventricular slice in normal control subjects. Although they have not specifically looked at isolated white matter voxels, they have shown a significant positive correlation between NAA and FA as well as a significant negative correlation between NAA with ADC values which is consistent with our interpretation of the FA index.
We also found significant correlations between FA and NAA in the left medial temporal white matter. Despite the co-occurrence of the reductions in NAA and fractional anisotropy these values were not significantly correlated on the right side (p = 0.07). These relatively weak significances were only observed when correlations were computed with both control subjects and patients combined. Therefore, these results between these two measures in the same region may be suggestive of different structural and/or metabolic changes co-occurring in the same region, but independent of each other, in contrast to what is observed in multiple sclerosis [81]. For example, patients with schizophrenia may be born with proper axonal formation but defective myelin maintenance infrastructure as reflected by reduced NAA/Cr. This interpretation is reasonable given that NAA is also found in large quantities in O-2A progenitor cells [35] putatively involved with glia repair processes [71]. In this scenario our results could be interpreted such that the reduction of anisotropy in this area might occur gradually whereas the low NAA/Cr levels could be constant throughout the lifespan of the patients. Low NAA may be a hallmark throughout the lifespan of the disease but its effect and other factors on FA may be more gradual.
One limitation of this study lies in the coregistration of the MRS ROIs with the DTI ROIs. Due to the lower sensitivity of MRS, the acquisition of MRS data is inherently slower and the resulting data is of lower resolution. We were only able to acquire two slices of MRS data. This made it impossible to accurately coregister the MRS slices to the DTI slices using conventional computer algorithms. The problem is compounded by the inherent distortions that exists in EPI based DTI acquisition schemes. In this study we have relied on our anatomy experts to visually identify the regions of interests. To minimize the error we have acquired the MRS slice for the DLPFC and the occipital regions in the same plane as the DTI which was along the AC-PC plane. This made it easier to identify the same regions on both modalities. Other limitations are the differences in slice thickness between the two modalities. The MRS was 10 mm thick whereas the DTI were 3 mm skip 1 mm. To minimize this difference we selected two DTI slices for every MRS slice.
Conclusion
Our study has provided further evidence of white matter abnormality in the medial temporal region in chronic schizophrenic patients. This was evidenced through MR Spectroscopy as well as Diffusion Tensor Imaging. Applying these two imaging modalities to an at risk population at a younger age before the disease is diagnosed and correlating the results with outcome would elucidate the sequence of events such as defective NAA preceding reduced FA. Differences in the MRS and DTI results may help one distinguish the nature of the white matter defects.
References
Benes FM: Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev. 2000, 31: 251-269. 10.1016/S0165-0173(99)00041-7.
Bunney WE, Bunney BG: Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res Brain Res Rev. 2000, 31: 138-146. 10.1016/S0165-0173(99)00031-4.
Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML: Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001, 41: 237-260. 10.1146/annurev.pharmtox.41.1.237.
Grace AA: Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev. 2000, 31: 330-341. 10.1016/S0165-0173(99)00049-1.
Heimer L: Basal forebrain in the context of schizophrenia. Brain Res Brain Res Rev. 2000, 31: 205-235. 10.1016/S0165-0173(99)00039-9.
Middleton FA, Strick PL: Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000, 31: 236-250. 10.1016/S0165-0173(99)00040-5.
Selemon LD, Rajkowska G, Goldman-Rakic PS: Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol. 1998, 392: 402-412. 10.1002/(SICI)1096-9861(19980316)392:3<402::AID-CNE9>3.0.CO;2-5.
Selemon LD, Rajkowska G, Goldman-Rakic PS: Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995, 52: 805-18; discussion 819-20.
Pakkenberg B: Total nerve cell number in neocortex in chronic schizophrenics and controls estimated using optical disectors. Biol Psychiatry. 1993, 34: 768-772. 10.1016/0006-3223(93)90065-L.
Williams RW, Rakic P: Elimination of neurons from the rhesus monkey's lateral geniculate nucleus during development. J Comp Neurol. 1988, 272: 424-436. 10.1002/cne.902720310.
Selemon LD, Goldman-Rakic PS: The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999, 45: 17-25. 10.1016/S0006-3223(98)00281-9.
Davis SR, Lewis DA: Local circuit neurons of the prefrontal cortex in schizophrenia: selective increase in the density of calbindin-immunoreactive neurons. Psychiatry Res. 1995, 59: 81-96. 10.1016/0165-1781(95)02720-3.
Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE, Jones EG: Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry. 1996, 53: 425-436.
Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET: Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000, 157: 16-25.
Cannon TD, van Erp TG, Huttunen M, Lonnqvist J, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG, Gur RE, Yan M: Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 1998, 55: 1084-1091. 10.1001/archpsyc.55.12.1084.
Foong J, Maier M, Barker GJ, Brocklehurst S, Miller DH, Ron MA: In vivo investigation of white matter pathology in schizophrenia with magnetisation transfer imaging. J Neurol Neurosurg Psychiatry. 2000, 68: 70-74. 10.1136/jnnp.68.1.70.
Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F: Brain morphology and schizophrenia. A magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry. 1992, 49: 921-926.
Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, Fukuda R, Ron M, Toone B: Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry. 2001, 158: 234-243. 10.1176/appi.ajp.158.2.234.
Buchanan RW, Vladar K, Barta PE, Pearlson GD: Structural evaluation of the prefrontal cortex in schizophrenia. Am J Psychiatry. 1998, 155: 1049-1055.
Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Feiner D, Rotrosen J, Wolkin A: Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry. 2000, 57: 471-480. 10.1001/archpsyc.57.5.471.
Foong J, Symms MR, Barker GJ, Maier M, Woermann FG, Miller DH, Ron MA: Neuropathological abnormalities in schizophrenia: evidence from magnetization transfer imaging. Brain. 2001, 124: 882-892. 10.1093/brain/124.5.882.
Maier M, Mellers J, Toone B, Trimble M, Ron MA: Schizophrenia, temporal lobe epilepsy and psychosis: an in vivo magnetic resonance spectroscopy and imaging study of the hippocampus/amygdala complex. Psychol Med. 2000, 30: 571-581. 10.1017/S0033291799001993.
Bertolino A, Esposito G, Callicott JH, Mattay VS, Van Horn JD, Frank JA, Berman KF, Weinberger DR: Specific relationship between prefrontal neuronal N-acetylaspartate and activation of the working memory cortical network in schizophrenia. Am J Psychiatry. 2000, 157: 26-33.
Rowland L, Bustillo JR, Lauriello J: Proton magnetic resonance spectroscopy (H-MRS) studies of schizophrenia. Semin Clin Neuropsychiatry. 2001, 6: 121-130. 10.1053/scnp.2001.21838.
Cecil KM, Lenkinski RE, Gur RE, Gur RC: Proton magnetic resonance spectroscopy in the frontal and temporal lobes of neuroleptic naive patients with schizophrenia. Neuropsychopharmacology. 1999, 20: 131-140. 10.1016/S0893-133X(98)00063-3.
Yurgelun-Todd DA, Renshaw PF, Gruber SA, Waternaux C, Cohen BM: Proton magnetic resonance spectroscopy of the temporal lobes in schizophrenics and normal controls. Schizophrenia Research. 1996, 19: 55-59. 10.1016/0920-9964(95)00071-2.
Fukuzako H, Kodama S, Fukuzako T, Yamada K, Doi W, Sato D, Takigawa M: Subtype-associated metabolite differences in the temporal lobe in schizophrenia detected by proton magnetic resonance spectroscopy. Psychiatry Res. 1999, 92: 45-56. 10.1016/S0925-4927(99)00035-9.
Buchsbaum MS, Tang CY, Peled S, Gudbjartsson H, Lu D, Hazlett EA, Downhill J, Haznedar M, Fallon J: MRI white matter diffusion anisotropy and PET metabolic rate in Schizophrenia. Neuroreport. 1998, 9: 425-430. 10.1097/00001756-199802160-00013.
Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A: Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999, 56: 367-374. 10.1001/archpsyc.56.4.367.
Kalus P, Buri C, Slotboom J, Gralla J, Remonda L, Dierks T, Strik WK, Schroth G, Kiefer C: Volumetry and diffusion tensor imaging of hippocampal subregions in schizophrenia. Neuroreport. 2004, 15: 867-871. 10.1097/00001756-200404090-00027.
Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, Kikinis R, Jolesz FA, McCarley RW, Shenton ME: Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biological Psychiatry. 2003, 54: 1171-1180. 10.1016/S0006-3223(03)00419-0.
Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO: MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003, 14: 2025-2029. 10.1097/00001756-200311140-00004.
Lim KO, Helpern JA: Neuropsychiatric applications of DTI - a review. NMR Biomed. 2002, 15: 587-593. 10.1002/nbm.789.
Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA: Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2000, 68: 242-244. 10.1136/jnnp.68.2.242.
Urenjak J, Williams SR, Gadian DG, Noble M: Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993, 13: 981-989.
Arnold DL, Matthews PM, Francis GS, O'Connor J, Antel JP: Proton magnetic resonance spectroscopic imaging for metabolic characterization of demyelinating plaques. Ann Neurol. 1992, 31: 235-241. 10.1002/ana.410310302.
Deicken RF, Feiwell R, Schuff N, Soher B: Evidence for altered cerebellar vermis neuronal integrity in schizophrenia. Psychiatry Res. 2001, 107: 125-134. 10.1016/S0925-4927(01)00103-2.
Auer DP, Wilke M, Grabner A, Heidenreich JO, Bronisch T, Wetter TC: Reduced NAA in the thalamus and altered membrane and glial metabolism in schizophrenic patients detected by 1H-MRS and tissue segmentation. Schizophr Res. 2001, 52: 87-99. 10.1016/S0920-9964(01)00155-4.
Ende G, Braus DF, Walter S, Henn FA: Lower concentration of thalamic n-acetylaspartate in patients with schizophrenia: a replication study. Am J Psychiatry. 2001, 158: 1314-1316. 10.1176/appi.ajp.158.8.1314.
Omori M, Murata T, Kimura H, Koshimoto Y, Kado H, Ishimori Y, Ito H, Wada Y: Thalamic abnormalities in patients with schizophrenia revealed by proton magnetic resonance spectroscopy. Psychiatry Res. 2000, 98: 155-162. 10.1016/S0925-4927(00)00049-4.
Deicken RF, Johnson C, Eliaz Y, Schuff N: Reduced concentrations of thalamic N-acetylaspartate in male patients with schizophrenia. Am J Psychiatry. 2000, 157: 644-647. 10.1176/appi.ajp.157.4.644.
Bertolino A, Callicott JH, Mattay VS, Weidenhammer KM, Rakow R, Egan MF, Weinberger DR: The effect of treatment with antipsychotic drugs on brain N-acetylaspartate measures in patients with schizophrenia. Biol Psychiatry. 2001, 49: 39-46. 10.1016/S0006-3223(00)00997-5.
Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR: Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000, 10: 1078-1092. 10.1093/cercor/10.11.1078.
Bertolino A, Breier A, Callicott JH, Adler C, Mattay VS, Shapiro M, Frank JA, Pickar D, Weinberger DR: The relationship between dorsolateral prefrontal neuronal N-acetylaspartate and evoked release of striatal dopamine in schizophrenia. Neuropsychopharmacology. 2000, 22: 125-132. 10.1016/S0893-133X(99)00096-2.
Ende G, Braus DF, Walter S, Weber-Fahr W, Soher B, Maudsley AA, Henn FA: Effects of age, medication, and illness duration on the N-acetyl aspartate signal of the anterior cingulate region in schizophrenia. Schizophr Res. 2000, 41: 389-395. 10.1016/S0920-9964(99)00089-4.
Szulc A, Galinska B, Tarasow E, Walecki J, Dzienis W, Kubas B, Czernikiewicz A: [Clinical and neuropsychological correlates of proton magnetic resonance spectroscopy detected metabolites in brains of first-episode and schizophrenic patients]. Psychiatr Pol. 2003, 37: 977-988.
Fukuzako H, Takeuchi K, Hokazono Y, Fukuzako T, Yamada K, Hashiguchi T, Obo Y, Ueyama K, Takigawa M, Fujimoto T: Proton magnetic resonance spectroscopy of the left medial temporal and frontal lobes in chronic schizophrenia: preliminary report. Psychiatry Res. 1995, 61: 193-200. 10.1016/0925-4927(95)02622-5.
Bitsch A, Bruhn H, Vougioukas V, Stringaris A, Lassmann H, Frahm J, Bruck W: Inflammatory CNS Demyelination: Histopathologic Correlation with In Vivo Quantitative Proton MR Spectroscopy. AJNR Am J Neuroradiol. 1999, 20: 1619-1627.
Basser PJ: Inferring Microstructural features and the Physiological State of Tissues from Diffusion Weighted Images. NMR in Biomedicine. 1995, 8: 333-344. 10.1002/nbm.1940080707.
Basser PJ: New histological and physiological stains derived from diffusion-tensor MR images. Ann N Y Acad Sci. 1997, 820: 123-138. 10.1111/j.1749-6632.1997.tb46192.x.
Basser PJ, Pierpaoli C: Microstructural and Physiological Features of Tissues Elucidated by Quantitative-Diffusion-Tensor MRI. Journal of Magnetic Resonance, Series B. 1996, 111 (3): 209-219. 10.1006/jmrb.1996.0086.
Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Chiro GD: Diffusion Tensor MR Imaging of the Human Brain. Radiology. 1996, 201: 637-648.
Pierpaoli C, Basser PJ: Toward a Quantitative Assessment of Diffusion Anisotropy. Magnetic Resonance in Medicine. 1996, 36: 893-906. 10.1002/mrm.1910360612.
Mori S, van Zijl PC: Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002, 15: 468-480. 10.1002/nbm.781.
Bammer R, Acar B, Moseley ME: In vivo MR tractography using diffusion imaging. European Journal of Radiology. 2003, 45: 223-234. 10.1016/S0720-048X(02)00311-X.
Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME: Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci. 1999, 96: 10422-10427. 10.1073/pnas.96.18.10422.
Wolkin A, Choi SJ, Szilagyi S, Sanfilipo M, Rotrosen JP, Lim KO: Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: a diffusion tensor imaging study. Am J Psychiatry. 2003, 160: 572-574. 10.1176/appi.ajp.160.3.572.
Hoptman MJ, Volavka J, Johnson G, Weiss E, Bilder RM, Lim KO: Frontal white matter microstructure, aggression, and impulsivity in men with schizophrenia: a preliminary study. Biological Psychiatry. 2002, 52: 9-14. 10.1016/S0006-3223(02)01311-2.
Sun Z, Wang F, Cui L, Breeze J, Du X, Wang X, Cong Z, Zhang H, Li B, Hong N, Zhang D: Abnormal anterior cingulum in patients with schizophrenia: a diffusion tensor imaging study. Neuroreport. 2003, 14: 1833-1836. 10.1097/00001756-200310060-00015.
Spalletta G, Tomaiuolo F, Marino V, Bonaviri G, Trequattrini A, Caltagirone C: Chronic schizophrenia as a brain misconnection syndrome: a white matter voxel-based morphometry study. Schizophrenia Research. 2003, 64: 15-23. 10.1016/S0920-9964(03)00010-0.
Agartz I, Andersson JL, Skare S: Abnormal brain white matter in schizophrenia: a diffusion tensor imaging study. Neuroreport. 2001, 12: 2251-2254. 10.1097/00001756-200107200-00041.
Andreasen NC, Flaum M, Arndt S: The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992, 49: 615-623.
Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M: Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000, 44: 259-268. 10.1002/1522-2594(200008)44:2<259::AID-MRM13>3.0.CO;2-6.
Nusbaum AO, Tang CY, Buchsbaum MS, Wei TC, Atlas SW: Regional and global changes in cerebral diffusion with normal aging. AJNR Am J Neuroradiol. 2001, 22: 136-142.
Saunders DE, Howe FA, van den Boogaart A, Griffiths JR, Brown MM: Aging of the adult human brain: in vivo quantitation of metabolite content with proton magnetic resonance spectroscopy. J Magn Reson Imaging. 1999, 9: 711-716. 10.1002/(SICI)1522-2586(199905)9:5<711::AID-JMRI14>3.0.CO;2-3.
Miller BL: A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed. 1991, 4: 47-52. 10.1002/nbm.1940040203.
Uren jak J, Williams SR, Gadian DG, Noble M: Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. J Neurochem. 1992, 59: 55-61. 10.1111/j.1471-4159.1992.tb08875.x.
Meyerhoff DJ, MacKay S, Bachman L, Poole N, Dillon WP, Weiner MW, Fein G: Reduced brain N-acetylaspartate suggests neuronal loss in cognitively impaired human immunodeficiency virus-seropositive individuals: in vivo 1H magnetic resonance spectroscopic imaging. Neurology. 1993, 43: 509-515.
Lu ZH, Chakraborty G, Ledeen RW, Yahya D, Wu G: N-Acetylaspartate synthase is bimodally expressed in microsomes and mitochondria of brain. Molecular Brain Research. 2004, 122: 71-78. 10.1016/j.molbrainres.2003.12.002.
Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD: bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004, 306: 2111-2115. 10.1126/science.1103709.
Chang A, Tourtellotte WW, Rudick R, Trapp BD: Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002, 346: 165-173. 10.1056/NEJMoa010994.
Mulkern RV, Zengingonul HP, Robertson RL, Bogner P, Zou KH, Gudbjartsson H, Guttmann CR, Holtzman D, Kyriakos W, Jolesz FA, Maier SE: Multi-component apparent diffusion coefficients in human brain: relationship to spin-lattice relaxation. Magn Reson Med. 2000, 44: 292-300. 10.1002/1522-2594(200008)44:2<292::AID-MRM17>3.0.CO;2-Q.
Mulkern RV, Gudbjartsson H, Westin CF, Zengingonul HP, Gartner W, Guttmann CR, Robertson RL, Kyriakos W, Schwartz R, Holtzman D, Jolesz FA, Maier SE: Multi-component apparent diffusion coefficients in human brain. NMR Biomed. 1999, 12: 51-62. 10.1002/(SICI)1099-1492(199902)12:1<51::AID-NBM546>3.0.CO;2-E.
Beaulieu C: The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002, 15: 435-455. 10.1002/nbm.782.
Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H: Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001, 13: 534-546. 10.1002/jmri.1076.
Steen RG, Hamer RM, Lieberman JA: Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005, 30: 1949-1962. 10.1038/sj.npp.1300850.
Buchsbaum MS, Friedman J, Buchsbaum BR, Chu KW, Hazlett EA, Newmark R, Schneiderman JS, Torosjan Y, Tang C, Hof PR, Stewart D, Davis KL, Gorman J: Diffusion Tensor Imaging in Schizophrenia. Biol Psychiatry. 2006
Steel RM, Bastin ME, McConnell S, Marshall I, Cunningham-Owens DG, Lawrie SM, Johnstone EC, Best JJK: Diffusion tensor imaging (DTI) and proton magnetic resonance spectroscopy (1H MRS) in schizophrenic subjects and normal controls. Psychiatry Research: Neuroimaging. 2001, 106 (3): 161-170. 10.1016/S0925-4927(01)00080-4.
Irwan R, Sijens PE, Potze JH, Oudkerk M: Correlation of proton MR spectroscopy and diffusion tensor imaging. Magn Reson Imaging. 2005, 23: 851-858. 10.1016/j.mri.2005.06.005.
Sijens PE, Irwan R, Potze JH, Mostert JP, De Keyser J, Oudkerk M: Analysis of the human brain in primary progressive multiple sclerosis with mapping of the spatial distributions using (1)H MR spectroscopy and diffusion tensor imaging. Eur Radiol. 2005, 15: 1686-1693. 10.1007/s00330-005-2775-0.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-244X/7/25/prepub
Acknowledgements
This work was supported by the following grants:
1. P50 MH 66392-01 awarded by the NIMH to Kenneth Davis.
2. VISN3 MIRECC.
3. GCRC grant M01-RR-00071 awarded to Mount Sinai School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
CYT was responsible for the DTI software development, MRI protocols, analysis and interpretation of the results. JF and DS was responsible for the recruitment and screening of the subjects. LC, TE and DS were responsible for the MRS protocols. AH, DC, JN and XM were responsible for ROI tracings. PRH was responsible for the anatomical localization of the ROIs.
The study was conceived by KD, MSB and JMG. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Tang, C.Y., Friedman, J., Shungu, D. et al. Correlations between Diffusion Tensor Imaging (DTI) and Magnetic Resonance Spectroscopy (1H MRS) in schizophrenic patients and normal controls. BMC Psychiatry 7, 25 (2007). https://doi.org/10.1186/1471-244X-7-25
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-244X-7-25