Abstract
Background
Inflammatory breast cancer (IBC) is a distinct and aggressive form of locally-advanced breast cancer with high metastatic potential. In Tunisia, IBC is associated with a high death rate. Among the major molecular subtypes, basal breast carcinomas are poorly differentiated, have metastatic potential and poor prognosis, but respond relatively well to chemotherapy. The aim of this study was to determine the distribution of molecular subtypes in IBC and identify factors that may explain the poor prognosis of IBC.
Methods
To determine breast cancer subtypes we studied by immunohistochemistry the expression of 12 proteins in a series of 91 Tunisian IBC and 541 non-IBC deposited in tissue microarrays.
Results
We considered infiltrating ductal cases only. We found 33.8% of basal cases in IBC vs 15.9% in non-IBC (p < 0.001), 33.3% of ERBB2-overexpressing cases in IBC vs 14.5% in non-IBC (p < 0.001), and 29.3% of luminal cases in IBC vs 59.9% in non-IBC (p < 0.001). The most differentially-expressed protein between IBCs and non-IBCs was P-cadherin. P-cadherin expression was found in 75.9% of all IBC vs 48.2% of all non-IBC (p < 0.001), 95% of IBC vs 69% of non-IBC (p = 0.02) in basal cases, and 82% of IBC vs 43% of non-IBC (p < 0.001) in luminal cases. Logistic regression determined that the most discriminating markers between IBCs and non-IBCs were P-cadherin (OR = 4.9, p = 0.0019) MIB1 (OR = 3.6, p = 0.001), CK14 (OR = 2.7, p = 0.02), and ERBB2 (OR = 2.3, p = 0.06).
Conclusion
Tunisian IBCs are characterized by frequent basal and ERBB2 phenotypes. Surprisingly, luminal IBC also express the basal marker P-cadherin. This profile suggests a specificity that needs further investigation.
Similar content being viewed by others
Background
Inflammatory breast cancer (IBC) represents less than 10% of all breast cancers but is the most lethal form of the disease [1]. Evolution of this distinct and aggressive form of locally-advanced breast cancer depends on the type of neo-adjuvant chemotherapy but is overall poor [2]. IBC, classified T4d (stage IIIB) according to the TNM classification of the American Joint Committee of Cancer [5], is generally a rapidly growing tumor associated with cutaneous erythema and edema [2–4]. The inflammatory aspect is associated with highly angiogenic and angio-invasive properties and with the presence of dermal lymphatic emboli [5]. The high metastatic potential of IBCs suggests a high propensity of the tumor cells to migrate.
IBCs in Tunisia are characterized by a higher frequency and a higher aggressiveness than in European countries [6–8]. However, we did not observe any difference between Tunisian and French IBCs in the expression of five representative proteins (E-cadherin, Estrogen receptor (ER), MIB1, MUC1 and ERBB2) [9, 10].
In non-IBCs the five molecular subtypes (luminal A and B, basal, ERBB2-overexpressing and normal-like) are associated with different features, including response to chemotherapy and clinical outcome [11]. Basal and ERBB2 subtypes have the worst prognosis, followed by luminal B subtype. The proportion of these subtypes has been determined by gene expression profiling in European IBC. Around half the cases are of basal and ERBB2 subtypes.
The subtypes defined by gene expression analysis have also been defined at the protein level using various markers. A panel of antibodies (directed against ER, ERBB2, EGFR, basal cytokeratin CK5/6 and/or KIT) identifies basal tumors with high specifity [12]. Other markers such as P-cadherin, CK14, P53, CAV1, PR, MIB1 and moesin also identify basal tumors [13–16]. Reciprocally, the expression of ER and ERBB2 exclude basal tumors.
The high frequency of the two subtypes associated with poor prognosis, basal and ERBB2, explains only in part the fatal evolution of IBC. In IBCs, the luminal cases also have a poor prognosis. Only a factor common to all subtypes could explain IBC poor evolution.
In breast cancer, P-cadherin is associated with enhanced cell invasion, tumor aggressiveness, motility [17] and with a poor prognosis [18, 19]. P-cadherin mRNA is essentially expressed in basal carcinomas [20, 21].
The aims of this study were to determine the importance of breast carcinoma subtypes in IBC and to identify a factor that could explain the poor prognosis of IBC. We show that this factor could be associated with P-cadherin expression.
Methods
Definition and selection of cases
We collected a series of 91 Tunisian T4d tumors (TNM, UICC) treated between 1994 and 1998 at the Salah Azaiz Institute (ISA, Tunis, Tunisia). Paraffin-embedded specimens were collected prior chemotherapy. The presence of dermal lymphatic emboli was not mandatory. All patients received neo-adjuvant chemotherapy with anthracyclin-based (FEC 100 or FAC 50) regimen. Forty-three patients were metastatic at diagnosis and did not have complementary mastectomy. External beam radiation was done for all patients.
This series was compared with a consecutive series of 547 non-IBC cases deposited in tissue microarrays and previously used in a study of IBC cases that established IBC immunohistochemical profile [22] and for other purposes [23]. The 547 non-IBC cases were selected from cases included in the database of the Paoli-Calmettes Institute (IPC, Marseille, France) treated between 1990 and 1999. They were defined as T1, T2, T3 tumors (TNM, UICC), collected prior to adjuvant chemotherapy and embedded in paraffin.
Clinicopathological study
The clinical records were reviewed to determine the following patient characteristics: age, metastatic status, therapeutic regimen and survival. Pathological slides were reviewed by two pathologists (JJ, KM) according to the European guidelines [24]. Criteria evaluated were histological type, Elston-and-Ellis grade and, whenever possible, peritumoral vascular invasion.
Tissue Microarray construction
Two tissue microarrays (TMA) were used, one for the IBCs (ISA set) and the other one for the non-IBCs (IPC set). TMAs were prepared as described, with slight modifications [22–25]. Briefly, for each tumor, three representative tumor areas were carefully selected from a hematoxylin-eosin-safran stained section of a donor block. Core cylinders with a diameter of 0.6 mm each were punched from each of these areas and deposited into a recipient paraffin block using a specific arraying device (Alphelys, Plaisir, France). In addition to tumor tissues, the recipient blocks also received normal breast tissues and cell lines. Five-μm sections of the resulting microarray blocks were made and used for IHC analysis after transfer to glass slides.
Markers and immunohistochemistry
Immunohistochemistry (IHC) was performed on 5-μm sections. The characteristics of the antibodies used and pre-treatment conditions are listed in Table 1. A good concordance of IHC results has been reported between standard full tissue sections and TMA [21]. TMA data were evaluated by the mean score of two cores biopsies minimum for each case. The slides were dewaxed, pre-treated according to the supplier's recommendations (Table 1). This was followed by the use of a streptavidin/biotin kit (Dako, Trappes, France). Diaminobenzidine, (DAB) or 3-amino-9-ethylcarbazole (AEC) was used as chromogen. Sections were counterstained with hematoxylin and coverslipped. Slides were evaluated under a light microscope by two independent observers on the Spot Browser device (Alphelys). Immunoreactivities were classified by estimating the percentage (P) of tumor cells showing characteristic staining (from 0%, undetectable level, to 100%, homogeneous staining) and by estimating the intensity (I) of staining 1, weak staining; 2, moderate staining; 3, strong staining). Results were scored by multiplying the percentage of positive cells by the intensity, i.e. by the so-called quick-score (Q). Internal positive controls such as epidermis or benign breast lobules were used. Hormone receptors (ER, PR) were positive when at least 1% of tumor cell nuclei were stained. For ERBB2, the Dako scale was used; staining was considered as positive when limited to a membrane staining of more than 10% of tumor cells and scored as 1+, 2+ or 3+ according to intensity and partial/complete staining. Protein overexpression was considered for 2+ and 3+. Cytokeratin 5/6, cytokeratin 14 and EGFR were scored positive if any (weak or strong) membranous invasive carcinoma cell staining was observed. Caveolin 1 (CAV1) and Caveolin 2 (CAV2) status of tumor cells was evaluated by light microscopy as either positive (any tumor cell with IHC staining) or negative. For CAV1 negative cases, endothelial cells and interstitial fibroblasts were used as internal positive controls.
Statistical analysis
Data were summarized by frequencies and percentages for categorical variables. Furthermore, for continuous variables the means, the median and range were computed. To investigate the association between categorical variables, univariate statistical analysis were done, using Pearson's Chi-2 test or Fisher's exact test for small sample size and using non-parametric Mann and Whitney test for continuous variables [26]. Multivariate analysis was done using logistic regression model with backward stepwise selection procedure to evaluate the effect of interactions between the different variables. All statistical tests were two-sided at the 5% level of significance. All the statistical analyses were done using R.2.4.0 statistical software[27].
Results
Characteristics of patients and tumors
A total of 91 IBC and 547 non-IBC informative cases from women patients were analyzed. Their characteristics are listed in Table 2. Age was different (p < 0.001) for the two series: for IBCs, age ranged from 22 to 76 years (mean 43.47 years) whereas for non-IBCs, age ranged from 25 to 94 years (mean 59.43 years).
Among the 91 IBCs, 86 were invasive ductal carcinomas (IDC) and 5 were invasive micropapillary carcinomas. The distribution of histological types in non-IBCs was representative of unbiased populations. IBCs were more frequently of grade II and III (65.93%, 30.77%) than non-IBCs (p < 0.001) (Table 2).
Protein expression profiles of IBC and non-IBC and immunohistochemical subtypes
IHC was done on IBCs and non-IBCs using three TMA slides for each marker. We compared the expression of the markers only for the infiltrating ductal cases, i.e. 86 IBC cases and 386 non-IBC cases. We observed differences between the two series for: ER negativity (53.85% IBCs, 26.26 % non-IBCs; p < 0.001), PR negativity (52.86% IBCs, 35.39% non-IBCs; p < 0.01), ERBB2 positivity (33.33% IBCs, 14.49% non-IBCs; p < 0.001), CK14 positivity (19.15% IBCs, 5.45% non-IBCs; p < 0.01), P-cadherin positivity (75.93% IBCs, 48.16% non-IBCs; p < 0.001) (Figure 1) and proliferative rate expressed by a Ki67 index (MIB1) higher than 20% (41.1% IBCs, 12.98% non-IBCs; p < 0.001) (Table 3).
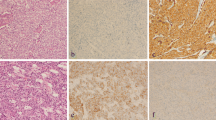
Immunohistochemistry on tissue microarray sections of breast cancers of the luminal subtype. A: Expression of estrogen receptor in 100% of tumor cells in a grade III inflammatory case. B: Strong expression of P-cadherin in 100% of tumor cells in the same case. C: Higher magnification showing the cell membrane localization. D: Expression of estrogen receptor in 100 % of tumor cells in a more differentiated (grade II) inflammatory case. E: Moderate expression of P- cadherin in 100% of tumor cells in the same case. F: Higher magnification showing the cell membrane localization. G: Luminal grade II non inflammatory case with 100% estrogen receptor-positive cells. H: Absence of expression of P-cadherin in the same non inflammatory case; note the positive internal control on myoepithelial cells of the normal duct. I: Higher magnification showing the cell membrane localization of the myoepithelial cells by contrast to the negativity of the luminal normal cells.
We classified tumors in terms of subtypes based on ER/PR and ERBB2 IHC expression [12, 28]. The analysis was restricted to the infiltrating ductal cases (86 IBC and 386 non-IBC), and allowed a classification in three groups (Table 4). ER-positive and PR-positive tumors were classified as luminal. ERBB2-positive tumors were classified as ERBB2-overexpressing. ER-negative and ERBB2-negative (double negative) were considered as basal (ER-/ERBB2-). Using this protein-based definition of subtypes, 33.8% of IBCs vs 15.93 % of non-IBCs (p < 0.001) were basal, 33.33 % of IBCs vs 14.49% of non-IBCs (p < 0.001) were ERBB2-overexpressing, and 29.33% of IBCs vs 58.93% of non-IBCs (p < 0.001) were luminal.
Expression of P-cadherin in IBC and non-IBC
Univariate analyses
Among various markers tested, the expression of P-cadherin was worth further investigation. The expression of P-cadherin varied according to the subtype and the IBC status in infiltrating ductal cases. The expression of P-cadherin in the basal subtype was 95% for IBCs vs 69% for non-IBCs (p = 0.02). There was no difference between IBCs and non-IBCs in the ERBB2-overexpressing subtype. In luminal tumors, P-cadherin was expressed in 82 % of IBCs vs 43% of non-IBCs (p < 0.01) (Table 5). Finally, we found a strong correlation between P-cadherin and Ki67/MIB1 (p = 0.0004).
Multivariate analyses
We used a logistic regression stepwise selection with clinical and biological factors (Table 6). The most discriminant factors for IBC status were grade (Odd ratio, OR = 6.28, p = 0.081), and P-cadherin (OR = 4.21, p = 0.013), MIB1 (OR = 2.82, p = 0.018), and ER (OR = 1.00, p = 0.053) expression. In a logistic regression by backward stepwise selection using biological factors only, the factors discriminating IBC from non-IBC were P-cadherin (OR = 4.9, p = 0.0019), MIB1 (OR = 3.6, p = 0.001), CK14 (OR = 2.7, p = 0.02), and ERBB2 (OR = 2.3, p = 0.06).
Discussion
The identification of a specific IBC profile could improve the diagnosis, treatment and evolution of this aggressive form of locally-advanced breast cancer with high metastatic potential. In Tunisia, IBCs are frequent and have a worse prognosis than in European countries. However, in a previous study, we did not observe any difference in protein expression between IBCs from France and Tunisia [10]. Five major molecular subtypes have been defined by gene expression profiling [11, 20, 29]. Basal and ERBB2 subtypes represent each about 20% of cases. These subtypes could also be recognized at the protein level, with a very good correlation with RNA analyses. IHC-defined subtypes also show differences in prognosis in non-IBCs: basal and ERBB2-overexpressing subtypes are associated with poor prognosis. To better understand the specificity and the poor prognostic of IBCs in general and Tunisian IBCs in particular, we studied by IHC on TMA the expression of proteins commonly used as markers for molecular subtypes.
We studied several markers but eventually used a simple operational definition based on ER and ERBB2 expression. The basal subtype was defined as negative for both ER and ERBB2. In non-IBCs basal and ERBB2 subtypes represent around 40% of cases. We compared only infiltrating ductal cases. We found differences in the proportion of subtypes in Tunisian IBCs. Basal and ERBB2 subtypes made up each about one-third of the cases. Overall, these data and those of the literature show an over-representation of basal and ERBB2 cases in IBCs as compared with non-IBCs [30, 31].
However, the high proportion of basal and ERBB2 subtypes is not sufficient per se to explain IBC prognosis. Only a factor common to all subtypes could explain IBC poor evolution. Surprisingly, we found that luminal IBCs express basal markers such as P-cadherin. Indeed, P-cadherin expression was not only higher in basal IBCs than in basal non-IBCs (95%/69%) but also higher in the IBC luminal cases (82%/42%). The logistic regression analysis showed that P-cadherin was the most representative marker of IBC.
P-cadherin is one of the most specific markers of myoepithelial cells and is associated with basal subtype. The frequency of P-cadherin IHC expression varies from 20% in initial works [32] to 40% in more recent series[33]. This percentage exceeds that of basal tumors suggesting that P-cadherin could also be expressed in non-basal cases. P-cadherin expression in breast cancer correlates with high grade, lack of ER/PR expression, increased tumor aggressiveness, high proliferation rate, and poor survival. P-cadherin is associated with MIB1, EGFR, ERBB2, P53 and CK5/6 expression [23]. This expression may be due to hypomethylation of the gene promoter [18]. Deregulated P-cadherin expression may alter epithelial cell behavior thereby contributing to a more aggressive tumor cell phenotype and poor survival [34]. P-cadherin has pro-invasive activity in the MCF-7/AZ luminal breast cancer cell line, through interaction with signaling proteins bound to its juxtamembrane domain. ICI182,780, which blocks ER, induces increased expression of P-cadherin, which is associated with in vitro invasion[35]. Overexpressed P-cadherin increases motility of pancreatic cancer cells by interacting with p120ctn and subsequent activation of RHO GTPases [36]. Activation of RHO GTPases has been observed in IBC. Overexpression of RHOC is associated with the loss of expression of WISP3 [37, 38], which acts as a tumor suppressor. RHOC is overexpressed in over 90% of IBCs vs 36% of non-IBCs. RHOC activity requires NFκB stimulation [39]. P-cadherin transgenic mice do not develop mammary tumors spontaneously. When mammary tumors are induced in the P-cadherin transgenic mice through breeding with the MMTV/neu transgenic mouse, the tumors do not express P-cadherin. This indicates that P-cadherin is not per se an oncogene [34].
Conclusion
Expression of P-cadherin in luminal IBC may indicate that the tumor cells derive from a stem/basal cell that has acquired ER expression and a luminal phenotype but retained some basal features. This suggests that IBC derives from a basal cell and progress along a specific oncogenic pathway allowing partial differentiation. This origin may be associated with specific IBC features and aggressiveness even in luminal cases. Although it may contribute, P-cadherin expression may be a sign of but not the reason for this aggressiveness.
Abbreviations
- CK:
-
Cytokeratin
- ER:
-
Estrogen receptor
- GTPase:
-
Guanosine triphosphate
- IBC:
-
Inflammatory breast cancer
- IHC:
-
Immunohistochemistry
- MMTV:
-
Mouse mammary tumor virus
- NF-kappa B:
-
Nuclear factor
- PR:
-
Progesterone receptor
- TMA:
-
Tissue microarrays.
References
Chevallier B, Asselain B, Kunlin A, Veyret C, Bastit P, Graic Y: Inflammatory breast cancer. Determination of prognostic factors by univariate and multivariate analysis. Cancer. 1987, 60: 897-902. 10.1002/1097-0142(19870815)60:4<897::AID-CNCR2820600430>3.0.CO;2-S.
Chang S, Parker SL, Pham T, Buzdar AU, Hursting SD: Inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program of the National Cancer Institute, 1975–1992. Cancer. 1998, 82: 2366-2372. 10.1002/(SICI)1097-0142(19980615)82:12<2366::AID-CNCR10>3.0.CO;2-N.
Chevallier B, Roche H, Olivier JP, Chollet P, Hurteloup P: Inflammatory breast cancer. Pilot study of intensive induction chemotherapy (FEC-HD) results in a high histologic response rate. Am J Clin Oncol. 1993, 16: 223-228. 10.1097/00000421-199306000-00006.
Cristofanilli M, Buzdar AU, Hortobagyi GN: Update on the management of inflammatory breast cancer. Oncologist. 2003, 8: 141-148. 10.1634/theoncologist.8-2-141.
Van dA I, Van Laere SJ, Van den Eynden GG, Benoy I, van Dam P, Colpaert CG, Fox SB, Turley H, Harris AL, Van Marck EA, Vermeulen PB, Dirix LY: Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin Cancer Res. 2004, 10: 7965-7971. 10.1158/1078-0432.CCR-04-0063.
Levine PH, Steinhorn SC, Ries LG, Aron JL: Inflammatory breast cancer: the experience of the surveillance, epidemiology, and end results (SEER) program. J Natl Cancer Inst. 1985, 74: 291-297.
Levine PH, Pogo BG, Klouj A, Coronel S, Woodson K, Melana SM, Mourali N, Holland JF: Increasing evidence for a human breast carcinoma virus with geographic differences. Cancer. 2004, 101: 721-726. 10.1002/cncr.20436.
Mourali N, Levine PH, Tabanne F, Belhassen S, Bahi J, Bennaceur M, Herberman RB: Rapidly progressing breast cancer (poussee evolutive) in Tunisia: studies on delayed hypersensitivity. Int J Cancer. 1978, 22: 1-3. 10.1002/ijc.2910220102.
Charafe-Jauffret E, Tarpin C, Bardou VJ, Bertucci F, Ginestier C, Braud AC, Puig B, Geneix J, Hassoun J, Birnbaum D, Jacquemier J, Viens P: Immunophenotypic analysis of inflammatory breast cancers: identification of an 'inflammatory signature'. J Pathol. 2004, 202: 265-273. 10.1002/path.1515.
Charafe-Jauffret E, Mrad K, Intidhar LS, Ben Hamida A, Ben Romdhane K, Ben Abdallah M, Ginestier C, Esterni B, Birnbaum D, Ben Ayed F, Xerri L, Viens P, Mezlini A, Jacquemier J: Inflammatory breast cancers in Tunisia and France show similar immunophenotypes. Breast. 2007, 16: 352-358. 10.1016/j.breast.2007.01.002.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de RM, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein LP, Borresen-Dale AL: Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001, 98: 10869-10874. 10.1073/pnas.191367098.
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de RM, Perou CM: Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004, 10: 5367-5374. 10.1158/1078-0432.CCR-04-0220.
Birnbaum D, Bertucci F, Ginestier C, Tagett R, Jacquemier J, Charafe-Jauffret E: Basal and luminal breast cancers: basic or luminous? (review). Int J Oncol. 2004, 25: 249-258.
Pinilla SM, Honrado E, Hardisson D, Benitez J, Palacios J: Caveolin-1 expression is associated with a basal-like phenotype in sporadic and hereditary breast cancer. Breast Cancer Res Treat. 2006, 99: 85-90. 10.1007/s10549-006-9184-1.
Charafe-Jauffret E, Monville F, Bertucci F, Esterni B, Ginestier C, Finetti P, Cervera N, Geneix J, Hassanein M, Rabayrol L, Sobol H, Taranger-Charpin C, Xerri L, Viens P, Birnbaum D, Jacquemier J: Moesin expression is a marker of basal breast carcinomas. Int J Cancer. 2007, 121: 1779-1785. 10.1002/ijc.22923.
Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, Perou CM: Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006, 19: 264-271. 10.1038/modpathol.3800528.
Sanders AP, Perry I, Hardy R, Jankowski J: Aberrant P-cadherin expression is a feature of clonal expansion in the gastrointestinal tract associated with repair and neoplasia. J Pathol. 2000, 190: 526-530. 10.1002/(SICI)1096-9896(200004)190:5<526::AID-PATH564>3.0.CO;2-9.
Paredes J, Albergaria A, Oliveira JT, Jeronimo C, Milanezi F, Schmitt FC: P-cadherin overexpression is an indicator of clinical outcome in invasive breast carcinomas and is associated with CDH3 promoter hypomethylation. Clin Cancer Res. 2005, 11: 5869-5877. 10.1158/1078-0432.CCR-05-0059.
Rakha EA, Putti TC, Abd EL-Rehim DM, Paish C, Green AR, Powe DG, Lee AH, Robertson JF, Ellis IO: Morphological and immunophenotypic analysis of breast carcinomas with basal and myoepithelial differentiation. J Pathol. 2006, 208: 495-506. 10.1002/path.1916.
Perou CM, Sorlie T, Eisen MB, van de RM, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D: Molecular portraits of human breast tumours. Nature. 2000, 406: 747-752. 10.1038/35021093.
Paredes J, Correia AL, Ribeiro AS, Albergaria A, Milanezi F, Schmitt FC: P-cadherin expression in breast cancer: a review. Breast cancer Res. 2007, 9: 214-10.1186/bcr1774.
Ginestier C, Charafe-Jauffret E, Bertucci F, Eisinger F, Geneix J, Bechlian D, Conte N, Adelaide J, Toiron Y, Nguyen C, Viens P, Mozziconacci MJ, Houlgatte R, Birnbaum D, Jacquemier J: Distinct and complementary information provided by use of tissue and DNA microarrays in the study of breast tumor markers. Am J Pathol. 2002, 161: 1223-1233.
Jacquemier J, Ginestier C, Rougemont J, Bardou VJ, Charafe-Jauffret E, Geneix J, Adelaide J, Koki A, Houvenaeghel G, Hassoun J, Maraninchi D, Viens P, Birnbaum D, Bertucci F: Protein expression profiling identifies subclasses of breast cancer and predicts prognosis. Cancer Res. 2005, 65: 767-779.
[European recommendations for quality assurance in the setting of mammographic screening for breast cancer. Report of pathologists of the working group "Breast Cancer Screening" of the European Union]. Ann Pathol. 1996, 16: 315-333.
Van den Eynden GG, Van dA I, Van Laere S, Colpaert CG, van Dam P, Merajver S, Kleer CG, Harris AL, Van Marck EA, Dirix LY, Vermeulen PB: Validation of a tissue microarray to study differential protein expression in inflammatory and non-inflammatory breast cancer. Breast Cancer Res Treat. 2004, 85: 13-22. 10.1023/B:BREA.0000021028.33926.a8.
Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958, 53: 457-481. 10.2307/2281868.
R Development Core Team: R: A language and environment for statistical computing. R Foundation for Statiscal Computing, Vienna, Austria. 2006, ISBN 3-900051-07-0, URL, [http://www.R-project.org]
Yehiely F, Moyano JV, Evans JR, Nielsen TO, Cryns VL: Deconstructing the molecular portrait of basal-like breast cancer. Trends Mol Med. 2006, 12: 537-544. 10.1016/j.molmed.2006.09.004.
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D: Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003, 100: 8418-8423. 10.1073/pnas.0932692100.
Sawaki M, Ito Y, Akiyama F, Tokudome N, Horii R, Mizunuma N, Takahashi S, Horikoshi N, Imai T, Nakao A, Kasumi F, Sakamoto G, Hatake K: High prevalence of HER-2/neu and p53 overexpression in inflammatory breast cancer. Breast Cancer. 2006, 13: 172-178. 10.2325/jbcs.13.172.
Turpin E, Bièche I, Bertheau P, Plassa LF, Lerebours F, de Roquancourt A, Olivi M, Espié M, Marty M, Lidereau R, Vidaud M, de Thé H: Increased incidence of ERBB2 overexpression and TP53 mutation in inflammatory breast cancer. Oncogene. 2002, 21: 7593-7597. 10.1038/sj.onc.1205932.
Palacios J, Benito N, Pizarro A, Suarez A, Espada J, Cano A, Gamallo C: Anomalous expression of P-cadherin in breast carcinoma. Correlation with E-cadherin expression and pathological features. Am J Pathol. 1995, 146: 605-612.
Kovacs A, Dhillon J, Walker RA: Expression of P-cadherin, but not E-cadherin or N-cadherin, relates to pathological and functional differentiation of breast carcinomas. Mol Pathol. 2003, 56: 318-322. 10.1136/mp.56.6.318.
Radice GL, Sauer CL, Kostetskii I, Peralta SA, Knudsen KA: Inappropriate P-cadherin expression in the mouse mammary epithelium is compatible with normal mammary gland function. Differentiation. 2003, 71: 361-373. 10.1046/j.1432-0436.2003.7106005.x.
Paredes J, Stove C, Stove V, Milanezi F, Van MV, Derycke L, Mareel M, Bracke M, Schmitt F: P-cadherin is up-regulated by the antiestrogen ICI 182,780 and promotes invasion of human breast cancer cells. Cancer Res. 2004, 64: 8309-8317. 10.1158/0008-5472.CAN-04-0795.
Taniuchi K, Nakagawa H, Hosokawa M, Nakamura T, Eguchi H, Ohigashi H, Ishikawa O, Katagiri T, Nakamura Y: Overexpressed P-cadherin/CDH3 promotes motility of pancreatic cancer cells by interacting with p120ctn and activating rho-family GTPases. Cancer Res. 2005, 65: 3092-3099.
Kleer CG, van Golen KL, Merajver SD: Molecular biology of breast cancer metastasis. Inflammatory breast cancer: clinical syndrome and molecular determinants. Breast Cancer Res. 2000, 2: 423-429. 10.1186/bcr89.
Kleer CG, Zhang Y, Merajver SD: CCN6 (WISP3) as a new regulator of the epithelial phenotype in breast cancer. Cells Tissues Organs. 2007, 185: 95-99. 10.1159/000101308.
Van Laere SJ, Van dA I, Van den Eynden GG, van Dam P, Van Marck EA, Vermeulen PB, Dirix LY: NF-kappaB activation in inflammatory breast cancer is associated with oestrogen receptor downregulation, secondary to EGFR and/or ErbB2 overexpression and MAPK hyperactivation. Br J Cancer. 2007, 97: 659-669. 10.1038/sj.bjc.6603906.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/8/28/prepub
Acknowledgements
This work was supported by Institut Paoli-Calmettes, Inserm, Institut National du Cancer (PL2006) and Ligue Nationale Contre le Cancer (Label DB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
ABH and JJ carried out the design of the study and the analysis and interpretation of the data and drafted the manuscript. JJ, ECJ and KM are the pathologists responsible for the diagnosis, dissection of the tumor material and interpretation of the immunohistochemical staining. ISL provided and analyzed clinical data. BE carried out the statistical analysis. PV, SBA, LX and FB were involved in the design. DB participated in the draft of the manuscript and the analysis and interpretation of results. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ben Hamida, A., Labidi, I.S., Mrad, K. et al. Markers of subtypes in inflammatory breast cancer studied by immunohistochemistry: Prominent expression of P-cadherin. BMC Cancer 8, 28 (2008). https://doi.org/10.1186/1471-2407-8-28
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-8-28