Abstract
Background
Antiphospholipid antibody syndrome is characterized by venous and/or arterial thrombosis, and is found in patients with systemic lupus erythematosus. Its diagnosis requires the presence of both clinical and laboratory findings, such as positive anti-cardiolipin and anti-β2 glycoprotein I antibodies and lupus anticoagulant. However, cardiolipin is a minor component of the vascular endothelial cells in human, and phosphatidylcholine and phosphatidylethanolamine are major components.
Case presentation
A 15-year-old female suddenly developed massive left intraretinal hemorrhaging due to central retinal vein occlusion. She also had a butterfly rash, and her laboratory findings revealed positive serum anti-nuclear antibodies and decreased serum complement. During this episode, she was diagnosed with systemic lupus erythematosus. Although she was negative for serum anti-cardiolipin IgG and anti-β2 glycoprotein I antibodies as well as lupus anticoagulant, her serum anti-phosphatidylcholine, anti-phosphatidylethanolamine, anti-phosphatidylinositol and phosphatidylserine IgG antibodies levels were increased.
Conclusion
Pediatric cases of central retinal vein occlusion are rare. Even in patients without anti-cardiolipin or anti-β2 glycoprotein I antibodies and lupus anticoagulant, there is the potential for the development of antiphospholipid antibody-related thrombosis.
Similar content being viewed by others
Background
Antiphospholipid antibody syndrome (APS) is characterized by venous and/or arterial thrombosis and recurrent fetal loss, and is found in patients with systemic lupus erythematosus (SLE) [1]. A diagnosis of APS requires the presence of both clinical and laboratory findings, such as positive anti-cardiolipin (CL) and anti-β2 glycoprotein I antibodies and lupus anticoagulant. However, CL is a minor component of phospholipids in humans [2].
This report describes central retinal vein occlusion (CRVO) in a pediatric patient with SLE and anti-phospholipids antibodies without anti-CL antibody.
Case presentation
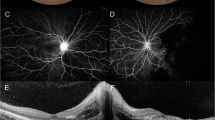
A female patient was born without any perinatal problems and her psychomotor and growth development had been normal. Her father had Crohn’s disease and her elder brother had atopic dermatitis. She developed allergic conjunctivitis when she was around 10 years old and was administered betamethasone eye-drops. She also developed a butterfly rash and photosensitivity at the age of 14. And then, she suddenly developed a left visual disturbance without any premonitory sign at the age of 15. Her visual acuity (left: 0.04, right: 1.2) showed asymmetry. Her eye fundus examination showed massive left intraretinal hemorrhaging due to CRVO (Figure 1). Her blood pressure was 100/70 mmHg and she had not history of any cardio-vascular disorders or diabetes.
Her laboratory findings showed pancytopenia with a WBC count of 4,680/mm3, RBC count of 351 × 104/mm3, Hb level of 9.9 g/dl, Ht level of 30.3% and PLT count of 16.1 × 104/mm3, an increased erythrocyte sedimentation rate of 54 mm/hr (<10), but APTT of 28.0 seconds was within the normal range. There was increased anti-nuclear antibody titer of x640 (<x40), ds-DNA antibody titer of 377.7 IU/ml (<12.0) and ss-DNA antibody titer of 722.4 AU/ml (<25.0) and a decline in complement levels of C3 14 mg/dl (86–160), C4 3 mg/dl (17–45) and CH50 < 10 U/ml (23–46). However, both her anti-CL IgG antibody of 9 U/ml (<10; EIA method), anti-β2 glycoprotein I antibody of 1.3 U/ml (<3.5; EIA method) and lupus anticoagulant of 1.2 (<1.4; Dilute Russell’s Viper Venom Time) were within the normal range.
Intravenous low-molecular weight heparin of reviparin was initiated, and she received steroid pulse therapy, oral fexofenadine and levocabastine eye-drops. The intraretinal hemorrhaging gradually improved, and the patient was treated with warfarin, aspirin, prednisolone and tacrolimus. Her anti-CL IgG antibody titer was examined four times every two weeks, however, all of evaluations showed negative results (2/3/1/1 U/ml). The second examination of the anti-β2 glycoprotein I antibody titer also showed a level less than 1.3 U/ml.
After informed consent was provided by the patient and parents, we examined the patient for other antiphospholipid antibodies according to the Guidelines of the European Consensus described the development of the antiphospholipid IgG antibody ELISA assay, as described previously [3]. Briefly, 1 ml of whole blood was drawn twice at an interval of 12 weeks. Microtitre plates were coated with 50 μg/ml phosphatidylcholine (PC, Nacalai tesque, Kyoto, Japan), phosphatidylethanolamine (PE, Sigma, St. Louis, USA), phosphatidylinositol (PI, Nacalai tesque, Kyoto, Japan) and phosphatidylserine (PS, ChromaDex, Inc, California, USA) and were incubated overnight. On the following day the plates were incubated with triplicate serum at a 1/10 dilution for one hour. After wash, the plates were incubated with goat anti-human IgG (Santa Cruz Biotechnology, Inc, California, USA) at a 1/2,000 dilution for one hour. Using substrate of o-phenylenediamine (Sigma, St. Louis, USA), the absorbance was read at 450 nm with ELISA reader. Age-matched three patients with APS with anti-CL IgG antibody, four patients with SLE and eight patients with non-collagen disease controls were also enrolled as disease controls. The positive cut-off value was defined as two standard deviations above the mean in the controls subjects. Since there were no positive control antiphospholipid antibodies, we calculated the optical density (OD) according to the method used in previous reports [3].
Her anti-PC IgG antibody (0.342 OD), anti-PE IgG antibody (0.190 OD), anti-PI IgG antibody (0.290 OD), and anti-PS IgG antibody (0.214 OD) were increased in comparison to not only normal controls (anti-PC; 0.095 ± 0.009 OD, anti-PE; 0.079 ± 0.019 OD, anti-PI; 0.082 ± 0.012 OD, anti-PS; 0.084 ± 0.033 OD) but also other patients with APS (anti-PC; 0.181 ± 0.076 OD, anti-PE; 0.117 ± 0.021 OD, anti-PI; 0.130 ± 0.039 OD, anti-PS; 0.129 ± 0.094 OD) and SLE (anti-PC; 0.117 ± 0.019 OD, anti-PE; 0.110 ± 0.038 OD), anti-PI; 0.097 ± 0.046 OD, anti-PS; 0.090 ± 0.037 OD). The elevation of anti-PC (0.202 OD), anti-PE (0.114 OD), anti-PI (0.181 OD), and anti-PS (0.139 OD) IgG antibodies persisted after 12 weeks.
Conclusion
CRVO in pediatric patients is rare and has been described to be caused by cardio-vascular disorders, such as hypertension, heart failure, diabetes, atherosclerosis and APS [4]. APS may be considered present in a patient with thrombotic events and positive antiphospholipid antibodies such as anti-CL and anti-β2 glycoprotein I antibodies and lupus anti-coagulant titer that is verified on at least two occasions at least 12 weeks apart [1].
However, CL is a minor component of phospholipids in humans and anti-CL antibodies have cross-reactivity with other major components of phospholipids. Takamura et al.[2] described that even in both arterial and venous endothelial cells, PC (49.0 ± 0.1/50.5 ± 0.1%) and PE (28.1 ± 0.3/25.5 ± 0.4%) are more extensively distributed than CL (2.0 ± 0.1/2.3 ± 0.2%). Reshetniak et al.[5] found that only 40% of patients with SLE had anti-CL antibody but 60% had anti-PC antibody. Kalashnikova et al.[6] described that anti-PE antibody was present in 46% of anti-CL and lupus anticoagulant-negative patients.
Von Schieven et al.[7] found no significant difference in the prevalence of anti- anti-β2 glycoprotein I between SLE children with or without thrombosis. Berard et al.[8] reported that 15 patients with anti-PE antibodies without anti-CL antibodies developed thromboembolic episodes. Bertolaccini ML et al.[9] also showed that anti-PS/prothrombin antibodies also act as risk factor for thrombosis. Moreover, Kuksis et al.[10] described that decreased plasma levels of the PC is an indicator of atherosclerosis. These previous reports indicated that anti-PC, -PE, and -PS increases the risk of thrombosis and atherosclerosis even in patients without anti-CL antibody.
The examination of patients for anti-CL and anti-β2 glycoprotein I antibodies and lupus anticoagulant is insufficient to understand the essential pathology of antiphospholipid antibody-related thrombosis. Even in patients without anti-CL and anti-β2 glycoprotein I antibodies or lupus anticoagulant, there is the potential for the development of antiphospholipid antibody-related thrombosis.
Consent
Informed consent was obtained from the patient and her parents for publication of this case report. A copy of the consent form is available for review from the Editor of this journal.
Abbreviations
- APS:
-
Antiphospholipid antibody syndrome
- SLE:
-
Systemic lupus erythematosus
- CL:
-
Cardiolipin
- CRVO:
-
Central retinal vein occlusion
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PI:
-
Phosphatidylinositol
- PS:
-
Phosphatidylserine
- OD:
-
Optical density.
References
Ravelli A, Martini A, Burgio GR: Antiphospholipid antibodies in paediatrics. Eur J Pediatr. 1994, 153: 472-479.
Takamura H, Kasai H, Arita H, Kito M: Phospholipid molecular species in human umbilical artery and vein endothelial cells. J Lipid Res. 1990, 31: 709-717.
Tincani A, Allegri F, Sanmarco M, Cinquini M, Taglietti M, Balestrieri G, Koike T, Ichikawa K, Meroni P, Boffa MC: Anticardiolipin antibody assay: a methodological analysis for a better consensus in routine determinations. A cooperative project of the European Antiphospholipid Forum. Thromb Haemost. 2001, 86: 575-583.
Suvajac G, Stojanovich L, Milenkovich S: Ocular manifestations in antiphospholipid syndrome. Autoimmun Rev. 2007, 6: 409-614.
Reshetniak TM, Boitsekhovscaoa A, Aleksandrova ZS, Kalashnikova LA, Mach ES, Zabek I: Antibodies to various phospholipids in SLE patients with primary antiphospholipid syndrome. Klin Med (Mosk). 1999, 77: 32-37. In Russian
Kalashnikova LA, Aleksandrova EN, Nivikov AA, Dobrynina LA, Nasonov EL, Sergeeva EV, Berkovskiĭ AL: Anti-phosphatidylethanolamine antibodies in patients with Sneddon’s syndrome. Klin Med (Mosk). 2005, 83: 46-49. In Russian
Von Schieven E, Gildden DV, Elder ME: Anti-β2 glycoprotein I antibodies in pediatric systemic lupus erythematosus and antiphospholipid syndrome. Arthiris Rheum. 2002, 47: 414-420.
Berard M, Chantome R, Marcelli A, Boffa MC: Antiphosphatidylethenolamine antibodies as the only antiphospholipid antibodies. I. Association with thrombosis and vascular cutaneous diseases. J Rheumatol. 1996, 23: 1369-1374.
Bertolaccini ML, Sciascia S, Murru V, Garcia-Fernandez C, Sanna G, Khamashta MA: Prevalence of antibodies to prothrombin in solid phase (aPT) and to phosphatidylserine-prothrombin complex (aPS/PT) in patients with and without lupus anticoagulant. Thromb Haemost. 2013, 109: 207-213.
Kuksis A, Roberts A, Thompson JS, Myher JJ, Geher K: Plasma phophatidylcholine/free cholesterol ratio as an indicator for atherosclerosis. Artheriosclerosis. 1983, 3: 389-397.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2431/14/116/prepub
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SK acted as the outpatient doctor for the present patient and performed the immunological examination. HG acted as the junior ward doctor for the patient. CG acted as the senior ward doctor for the patient. RO and TK acted as doctors in duty of the Department of Ophthalmology. TI is a professor in Department of Pediatrics and Child Neurology and made a significant contribution to the diagnosis and treatment of the patient. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Korematsu, S., Goto, H., Gotoh, C. et al. Central retinal vein occlusion in a pediatric patient with SLE and antiphospholipid antibodies without anti-cardiolipin or anti-β2 glycoprotein I antibodies. BMC Pediatr 14, 116 (2014). https://doi.org/10.1186/1471-2431-14-116
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2431-14-116