Abstract
Background
Central nervous system is a common site of metastasis in NSCLC and confers worse prognosis and quality of life. The aim of this prospective study was to evaluate the prognostic significance of clinical-pathological factors (CPF), serum CEA levels, and EGFR and HER2 tissue-expression in brain metastasis (BM) and overall survival (OS) in patients with advanced NSCLC.
Methods
In a prospective manner, we studied 293 patients with NSCLC in IIIB-IV clinical stage. They received standard chemotherapy. CEA was measured prior to treatment; EGFR and HER2 were evaluated by immunohistochemistry. BM development was confirmed by MRI in symptomatic patients.
Results
BM developed in 27, and 32% of patients at 1 and 2 years of diagnosis with adenocarcinoma (RR 5.2; 95% CI, 1.002–29; p = 0.05) and CEA ≥ 40 ng/mL (RR 11.4; 95% CI, 1.7–74; p < 0.01) as independent associated factors. EGFR and HER2 were not statistically significant. Masculine gender (RR 1.4; 95% CI, 1.002–1.9; p = 0.048), poor performance status (RR 1.8; 95% CI, 1.5–2.3; p = 0.002), advanced clinical stage (RR 1.44; 95% CI, 1.02–2; p = 0.04), CEA ≥ 40 ng/mL (RR 1.5; 95% CI, 1.09–2.2; p = 0.014) and EGFR expression (RR 1.6; 95% CI, 1.4–1.9; p = 0.012) were independent associated factors to worse OS.
Conclusion
High CEA serum level is a risk factor for BM development and is associated with poor prognosis in patients with advanced NSCLC. Surface expression of CEA in tumor cells could be the physiopathological mechanism for invasion to CNS.
Similar content being viewed by others
Background
Lung cancer is the first cause of cancer death in the world. Eighty five percent of patients are diagnosed yearly with non-small cell lung cancer (NSCLC). Despite efforts, innovations, and progress in diagnosis and treatment of these patients, overall survival (OS) at 5 years of diagnosis is only 15% [1].
The central nervous system (CNS) is a devastating and frequent site of metastasis development in NSCLC. The reported incidence of CNS metastasis in patients with NSCLC is 54% [2] with an OS of <1 year after diagnosis [3–5]. Age [3], clinical stage [6], gender [7], and initial treatment period [8] are some of the reported with CNS metastasis development-related factors in patients with NSCLC; however, due to their lack of specificity, we are required to detect biomarkers to predict brain metastasis in patients with NSCLC.
Carcinoembryonic antigen (CEA, CEA-related cell adhesion molecule 5, CEACAM5) is an oncofetal protein attached to epithelial-cell apical membrane via its c-terminal glycosylphosphatidylinositol anchor, a member of the immunoglobulin superfamily of cell adhesion molecules (IgCAMs) [9]. CEA is usually over-expressed in a variety of neoplasms, such as colorectal, breast, bladder, gastric, pancreatic, and lung carcinomas [10]. CEA protein levels were found to correlate with its mRNA levels in cells and tissues examined, suggesting that CEA overexpression in cancer cells involves CEA-gene transcriptional activation [9]. CEA-related cell adhesion molecules (CAMs) are involved in cell-cell recognition and modulate cellular processes [11]. High serum CEA levels have been associated with advanced disease and tumor relapse in resected NSCLC [12–19]. Despite this widely reported information, there are no studies on serum CEA levels in advanced NSCLC and brain metastasis development.
The family of epidermal growth factor receptors (EGFRs) plays an important role in proliferation and cell survival. It is composed of the following four different receptors: 1) epidermal growth factor receptor (EGFR, erbb1); 2) HER2/neu (erbb2); 3) HER3 (erbb3), and 4) HER4 (erbb4). In NSCLC, reported incidence of EGFR tissue expression is 43–89% [20] and it has been associated with a worse prognosis [21]; nonetheless, evidence concerning its role as a prognostic factor remains controversial [22–26]. With an incidence of 25% in breast cancer, HER2 tissue expression is associated with poor disease-free survival and OS compared with patients who are HER2-negative [27]. Recent reports correlate this with brain metastasis development [28]. In patients with NSCLC, HER2 tissue expression entertains an incidence of 11–32% [29] and has been linked with worse OS at 3 and 5 years of diagnosis [30].
The objective of this study was to evaluate prospectively manner the prognostic significance of clinical-pathological factors, serum CEA levels, and EGFR and HER2 lung expression in brain metastasis development and OS in patients with recent diagnosis of advanced NSCLC (clinical stage IIIB-IV) treated with platin-based cytotoxic chemotherapy or EGFR inhibitors.
Methods
Patients
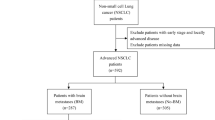
Between March 2005 and June 2007, patients with recent histological diagnosis of advanced NSCLC without previous treatment and referred to the Lung cancer and Thoracic Tumors Clinic (Instituto Nacional de Cancerología and Instituto Nacional de Enfermedades Respiratorias, Mexico City, Mexico) were enrolled in this prospective study. This work was approved by bioethical and research committees of each institution, and patient informed consent was obtained before enrollment. Histological diagnosis of primary NSCLC was established according to the revised classification of lung tumors of World Health Organization and the International Association for Lung Cancer Study. During pre-treatment clinical evaluation, we focused on physical examination, weigh loss, and performance status (according to the Eastern Cooperative Oncologic Group scale, ECOG). Thoracic, upper abdominal computed tomography (CT) and bone scintillography were used in disease staging. Only patients with IIIB (T4 N0-1-2 M0 or any T N3 M0: tumor of any size that invades mediastinum, heart, great vessels trachea, esophagus, vertebral body, carina; or tumor with a malignant pleural or pericardial effusion, or with satellite tumor nodule(s) within the ipsilateral primary-tumor lobe of the lung [T4], no regional lymph node metastasis [N0], metastasis to ipsilateral peribronchial and/or ipsilateral hiliar lymph nodes, and intrapulmonary lymph nodes involved by direct extension of the primary tumor [N1], metastasis to ipsilateral mediastinal and/or subcarinal lymph node(s) [N2], or metastasis to contralateral mediastinal, contralateral hiliar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s) [N3], and no distant metastasis [M0]) and IV clinical stage (any T any N M1: distant metastasis present) were enrolled [31, 32]. Chemotherapy was based on platin or EGFR inhibitors. Six different schedules were instituted, including gemcitabine, vinorelbine, or paclitaxel combined with cisplatin or carboplatin and erlotinib.
Samples
Analyzed variables comprised smoking history, gender, general condition, histology, brain metastasis development, serum CEA levels, and EGFR and HER2 tissue expression.
CEA levels in serum were evaluated prior to chemotherapy. CEA was measured by solid-phase, two-site sequential chemiluminescent immunometric assay (IMMULITE 2000-CEA Analyzer, Siemens, Inc., Los Angeles, CA) with an analytical sensibility of 0.15 ng/mL and a high-dose Hook effect at levels of >250,000 ng/mL. Serum samples (15 μL serum) were collected using the same method for each patient and stored if necessary at -20°C before processing. Cut-off points for resuming tables were selected according to the previously reported normal value for CEA serum level (< 10 ng/mL) [10].
EGFR and HER2 tissue expression were evaluated by immunohistochemistry (IHC). IHC was performed on formalin-fixed, paraffin-embedded tissue sections from primary lung tumors. 5 μm sections were placed on chemically charged slides. Sections were deparaffinized and rehydrated in a series of alcohols and xylene according to established procedures. Following deparaffination, sections were immersed in an antigen-retrieval solution (Dako Corporation) for 40 min at 95°C. Endogen peroxidase was blocked with 3% H2O2 in absolute methanol for 5 min. Slides were incubated with rabbit antibody human HER2 (Hercep Test, Dako Corporation) for 30 min, and staining was completed using Dako Rabbit Evision Plus Kit (Dako Corporation). The antibody binding site was visualized using diaminobenzidine reagent for 5 min. The slides were counterstained with Mayer's hematoxylin. Immunostainig for EGFR was accomplished using the monoclonal mouse antibody to EGFR (1:100, Zymed Laboratories, San Francisco, CA). The slides were incubated with peroxide block for 10 min, this followed by incubation for 10 min with proteinase K. After incubation of slides with primary antibody for 30 min, staining was completed by incubation with monoclonal-labeled polymer for 10 min. The antibody binding site was visualized using diaminobenzidine reagent. Finally, the slides were counterstained with Mayer's hematoxylin.
EGFR and HER2 were examined using light microscopy. For EGFR, we considered negative tissue expression if the stain was heterogeneous in ≤ 25% of the sample, and positive tissue expression if stain was homogeneous >25% of sample. For semi-quantitative evaluation of HER2, all slides were scored following the guidelines for scoring HercepTest: 0, completely negative or membrane staining in fewer than 10% of tumor cells; 1+, faint membranous staining in >10% of the tumor cells; 2+, weak or moderate complete staining in >10% of tumor cells, and 3+, strong complete membrane staining in >10% of the tumor cells. IHC was graded by a single pathologist (AAS), who was blinded to clinical characteristics and outcomes.
Follow-up
In presence of neurological symptoms (persistent headache, neurological focalization, motor deficits, or abnormal behaviour), brain magnetic resonance imaging (MRI) was performed. Monitoring of disease (including primary endpoints, e.g., OS and brain metastasis development) was carried out by means of clinical follow-up.
Statistical analysis
With a descriptive purpose, we resumed each continuous variable as arithmetic mean, median and standard deviation (SD), and categorical variables as proportion with 95% confidence interval (95% CI). For inferential comparisons, we employed Student t or Mann-Whitney U test, according to data distribution (normal or non-normal, determined with Kolmogorov-Smirnov test). To calculate statistical significance between categorical variables, we used chi-square or Fisher exact test. Statistically significant and borderline results (p < 0.1) were included in multivariate logistic regression analysis. CNS metastasis development and OS were defined as the period from date of histological diagnosis to date of confirmed diagnosis of brain metastasis by MRI and to date of death, respectively; both were analyzed with Kaplan-Meier method, and sub-groups were compared with log-rank test. To analyze survival curves, each variable was dichotomized. HER2 tissue expression was considered negative if IHC was classified as 0 or +1, and positive if classified as +2 and +3. For EGFR, tissue expression was negative or positive if IHC was positive in <25% or ≥ 25% of tumor cells, respectively. Statistically significant or borderline (p < 0.1) variables on univariate analysis were included in multivariate analysis utilizing Cox proportional hazards model. Statistical significance was determined with a p < 0.05 in a two-sided test. SPSS software package (version 14.0; SPSS, Inc., Chicago, IL) was employed for data analysis.
Results
Patients and samples
Two hundred ninety three consecutive patients were prospectively studied. Patient characteristics are shown in Table 1. Mean for age was 60.7 ± 0.7 years. Only 54% of patients had smoking history. The majority of tumors were adenocarcinoma (65%) classified as moderate or high histological grade (36 and 54%, respectively). Seventy one percent of patients were diagnosed in a metastatic stage, and 29% were in IIIB clinical stage. Of patients with metastasis at time of diagnosis, 18.6% had CNS metastasis and 7.5%, liver metastasis. Most patients were referred to our Institute without paraffin blocks and in others the diagnosis was based on cytopathological samples; even, when tissue samples were available, some of them were insufficient to perform the immunohistochemistry. Because of these reasons, we only analyzed 85 biopsies of primary tumor to determine tissue expression of EGFRs types 1 and 2, of which, 59% were positive for EGFR and only 7% for HER2 tissue expression.
Serum levels of CEA
In 42.8, 32.3, 22.2, and 21.4% of patients, basal serum CEA level was ≥ 10, 20, 40, and 50 ng/mL at diagnosis, respectively (median ± standard deviation, 6.3 ± 1,021 ng/mL; range, 0.2–15,475 ng/mL). Age, gender, positive smoking history, status performance, histological-grade of differentiation and presence of liver metastasis at diagnosis were not associated to basal CEA serum levels ≥ 40 ng/mL. However, at the bi- and multivariate analysis, factors associated with basal CEA serum levels ≥ 40 ng/mL were adenocarcinoma histological type (frequency of 41.3% compared with 14.1% of patients with squamous or large-cell histologies, RR 1.6; 95% CI, 1.4–3.4; p = 0.005) and presence of CNS metastasis at diagnosis (frequency of 77.7% compared with 12.3% of patients without CNS metastasis at diagnosis, RR 14.05; 95% CI, 5.7–34.4; p < 0.001).
CNS metastasis
At 1 year of diagnosis, 27% (95% CI, 23–30%) of patients developed brain metastasis, and at 2 years, 32% (95% CI, 21–43%). As treatment for brain metastasis, only 4 patients achieved criteria to undergo radiosurgery, none of the patients was submitted to brain surgery, and all other patients received whole brain irradiation. Independent associated factors comprised adenocarcinoma histological type (RR 5.2; 95% CI, 1.002–29; p = 0.0002) and CEA serum levels ≥ 40 ng/mL (RR 11.4; 95% CI, 1.7–74; p < 0.001) (Table 2). In the subgroup analysis of patients with adenocarcinoma histologycal type, frequency ± standard error of CNS metastasis development at 12 months of diagnosis were 16.4 ± 0.03% (95% CI, 16.34 – 16.46%) and 67 ± 0.09% (95% CI, 66.91 – 67.09), in patients with CEA serum levels < 40 ng/mL, and ≥ 40 ng/mL, respectively. And at 24 months of diagnosis, frequency ± standard error of CNS metastasis development were 20.2 ± 0.05% (95% CI, 20.19 – 20.21%) and 67 ± 0.09% (95% CI, 66.82 – 67.18%) in patients with CEA serum levels < 40 ng/mL and ≥ 40 ng/mL, respectively. EGFR tissue expression was not statistically significant in terms of CNS metastasis development.
Overall survival
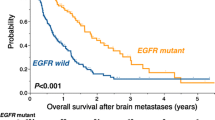
OS was 7 ± 0.48 months. Factors associated with statistical significance at univariate analysis with poor OS were male gender (p = 0.02), age ≥ 60 years (p = 0.028), poor performance status (ECOG III, p < 0.001), serum CEA levels ≥ 40 ng/mL (p = 0.002), and EGFR-positive tissue expression (p = 0.023). Clinical stage IV demonstrated solely a tendency toward significance (p = 0.056) (Table 3). On multivariate analysis, male gender (RR = 1.4; 95% CI, 1.002–1.9; p = 0.048) [Fig 1a], poor performance status (RR 1.8; 95% CI, 1.5–2.3; p = 0.002) [Fig 1b], clinical stage IV (RR 1.4; 95% CI, 1.02–2; p = 0.04) [Fig 1c], serum CEA levels ≥ 40 mg/mL (RR 1.5; 95% CI, 1.09–2.2; p = 0.014) [Fig 1d], and EGFR tissue expression (RR 1.6; 95% CI, 1.4–19; p = 0.012) [Fig 1e] were statistically significant.
Discussion
Carcinoembryonic antigen, a glycoprotein expressed during early fetal life, is the product of the CEACAM5-gen. Its expression is restricted to epithelial cells, and it is found more abundantly on apical surface of gastrointestinal epithelium, but can also be found in other mucosal epithelia, such as lung [9]. In this prospective study, we found abnormal serum CEA levels (≥ 10 ng/mL) in 43% and levels ≥ 40 ng/mL in 22% of patients with advanced NSCLC. However, 41% of patients with pathological diagnosis of adenocarcinoma had serum CEA levels ≥ 40 ng/mL. In our study, we found a significant association between high CEA serum levels and adenocarcinoma in comparison with other histological types. This observation is consistent with a Japanese study, which showed association between high CEA serum levels and primary lung adenocarcinoma in clinical stage I, compared with squamous type [33].
We found that high CEA serum levels and histological type adenocarcinoma were associated with brain metastasis at time of diagnosis. Furthermore, we found that a CEA serum level ≥ 40 ng/mL is the more important factor associated with CNS metastasis development during follow-up (Table 2), independent of clinical stage (IIIB or IV) and absence or presence of liver metastasis, suggesting that association between high CEA serum levels and brain metastasis development are not due to tumor charge but rather to a more invasive phenotype.
Tumors with high CEA expression could possess an increased capacity to develop brain metastasis, and this could be due to vascular-tumoral cell-cell adhesion processes. CEA is a member of the IgCAM superfamily and is involved in homo and heterotypic interactions with other closely related IgCAMs, which possess at least one immunoglobulin-like domain [11]. Furthermore, CEA is usually found at higher concentrations in cerebrospinal fluid (CSF) of patients with metastatic tumors to CNS [34]. There is strong evidence regarding the capacity of CEA to penetrate the blood-brain barrier, behaving in a similar manner to Igs due to their homologous molecular weights [35, 36]. Thus, CEA-positive tumor cells could bond to brain vasculature, favoring CNS metastasis development, similar to leukocyte transendothelial arrest and migration through blood-brain barrier mediated by integrin-Ig adhesion interactions [37]; in addition, this could be explained by paraprotein-secreting cells, such as mononuclear cells with Ig-kappa and lambda light chains, which preferentially pass from peripheral blood to CSF [38]. CEA could represent a molecular target in patients with lung adenocarcinoma. CEA blockage or expression inhibition could slow or even prevent brain metastasis development in this group of patients. Target blockade of CEA with antibodies inhibits the cell migration, invasion, and adhesion in vitro and in vivo in several tumor cell lines [39]. Moreover, CEA serum levels could be a recognition tool of patients with high risk of metastasis development, who may benefit from CNS imaging tests prior to development of neurological symptoms. Other cell-adhesion-molecular markers associated to lymph-node metastasis, as chemokine receptors CCR7, CXCR3 and CCL21 [40, 41], could be related to brain metastasis development, thus, studies about analysis of their association with brain metastasis development are justified.
We found that OS-associated factors were gender, functional state, clinical stage, serum CEA levels, and EGFR tissue expression. Masculine gender is associated with a higher mortality risk than feminine gender. The eastern cooperative oncology group (ECOG) analyzed 1,594 patients, obtaining median survival of 9.2 months for females and 7.3 months for males (p = 0.004) [42], and Visbal et al. in 2004 conducted a study of 4,618 patients, finding a mortality risk for males of 1.2 (95% CI, 1.11–1.3) compared with females [43]. These findings are similar to our results [Fig. (A)]; however, they remain unexplained, and recent studies report contradictory results [44, 45]. Evidence oriented in favor of females (best survival and local control post-surgery) is based on a different etiologic possibility: best tolerance to chemotherapy and possibly an estrogen role in lung oncogenesis. EGFR tissue expression increased the mortality risk in our patients by 60% compared with those not expressing EGFR. As showed by meta-analysis in 2002, detection of EGFR tissue expression by IHC was associated with a worse prognosis (mortality, RR 1.13; 95% CI, 1–1.28) [46]. As previously reported, HER2 tissue expression had a low incidence in our population, and we found no association between HER2 expression and brain metastasis development or survival. Nonetheless, this may be due to low frequency; thus, we cannot conclude about its impact on brain metastasis development or survival.
It is convenient to mention that we found differences between CNS metastasis prensence at diagnosis and overall survial rates in our cohort patients, according to cut-off levels of CEA serum levels ≥ 10, 20 30 y 40 ng/mL; however, related differences to both variables were more notorious with a cut-off point of CEA serum level ≥ 40 ng/mL, thus we selected this value to perform our subsequent analyses.
In many neoplasms, high serum CEA levels have been previously described as a predictor of residual disease or tumor relapse in patients without normal-range serum levels after surgery [47]. We prospectively reported that patients with CEA serum levels ≥ 40 ng/mL had an increased mortality risk of 50% as an independent feature. In a retrospective study in 70 patients in early stages of NSCLC, those with high CEA serum levels demostrated an OS at 3 years of diagnosis of 0%, compared to 18% in patients with normal levels. In fact, Iwasaki et al. proposed a formula to evaluate mortality risk based on CEA serum levels, histological type, and presence of positive mediastinal lymph nodes [48]. High CEA serum levels may reflect micrometastatic disease, although we detect no differences of CEA serum levels between patients in IIIB and in IV clinical stage. This observation suggests that the prognostic role of high CEA serum levels could be not due to other factors aside from tumor charge. CEA comprises an important tumor marker associated with several physiopathological processes of tumorogenesis, such as immunological defense, cell adhesion, cell survival, and metastasis, and its expression is induced by hypoxia-inducible factor α (HIF-α), suggesting that CEA plays an important role as a microenvironmental factor during tumorogenesis and confers a worse prognosis [9, 49, 50].
Conclusion
High serum CEA level at diagnosis is an independent prognostic factor of CNS metastasis development and survival in patients with NSCLC. Surface expression of CEA in tumor cells could be a mechanism of invasion to CNS through immunoglobulin-related transport in blood-brain barrier. CEA may represent a potential molecular target.
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ: Cancer Statistics, 2008. CA Cancer J Clin. 2008, 58: 71-96. 10.3322/CA.2007.0010.
Cho LC, Dowell JE, Garwood D, Spangler A, Choy H: Prophylactic cranial irradiation with combined modality therapy for patients with locally advanced non-samll cell lung cancer. Semin Oncol. 2005, 32: 293-298. 10.1053/j.seminoncol.2005.02.017.
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R: Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997, 37: 745-751.
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B: A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990, 322: 494-500.
Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR, et al: Treatment of single brain metastases: radiotherapy alone or combined with neurosurgery?. Ann Neurol. 1993, 33: 583-590. 10.1002/ana.410330605.
Robnett TJ, Machtay M, Stevenson JP, Algazy KM, Hanh SM: Factors affecting the risk of brain metastases after definitive chemoradiation for locally advanced non-samll cell-lung carcinoma. J Clin Oncol. 2001, 19: 1344-1349.
Keith B, Vincent M, Stitt L, Tomiak A, Malthaner R, Yu E, Truong P, Inculet R, Lefcoe M, Dar AR, Kocha W, Craig I: Subsets more likely to benefit from surgery or prophylactic cranial irradiation after chemoradiation for localized non-small-cell lung cancer. Am J Clin Oncol. 2002, 25: 583-587. 10.1097/00000421-200212000-00011.
Komaki R, Scott CB, Byhardt R, Emami B, Asbell SO, Russell AH, Roach M, Parliament MB, Gaspar LE: Failure patterns by prognostic group determined by recursive partitioning analysis (RPA) of 1547 patients on four Radiation Therapy Oncology Group (RTOG) studies in inoperable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1998, 42: 263-267.
Kokkonen N, Ulibarri IF, Kauppila A, Luosujärvi H, Rivinoja A, Pospiech H, Kellokumpu I, Kellokumpu S: Hypoxia upregulates carcinoembryonic antigen expression in cancer cells. Int J Cancer. 2007, 121: 2443-2450. 10.1002/ijc.22965.
Hammarstrom S: The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999, 9: 67-81. 10.1006/scbi.1998.0119.
Kuespert K, Pils S, Hauck CR: CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol. 2006, 18: 565-571. 10.1016/j.ceb.2006.08.008.
Ford CH, Stokes HJ, Newman CE: Carcinoembryonic antigen and prognosis after radical surgery for lung cancer: immunocytochemical localization and serum levels. Br J Cancer. 1981, 44: 145-153.
Dent PB, McCulloch PB, Wesley-James O, MacLaren R, Muirhead W, Dunnett CW: Measurement of carcinoembryonic antigen in patients with bronchogenic carcinoma. Cancer. 1978, 42: 1484-1491. 10.1002/1097-0142(197809)42:3+<1484::AID-CNCR2820420819>3.0.CO;2-J.
Stokes TC, Stevens JF, Long P, Lockey E, Miller AL: Preoperative carcinoembryonic antigen and survival after resection of lung cancer. Br J Dis Chest. 1980, 74: 390-394.
Vincent RG, Chu TM, Fergen TB, Ostrander M: Carcinoembryonic antigen in 228 patients with carcinoma of the lung. Cancer. 1975, 36: 2069-2076. 10.1002/1097-0142(197509)36:3<873::AID-CNCR2820360308>3.0.CO;2-9.
Concannon JP, Dalbow MH, Hodgson SE, Headings JJ, Markopoulos E, Mitchell J, Cushing WJ, Liebler GA: Prognostic value of preoperative carcinoembryonic antigen (CEA) plasma levels in patients with bronchogenic carcinoma. Cancer. 1978, 42: 1477-1483. 10.1002/1097-0142(197809)42:3+<1477::AID-CNCR2820420818>3.0.CO;2-E.
Icard P, Regnard JF, Essomba A, Panebianco V, Magdeleinat P, Levasseur P: Preoperative carcinoembryonic antigen level as a prognostic indicator in resected primary lung cancer. Ann Thorac Surg. 1994, 58: 811-814.
Hotta K, Segawa Y, Takigawa N, Kishino D, Saeki H, Nakata M, Mandai K, Eguchi K: Evaluation of the relationship between serum carcinoembryonic antigen level and tratment outcome in surgically resected clinical-stage I patients with non-small-cell lung cancer. Anticancer Res. 2000, 20: 2177-2180.
Graziano SL, Tatum AH, Newman NB, Oler A, Kohman LJ, Veit LJ, Gamble GP, Coleman MJ, Barmada S, O'Lear S: The prognostic significance of neuroendocrine markers and carcinoembryonic antigen in patients with resected stage I ans II non-small cell lung cancer. Cancer Res. 1994, 54: 2908-2913.
Isobe T, Herbst RS, Onn A: Current management of advanced non-small cell lung cancer: targeted therapy. Semin Oncol. 2005, 32: 315-328. 10.1053/j.seminoncol.2005.02.016.
Mendelsohn J: Targeting the epidermal growth factor receptor for cancer therapy. J Clin Oncol. 2002, 20: 1-13.
Silvestri GA, Rivera MP: Targeted therapy for the treatment of advanced non-small cell lung cancer. A review of the epidermal growth factor receptor antgonists. Chest. 2005, 128: 3975-3984. 10.1378/chest.128.6.3975.
Salomon DS, Brandt R, Ciardiello F, Normanno N: Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995, 19: 183-232. 10.1016/1040-8428(94)00144-I.
Nakamura H, Kawasaki N, Taguchi M, Kabasawa K: Association of HER-2 overexpression with prognosis in non-small cell lung carcinoma: a metaanalysis. Cancer. 2005, 103: 1865-1873. 10.1002/cncr.20957.
Suzuki S, Dobashi Y, Sakurai H, Nishikawa K, Hanawa M, Ooi A: Protein overexpression and gene amplification of epidermal growth factor receptor in non-small cell lung carcinomas: an immunohistochemical and fluorescent in situ hybridation study. Cancer. 2005, 103: 1265-1273. 10.1002/cncr.20909.
Jeon YK, Sung SW, Chung JH, Park WS, Seo JW, Kim CW, Chung DH: Clinicopathologic features and prognostic implications of epidermal growth factor receptor (EGFR) gene copy number and protein expression in non-small cell lung cancer. Lung Cancer. 2006, 54: 387-398. 10.1016/j.lungcan.2006.08.015.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL: Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987, 235: 177-182. 10.1126/science.3798106.
Palmieri D, Chambers AF, Felding-Habermann B, Huang S, Steeg PS: The biology of metastasis to a sanctuary site. Clin Cancer Res. 2007, 13: 1656-1662. 10.1158/1078-0432.CCR-06-2659.
Swanton C, Futreal A, Eisen T: Her2-targeted therapy in non-small cell lung cancer. Clin Cancer Res. 2006, 12: 4377-4383. 10.1158/1078-0432.CCR-06-0115.
Nakamura H, Kawasaki N, Taguchi M, Kabasawa K: Survival impact of epidermal growth factor receptor overexpression in patients with non-small cell lung cancer: a meta-analysis. Thorax. 2006, 61: 140-145. 10.1136/thx.2005.042275.
Greene FL, Page DL, Fleming ID, eds, et al: American Joint Committee on Cancer Staging Manual. 2002, Philadelphia: Springer, 6
Sobin LH, Wittekind Ch, eds: TNM Classification of malignant tumors. 1997, New York: John Wiley & Sons, Inc, 5
Okada M, Nishio W, Skaamoto T, Uchino K, Yuki T, Nakagawa A, Tsubota N: Effect of hystologic type and smoking status on interpretation of serum carcinoembryonic antigen value in non-small cell lung carcinoma. Ann Thorac Surg. 2004, 78: 1004-1009. 10.1016/j.athoracsur.2004.03.019.
Noris-Garcia E, Escobar-Pérez X: Brain metastasis and the carcinoembryonic antigen. Rev Neurol. 2004, 38: 267-270.
Reiber H, Jacobi C, Felgenhauer K: Sensitive quantitation of carcinoembrionic antigen in cerebrospinal fluid and its barrier-dependent differentiation. Clin Chim Acta. 1986, 156: 259-269. 10.1016/0009-8981(86)90069-0.
Reiber H: Dynamics of brain-derived proteins in cerebrospinal fluid. Clin Chim Acta. 2001, 310: 173-186. 10.1016/S0009-8981(01)00573-3.
Pachter JS, de Vries HE, Fabry Z: The blood-brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol. 2003, 62: 593-604.
Vrethem M, Henriksson A, Malm C, Olsson T, Emerudh J: Ig-secreting cells pass the blood-brain barrier: studies on kappa and lambda light chain secreting cells in plasma cell dyscrasia. J Neuroimmunol. 1992, 41: 189-194. 10.1016/0165-5728(92)90069-W.
Blumenthal RD, Hansen HJ, Goldenberg DM: Inhibition of adhesion, invasion, and metastasis by antibodies targeting CEACAM 6(NCA-90) and CEACAM5 (Carcinoembryonic Antigen). Cancer Res. 2005, 65: 8809-8817. 10.1158/0008-5472.CAN-05-0420.
Maekawa S, Iwasaki A, Shirakusa T, Kawakami T, Yanagisawa J, Tanaka T, Shibaguchi H, Kinugasa T, Kuroki M, Kuroki M: Association between the expression of chemokine receptors CCR7 and CXCR3, and lymph node metastatic potential in lung adenocarcinoma. Oncol Rep. 2008, 19: 1461-1468.
Koizumi K, Kozawa Y, Ohashi Y, Nakamura ES, Aozuka Y, Sakurai H, Ichiki K, Doki Y, Misaki T, Saiki I: CCL21 promotes the migration and adhesion of highly lymph node metastatic human non-small cell lung cancer Lu-99 in vitro. Oncol Rep. 2007, 17: 1511-1516.
Wakelee HA, Wang W, Schiller JH, Langer CJ, Sandler AB, Belani CP, Johnson DH, for the Eastern Cooperative Oncology Group: Survival differences by sex for patients with advanced non-small cell lung cancer on Eastern Cooperative Oncology Group trial 1594. J Thorac Oncol. 2006, 1: 441-446. 10.1097/01243894-200606000-00011.
Visbal AL, Williams BA, Nichols FC, Marks RS, Jett JR, Aubry MC, Edell ES, Wampfler JA, Molina JR, Yang P: Gender differences in non-small cell lung cancer survival: an analysis of 4,618 patients diagnosed between 1997 and 2002. Ann Thorac Surg. 2004, 78: 209-215. 10.1016/j.athoracsur.2003.11.021.
Caldarella A, Crocetti E, Comin CE, Janni A, Pegna AL, Paci E: Gender differences in non-small cell lung cancer: a population-based study. Eur J Surg Oncol. 2007, 33: 763-768.
Moore R, Doherty D, Chamberlain R, Khuri F: Sex differences in survival in non-small cell lung cancer patients 1974–1998. Acta Oncologica. 2004, 43: 57-64. 10.1080/02841860310017973.
Meert AP, Martin B, Delmotte P, Berghmans T, Lafitte JJ, Mascaux C, Paesmans M, Steels E, Verdebout JM, Sculier JP: The role of EGF-R expression on patient survival in lung cancer: a systematic review with meta-analysis. Eur Respir J. 2002, 20: 975-981. 10.1183/09031936.02.00296502.
Macdonald JS: Carcinoembryonic antigen screening: pros and cons. Semin Oncol. 1999, 26: 556-560.
Iwasaki A, Shirakusa T, Yoshinaga Y, Enatsu S, Yamamoto M: Evaluation of the treatment of non-small cell lung cancer with brain metastasis and the role of risk score as a survival predictor. Eur J Cardiothoracic Surg. 2004, 26: 488-493. 10.1016/j.ejcts.2004.05.049.
Nittka S, Böhm C, Zentgraf H, Neumaier M: The CEACAM1-mediated apoptosis pathway is activated by CEA and triggers dual cleavage of CEACAM1. Oncogene. 2008, 27: 3721-3728. 10.1038/sj.onc.1211033.
Itlantzis C, DeMarte L, Screaton RA, Stanners CP: Deregulated expression of the human tumor marker CEA and CEA family member CEACAM6 disrupts tissue architecture and blocks colonocyte differentiation. Neoplasia. 2002, 4: 151-63. 10.1038/sj.neo.7900201.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/9/119/prepub
Acknowledgements
This work was partly supported by the National Council of Science and Technology of Mexico (CONACyT-014147 and CONACyT 044395).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DSP, RK, LM, DMP, PC, AA, EG and JDG participated in the design and follow-up of patients. AAS performed the analysis of tumor specimens. DSP helped to draft the manuscript and contributed to the statistical analysis. OA conceived of the study, and participated in its design and coordination, performed the statistical analysis and helped to draft the manuscript. All authors read and approved the final manuscript.
Roberto Kuri, Luis Martinez, Daniel Mendoza-Posada, Patricia Castillo, Alma Astorga, Enrique Guzman and Jaime De la Garza contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Arrieta, O., Saavedra-Perez, D., Kuri, R. et al. Brain metastasis development and poor survival associated with carcinoembryonic antigen (CEA) level in advanced non-small cell lung cancer: a prospective analysis. BMC Cancer 9, 119 (2009). https://doi.org/10.1186/1471-2407-9-119
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-9-119